Abstract
Objective
The addition of androgen deprivation therapy (ADT) to conventional radiation therapy improves overall survival (OS) in intermediate- and high-risk prostate cancer. The benefit of ADT to added to dose-escalated radiotherapy is less clear. The aim of this study was to report disease control outcomes and to identify prognostic variables associated with favorable outcomes in patients with intermediate- and high-risk prostate cancer treated with dose-escalated radiation therapy without ADT.
Methods and materials
From September 2001 to March 2010, 127 patients with intermediate- or high-risk prostate cancer were treated with dose-escalated radiation otherapy without ADT. Biochemical recurrence-free survival (bRFS), distant metastases-free survival (DMFS), prostate cancer–specific mortality, and OS were assessed. Univariate and multivariate analyses using Cox regression modeling were performed.
Results
The median follow-up was 6.5 years, and the 5-year estimated bRFS, DMFS, prostate cancer–specific mortality, and OS for all patients was 89%, 96.1%, 98.4%, and 96.9% respectively. On multivariate analysis, factors that predict bRFS include risk group and PSA nadir, and factors that predict DMFS include perineural invasion, risk group, and PSA nadir.
Conclusions
Patients with favorable intermediate-risk cancer could likely be treated with dose-escalated radiation therapy without ADT. Patients with high-risk and unfavorable intermediate-risk cancer, perineural invasion, and PSA nadir ≥1ng/dL had worse outcomes and likely need distinct therapeutic approaches.
Summary.
Most studies on prostate cancer report that the addition of androgen deprivation therapy (ADT) to conventional radiation therapy (RT) improves overall survival. Nevertheless, the benefit of ADT when added to dose-escalated RT is less clear in patients with intermediate- or high-risk prostate cancer. We found that patients with favorable intermediate-risk prostate cancer likely can be treated with dose-escalated RT without ADT. However, for patients with high-risk or unfavorable intermediate-risk prostate cancer, perineural invasion and prostate-specific antigen nadir ≥1ng/dL had worse outcomes and these patients would likely benefit from both approaches.
Introduction
The addition of androgen deprivation therapy (ADT) to radiation therapy (RT) for patients with intermediate (ie, prostate-specific antigen [PSA] 10-20 ng/mL; Gleason score 7; or tumor stage T2b-T2c) or high-risk (PSA ≥20 ng/mL; tumor stage ≥T3a; or Gleason score ≥8) prostate cancer has become the standard of care based on several randomized trials that have demonstrated improved outcomes.1, 2, 3 An important criticism of these studies is that the majority of patients who were treated had locally advanced prostate cancer (≥T3). Furthermore, given the available technology at the time, most patients received radiation doses that were below 74 Gy.
Since the publication of these studies, significant advances in conformal treatment delivery in the form of intensity modulated radiation therapy (IMRT) have allowed for excellent target coverage with high doses of radiation.4 RT with doses above 74 Gy is an important, well-established treatment for prostate cancer control. A meta-analysis of randomized clinical trials that compared high- versus low-dose RT for patients with clinically localized prostate cancer showed a significant reduction in biochemical failure over a period of 5 years for the group of patients who were treated with high doses of RT.5
However, in the results of a large phase 3 trial, published in abstract form, 1532 patients with localized prostate cancer were randomized to 79.2 Gy and 70.2 Gy treatment groups; after a median follow-up of 7 years, dose escalation did not improve overall survival (OS) despite significant improvement in biochemical failure, distant metastases, and local progression.6
In addition to the potential benefits in disease control, ADT is associated with a variety of adverse effects that include erectile dysfunction, diabetes, risk of fracture, and in some patients an increased risk of cardiovascular disease.7, 8 When considering these possible harmful side effects and the improved outcomes from dose escalation, the benefit of ADT for patients with unfavorable localized prostate cancer and small volume of disease (ie, tumor stage T1-T2) is called into question.9, 10
Several retrospective series have shown no benefit of adding ADT to high-dose RT for patients with intermediate-risk prostate cancer.11, 12 In contrast, the preliminary results of two prospective randomized trials showed that ADT with dose-escalated RT improves disease control for patients with intermediate-risk prostate cancer.13, 14
The aim of this study was to report the results of disease control and identify the prognostic factors that are associated with biochemical recurrence-free survival (bRFS), distant metastases-free survival (DMFS), prostate cancer-specific mortality (PCSM), and OS in patients with intermediate- or high-risk clinically localized prostate cancer with less bulky disease who are treated with dose-escalated RT without hormone therapy.
Methods
Study design and population
This is a retrospective cohort study of consecutive patients with histologically proven prostate cancer who fulfilled the inclusion criteria and were treated with IMRT between September 2001 and March 2010 at a single institution. During this period, 671 patients with prostate cancer were treated at the institution. Patients were excluded if they were treated with doses of RT <74 Gy, underwent any type of hormone therapy, or had RT to pelvic lymph nodes. Patients with low-risk prostate cancer (T1-T2a; PSA <10 ng/mL; and Gleason score <7), locally advanced disease (≥T3), metastatic disease, or lymph node involvement were also excluded. A total of 127 patients were included in the study. Using the National Comprehensive Cancer Network (NCCN) criteria,15 75 patients with intermediate-risk and 52 patients with high-risk prostate cancer were selected. Although most of these patients had oncologic reasons for receiving ADT, they did not receive ADT either due to potential side effects, comorbidities, or patient preference. The medical records of these patients were reviewed with the approval of the Ethics Committee of the Sírio-Libanês Hospital.
Treatment
The simulation and treatment procedures have been reported in a previous publication.16 All patients were treated with computed tomography planning. The clinical target volume (CTV) for patients with intermediate-risk prostate cancer included the prostate and the proximal portion of the seminal vesicles. CTV for patients with high-risk prostate cancer included the prostate and the entire seminal vesicles. The planning target volume consisted of the CTV plus a 10-mm margin in all directions.
RT was planned in two phases. The first phase was carried out with five fields using the IMRT step-and-shoot technique. A total dose of 60 Gy to the prostate and 54 Gy to the seminal vesicles (30 fractions of 2 Gy and 1.8 Gy, respectively) was delivered in this phase. In the second phase, a three-field 3-dimensional conformal RT technique was used to deliver 7 to 10 fractions of 2 Gy in the prostate only, totaling 74 Gy to 80 Gy.
Outcomes
The primary endpoint was bRFS. Biochemical failure was defined in accordance with the Phoenix criteria (PSA nadir + 2.0 ng/mL).17 Additional endpoints were DMFS, PCSM, and OS. The intervals to PSA recurrence, metastasis, and death were all defined relative to the start of RT until the event of interest, death, or last-follow-up visit.
Covariates
Variables that represent potential risk factors were examined in a multiple regression analysis according to a conceptual hierarchical framework.18 This framework was composed of five levels: 1) pretreatment PSA level (<10 ng/mL and ≥10 ng/mL), age (<70 years and ≥70 years), tumor stage (T1a-T2c), proportion of positive biopsy cores (<50% and ≥50%), NCCN risk classification, Gleason score (3+4, 4+3, or >7), and perineural invasion; 2) PSA nadir (<1 ng/mL and ≥1 ng/mL); 3) biochemical failure; 4) distant metastases; and 5) death.
A risk classification variable was created by combining the values of PSA, tumor stage, and Gleason score.15 When the risk classification model was included in the models, its component variables were not. This variable was initially created with three categories: intermediate risk with one NCCN risk factor, intermediate risk with >1 NCCN risk factor, and high risk. However, because the two upper categories were found to produce similar results, indicating no statistical difference, a new binary variable was created to retain the baseline category (intermediate risk with one NCCN risk factor) and combine the two upper categories (intermediate risk with >1 NCCN factor and high risk). Multiple regression results are presented only for this binary variable.
Statistical analysis
Univariate analysis was conducted and considered all outcomes and potential risk factors. The χ2 or Fisher exact test was used as appropriate. Multiple Cox regressions were used to derive hazard ratios (HRs) for the outcomes studied and adjusted for confounders in accordance with the logic of the conceptual framework. Stepwise backward procedures were used to remove potential risk factor variables one by one from the models. Variables that were removed were those with P ≥ .2 in the Wald test. The remaining variables were then successively removed based on their confounding effect and their contribution to the models. Variables whose removal from the model caused substantial changes (>10%) in the HRs were retained, as were the variables whose removal incurred significant likelihood ratio tests (P < .05).
The Kaplan-Meier method was used to analyze the differences in survival related to the explanatory variables. These differences were compared with log-rank tests. Significance level was set at 5% (P < .05). Statistical analyses were performed using the STATA 13 software (Statacorp, College Station, TX).
Results
A total of 127 patients were included in the cohort. Table 1 summarizes patient characteristics, including tumor stage, pretreatment PSA value, Gleason score, NCCN risk group, prescription dose, presence of perineural invasion, and proportion of positive cores. The median follow-up time was 6.7 years (1.8-10.4 years). During the entire follow-up period, of the total number of patients, 24 (18.9%) developed biochemical failure; 11 (8.7%) developed metastasis; and 8 (6.3%) died due to all causes, of whom 4 (3.2%) died due to prostate cancer. The 5-year bRFS, DMFS, PCSM, and OS for all patients was 89%, 96.1%, 98.4%, and 96.9% respectively.
Table 1.
Characteristics of the all 127 study patients
| Characteristic | N | % |
|---|---|---|
| Age (y) | ||
| Median | 70 | |
| Mean | 68 | |
| Range | 45-87 | |
| Median follow-up (y) | 6.5 | |
| Race | ||
| White | 115 | 90 |
| Black | 5 | 4 |
| Other | 7 | 5 |
| Tumor Stage | ||
| T1b-c | 59 | 47 |
| T2a | 48 | 38 |
| T2b | 13 | 10 |
| T2c | 7 | 5 |
| Gleason score | ||
| ≤6 | 16 | 13 |
| 7 (3+4) | 24 | 19 |
| 7 (4+3) | 40 | 31 |
| 8-10 | 47 | 37 |
| PSA (ng/ml) | ||
| Median | 7.7 (1.3-27) | |
| ≤10 | 43 | 34 |
| >10 | 84 | 66 |
| NCCN Risk Group | ||
| Intermediate 1 NCCN risk factor | 55 | 43 |
| Intermediate >1 NCCN risk factor | 20 | 16 |
| High-risk | 52 | 41 |
| Prescribed Dose Levels (Gy) | ||
| Median | 80 (74-80) | |
| 74 | 3 | 2 |
| 76 | 11 | 9 |
| 78 | 43 | 34 |
| 80 | 70 | 55 |
| Perineural Invasiona | ||
| Positive | 33 | 32 |
| Negative | 70 | 68 |
| % Positive Biopsy Cores | ||
| <50% | 84 | 66 |
| ≥50% | 43 | 34 |
NCCN = National Comprehensive Cancer Network; PSA = prostate-specific antigen.
Not available for all patients.
For bRFS, only PSA nadir levels and the risk classification variable retained the statistical significance that was observed in the univariate analysis in the multiple regression analysis (Table 2). The HR of developing biochemical failure was 5.5 times higher for patients with higher PSA nadir levels compared with patients who had lower PSA nadir levels. The HR of developing biochemical failure was 3.9 times higher for patients classified as having intermediate-risk prostate cancer with more than one risk factor (intermediate unfavorable) or high-risk prostate cancer compared with patients classified as having intermediate-risk prostate cancer with one risk factor (intermediate favorable).
Table 2.
Results of univariate and multivariate analyses for the associations between potential risk factors and biochemical failure
| Potential Risk Factors | Biochemical Failure − (n = 103) |
Biochemical Failure + (n = 24) |
Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | |||
| Pretreatment PSA Level | 0.002 | - | NS | ||
| <10 ng/mL | 73 (70.9%) | 11 (45.8%) | |||
| ≥10 ng/mL | 30 (29.1%) | 13 (54.2%) | |||
| Age | 0.218 | - | NS | ||
| <70 y | 53 (51.5%) | 9 (37.5%) | |||
| ≥70 y | 50 (48.5%) | 15 (62.5%) | |||
| Tumor Stage | 0.131 | - | NS | ||
| T1b-T1c | 52 (50.5%) | 7 (29.2%) | |||
| T2a | 34 (33%) | 14 (58.3%) | |||
| T2b | 11 (10.7%) | 2 (8.3%) | |||
| T2c | 6 (5.8%) | 1 (4.2%) | |||
| Gleason Score | 0.632 | - | NS | ||
| ≤6 | 14 (13.6%) | 2 (8.3%) | |||
| 7 (3 + 4) | 21 (20.4%) | 3 (12.5%) | |||
| 7 (4 + 3) | 32 (31%) | 8 (33.3%) | |||
| ≥8 | 36 (35%) | 11 (45.9%) | |||
| % Positive Biopsy Cores | 0.675 | - | NS | ||
| <50% | 69 (67%) | 15 (62.5%) | |||
| ≥50% | 34 (33%) | 9 (37.5%) | |||
| Perineural Invasiona | 0.086 | - | NS | ||
| Positive | 59/82 (72%) | 11/21 (52.4%) | |||
| Negative | 23/82 (28%) | 10/21 (47.6%) | |||
| Risk classificationb | 0.033 | 3.9 (1.4-10.8) | 0.007 | ||
| Intermediate 1 NCCN risk factor | 50 (48.5%) | 5 (20.8%) | |||
| Intermediate >1 NCCN risk factor | 14 (13.6%) | 6 (25%) | |||
| High-risk | 39 (37.9%) | 13 (54.2%) | |||
| PSA Nadira | < 0.001 | 5.5 (2.3-12.9) | < 0.001 | ||
| <1 ng/mL | 83/96 (86.5%) | 12/23 (52.2%) | |||
| ≥1 ng/mL | 13/96 (13.5%) | 11/23 (47.8%) | |||
CI = confidence interval, HR = hazard ratio, NCCN = National Comprehensive Cancer Network; NS = non-significant; PSA = prostate-specific antigen.
Not available for all patients. Group denominators are shown.
Intermediate >1 NCCN risk factor and high-risk patients were analyzed combined.
Rates of progression to biochemical failure over the study years are represented in Figure 1. bRFS was better for patients with lower PSA nadir levels (log-rank P < .001) and a lower risk classification (log-rank P = .004). In addition to PSA nadir levels (HR = 10.9) and the risk classification variable (HR = 9.1), perineural invasion (HR = 18.2) was also found to be independently associated with metastasis (Table 3).
Figure 1.
Kaplan-Meier curves for biochemical failure by relevant risk factors: Nadir prostate-specific antigen levels (A) and risk classification (B).
Table 3.
Results of univariate and multivariate associations between potential risk factors and occurrence of distant metastasis
| Potential Risk Factors | Metastasis − (n = 109) |
Metastasis + (n = 10) |
Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | |||
| Pretreatment PSA Level | 0.181 | - | NS | ||
| <10 ng/mL | 79 (68.1%) | 4 (45.5%) | |||
| ≥10 ng/mL | 37 (31.9%) | 6 (54.6%) | |||
| Age | 0.055 | - | NS | ||
| <70 y | 60 (51.7%) | 2 (18.2%) | |||
| ≥70 y | 56 (48.3%) | 8 (81.8%) | |||
| Tumor Stage | 0.574 | - | NS | ||
| T1b-T1c | 55 (47.4%) | 4 (36.4%) | |||
| T2a | 43 (37.1%) | 5 (45.4%) | |||
| T2b | 11 (9.5%) | 2 (18.2%) | |||
| T2c | 7 (6%) | 0 (0%) | |||
| Gleason Score | 0.247 | - | NS | ||
| ≤6 | 16 (13.8%) | 0 (0%) | |||
| 7 (3 + 4) | 22 (18.9%) | 2 (18.2%) | |||
| 7 (4 + 3) | 38 (32.8%) | 2 (18.2% | |||
| ≥8 | 40 (34.5%) | 7 (63.6%) | |||
| % positive biopsy cores | 0.749 | - | NS | ||
| <50% | 76 (65.5%) | 8 (72.7%) | |||
| ≥50% | 40 (34.5%) | 3 (27.3%) | |||
| Perineural Invasiona | 0.005 | 18.2 (1.2-71.5) | 0.003 | ||
| Positive | 68/94 (72.3%) | 2/9 (22.2%) | |||
| Negative | 26/94 (27.7%) | 7/9 (75%) | |||
| Risk Classificationb | 0.035 | 9.1 (1.2-71.5) | 0.035 | ||
| Intermediate 1 NCCN risk factor | 54 (46.5%) | 1 (9.1%) | |||
| Intermediate >1 NCCN risk factor | 17 (14.7%) | 3 (27.3%) | |||
| High-risk | 45 (38.8%) | 7 (63.3%) | |||
| PSA Nadira | 0.005 | 10.9 (2.1-57.7) | 0.005 | ||
| <1 ng/mL | 91/109 (83.5%) | 4/10 (40%) | |||
| ≥1 ng/mL | 18/109 (16.5%) | 6/10 (60%) | |||
| Biochemical Failure | <0.001 | NT | NT | ||
| No | 103 (88.8%) | 0 (0%) | |||
| Yes | 13 (11.2%) | 11 (100%) | |||
CI = confidence interval, HR = hazard ratio, NCCN = National Comprehensive Cancer Network; NS = non-significant; PSA = prostate-specific antigen.
not available for all patients. Group denominators are shown.
Intermediate >1 NCCN risk factor and high-risk patients were analyzed combined.
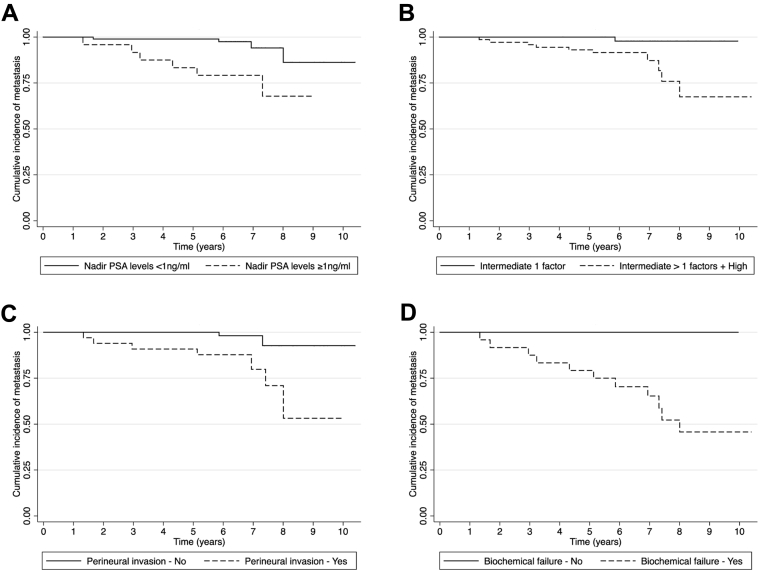
Because all patients with metastases had biochemical failure, indicating a strong association between the two, the HR of metastases for biochemical failure could not be measured. However, it was possible to plot the Kaplan-Meier curve and test the difference of the curves of patients with and without biochemical failure with the log-rank test (Fig 2). DMFS was better for patients with lower PSA nadir levels (log-rank P = .002), lower risk classification (log-rank P = .011), and no perineural invasion (log-rank P = .002).
Figure 2.
Kaplan-Meier curves for distant metastases by relevant risk factors: prostate-specific antigen nadir levels (A), risk classification (B), perineural invasion (C), and biochemical failure (D).
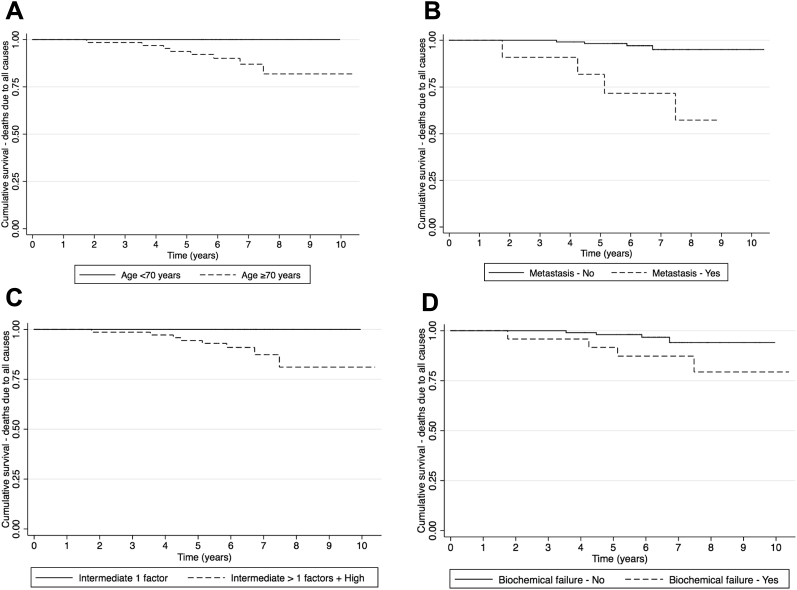
Table 4 shows the univariate and multivariate associations of potential risk factors and OS. In the multiple regression analysis, age (HR = 7.0) and metastases (HR = 10.4) were the only two variables found to be independently associated with OS. Figure 3 shows that OS was better for patients who were younger (log-rank P = .006), had no metastases (log-rank P < .001), had a lower risk classification (log-rank P = .008), and did not develop biochemical failure (log-rank P = .058). Even though the variables risk classification and biochemical failure were not found to be independently associated with OS in the multiple regression analysis, differences in the curves were statistically significant (or borderline significant for biochemical failure) in the univariate logic of the Kaplan-Meier and log-rank test analyses.
Table 4.
Results of univariate and multivariate associations between potential risk factors and death due to all causes
| Potential Risk Factors | Death − (n = 119) |
Death + (n = 8) |
Univariate Analysis |
Multivariate Analysis |
|
|---|---|---|---|---|---|
| P-value | HR (95%CI) | P-value | |||
| Pretreatment PSA Level | 0.716 | - | NS | ||
| <10 ng/mL | 78 (65.6%) | 6 (75 %) | |||
| ≥10 ng/mL | 41 (34.4%) | 2 (25 %) | |||
| Age | 0.006 | 7.0 (1.5-31.5) | 0.012 | ||
| <70 y | 62 (52.1%) | 0 (0%) | |||
| ≥70 y | 57 (47.9%) | 8 (100%) | |||
| Tumor Stage | 0.178 | - | NS | ||
| T1b-T1c | 57 (47.9%) | 2 (25%) | |||
| T2a | 45 (37.8%) | 3 (37.5%) | |||
| T2b | 11 (9.2%) | 2 (25%) | |||
| T2c | 6 (5.1%) | 1 (12.5%) | |||
| Gleason Score | 0.616 | - | NS | ||
| ≤6 | 16 (13.5%) | 0 (0%) | |||
| 7 (3 + 4) | 23 (19.3%) | 1 (12.5%) | |||
| 7 (4 + 3) | 38 (31.9%) | 2 (25%) | |||
| ≥8 | 42 (35.3%) | 5 (62.5%) | |||
| % Positive Biopsy Cores | 1.000 | - | NS | ||
| <50% | 79 (66.4%) | 5 (62.5%) | |||
| ≥50% | 40 (33.6%) | 3 (37.5%) | |||
| Perineural Invasiona | 0.082 | - | NS | ||
| Positive | 68/97 (70.1%) | 2/6 (33.3%) | |||
| Negative | 29/97 (29.9%) | 4/6 (66.7%) | |||
| Risk Classificationb | 0.008 | - | NS | ||
| Intermediate 1 NCCN risk factor | 55 (46.2%) | 0 (0%) | |||
| Intermediate >1 NCCN risk factor | 19 (16%) | 1 (12.5%) | |||
| High-risk | 45 (37.8%) | 7 (87.5%) | |||
| PSA Nadira | 0.628 | - | NS | ||
| <1 ng/mL | 90/112 (80.4%) | 5/7 (71.4%) | |||
| ≥1 ng/mL | 22/112 (19.6%) | 2/7 (28.6%) | |||
| Biochemical Failure | 0.041 | - | NS | ||
| No | 99 (83.2%) | 4 (50%) | |||
| Yes | 20 (16.8%) | 4 (50%) | |||
| Metastasis | 0.002 | 10.4 (2.6-42.7) | 0.001 | ||
| No | 112 (94.1%) | 4 (50%) | |||
| Yes | 7 (5.9%) | 4 (50%) | |||
CI = confidence interval, HR = hazard ratio, NCCN = National Comprehensive Cancer Network; NS = non-significant; PSA = prostate-specific antigen.
Not available for all patients. Group denominators are shown.
Intermediate >1 NCCN risk factor and high-risk patients were analyzed combined.
Figure 3.
Kaplan-Meier curves for deaths due to all causes by relevant risk factors: age (A), metastases (B), risk classification (C), and biochemical failure (D).
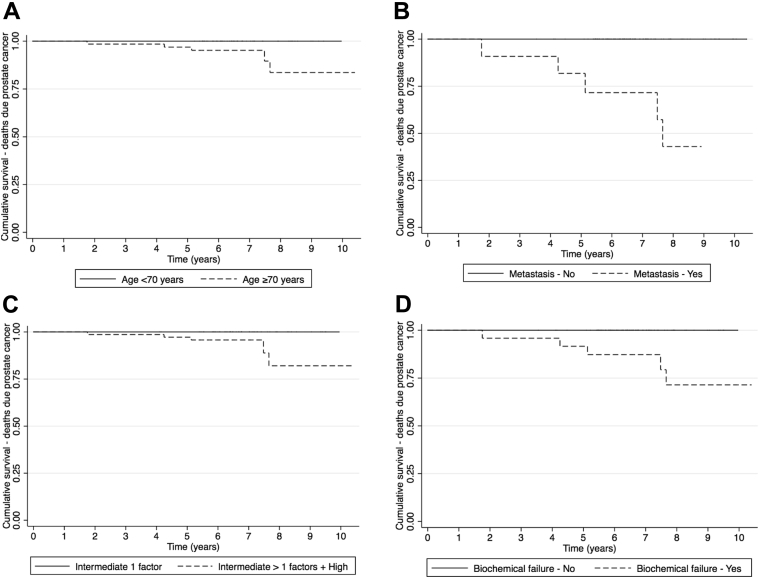
Because of the small number of deaths due to prostate cancer, it was not possible to model the effect of potential risk factors using multiple Cox regression models. However, it was possible to plot Kaplan-Meier curves and test the difference of the curves. Figure 4 shows that PCSM was lower for patients who were younger (log-rank P = .032), had no metastases (log-rank P < .001), had a lower risk classification (log-rank P = .032), and did not develop biochemical failure (log-rank P < .001). None of the models showed evidence against the proportional hazards assumption.
Figure 4.
Kaplan-Meier curves for deaths due to prostate cancer by relevant risk factors: age (A), metastases (B), risk classification (C), and biochemical failure (D).
Discussion
Even though several phase 3 trials have shown improved outcomes with the addition of short-term and long-term ADT to RT compared with RT alone,1, 2, 3 an important question remains unanswered: Is ADT necessary for all patients with intermediate- or high-risk clinically localized prostate cancer treated with dose-escalated RT?
With a median follow-up time of 6.7 years, in a cohort of patients with intermediate- or high-risk prostate cancer who received dose-escalated RT without ADT, the 5-year estimated bRFS and OS for all patients was 89.0% and 96.9%, respectively. At 5 years, only 2 patients (1.6%) died of prostate cancer and 5 (3.9%) presented distant metastases. In a classic prospective randomized trial that included patients with the same characteristics as patients in the present study, after a median follow-up period of 4.5 years, Kaplan-Meier estimates of the 5-year survival rates were 88%, and no deaths occurred due to prostate cancer in the group of patients who received low-dose 3D-RT (70 Gy) plus 6 months of ADT.2
To date, two phase 3 trials with results published only in abstract form have attempted to answer this question, but only for patients with intermediate-risk prostate cancer.13, 14 Both trials demonstrated better disease control results but not better OS. Despite these results, it is important to note that there is a great heterogeneity within the group of patients with intermediate-risk prostate cancer.
Zumnsteg et al proposed a new risk stratification system for patients with intermediate-risk prostate cancer, separating them into favorable and unfavorable subsets.19 Patients with unfavorable intermediate-risk prostate cancer were defined as those with primary Gleason patterns of 4, ≥50% positive biopsy cores, or multiple NCCN intermediate-risk factors. Patients with favorable intermediate-risk prostate cancer were defined as those with a single NCCN risk factor, Gleason ≤3+4 = 7, and <50% of biopsy cores that contain cancer. In this study, patients with unfavorable intermediate-risk prostate cancer had inferior PSA recurrence-free survival, DMFS, and PCSM compared with those who had favorable intermediate-risk disease.
As proposed by Zumnsteg et al, we divided the study patients in two groups of intermediate-risk prostate cancer: the first group with patients who had one NCCN adverse factor (favorable group) and the second group with patients who had two or more NCCN adverse factors (unfavorable group). In the multivariate analyses, patients in the unfavorable group had an HR of developing biochemical failure that was 3.9 times higher and an HR of developing metastases that was 9.1 times higher than patients in the favorable group. When considering exclusively patients in the favorable group, 5 (9%) had biochemical failure and only 1 (1.8%) had metastases almost 6 years after treatment initiation. These outcomes are similar to those of patients with low-risk prostate cancer as reported in other studies,20 suggesting that there may be a subset of patients within the intermediate-risk group for whom radiation treatment alone is adequate and ADT may be withheld.
On the other hand, patients with intermediate unfavorable prostate cancer had outcomes that were similar to those of patients with high-risk prostate cancer. It is without question that these patients deserve some form of treatment with ADT even when they have already received treatment with high-dose RT. However, the question of how long ADT should be used for these patients remains.
In a recent publication, Zapatero et al21 randomized patients with intermediate- or high-risk prostate cancer into 2 groups to receive short-term ADT (4 months) combined with RT at a minimum dose of 76 Gy or the same treatment followed by long-term ADT (24 months). Patients with high-risk disease who were treated with long-term ADT had significant benefits in biochemical disease-free, metastasis-free, and overall survival.
Two prognostic variables that are independently associated with biochemical progression and metastases-free survival were found in the present study: PSA nadir and presence of perineural invasion. In the multivariate analysis, patients with PSA nadir ≥1.0 ng/mL had a 5.5-times higher HR of biochemical recurrence and 10.9-times higher HR of developing distant metastases than patients with PSA nadir <1.0 ng/mL. It is well known that PSA nadir after RT is a significant determinant factor of outcomes. Zagars et al showed that for patients with PSA nadir <1 ng/mL, the 5-year FFBF was 86% compared with 33% to 58% for patients with PSA nadir ≥1 ng/mL.22 These results suggest that for patients with intermediate- or high-risk clinically localized prostate cancer treated with RT alone and with PSA nadir ≥1 ng/mL, a strict follow-up and early introduction of ADT with or without salvage local therapy after biochemical failure could be important.
Perineural invasion implies a potential extension of the tumor to the periprostatic soft tissue and represents a marker of a more aggressive tumor biology.23 Some studies report the presence of perineural invasion as an independent risk factor for biochemical failure or prostate cancer death.24 In our study, perineural invasion was predictive for metastases (HR = 18.2), which confirms that, despite the use of high-dose radiation, it is an important prognostic variable predictor of worse clinical outcome.
Limitations
As a retrospective cohort, our study could be limited by information bias with regard to the assessment of the outcome and exposure variables, but we believe that our accurate recordkeeping over the years has prevented such a problem. There is also the potential for residual confounding because not many patient characteristics were recorded and analyzed. Additionally, given the restricted sample size, the low mortality rate in our cohort, and the short follow-up time, the study had low power to detect DMFS and PCSM predictors. Despite these limitations, we have shown evidence that a subgroup of patients with intermediate-risk prostate cancer may be considered for RT alone and spared from unnecessary ADT treatment-related morbidity.
In addition, it is well known that ADT is related to a diminished quality of life (QoL) that negatively affects patients' psychosocial well-being and physical health. A shortcoming of this study is the lack of QoL-related data. It will be important for future studies to evaluate QoL using standardized, universally accepted scoring systems.
The median RT delivered dose was 80 Gy, and 89% of patients received a dose of 78 Gy or more. Thus, when considering treatment of patients with RT alone, the prescription of high doses around this level should be considered.
Future prospective trials are necessary to identify other clinical and biologic characteristics that can predict the best treatment combination. We hope that the aforementioned issues will definitively be solved with the results of the phase 3 RTOG0815 study to utilize dose-escalated RT with or without ADT for patients with intermediate-risk prostate cancer.
Conclusions
Selected patients with intermediate-risk clinically localized prostate cancer with less bulky disease have excellent outcomes after treatment with high-dose IMRT without ADT. Patients with intermediate- or high-risk unfavorable prostate cancer should receive at least short-term ADT with high-dose RT in light of their predilection for treatment failure. Perineural invasion was an important prognostic factor for this population and warrants further study to determine if it should include the intermediate-risk grouping stratification.
Footnotes
Conflicts of interest: None.
References
- 1.Bolla M., Collette L., Blank L. Long-term results with immediate androgen suppression and external irradiation in patients with locally advance prostate cancer (an EORTC study): A phase III randomized trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico A.V., Manola J., Loffredo M., Renshaw A.A., DellaCroce A., Kantoff P.W. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer. JAMA. 2004;292:821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz E.M., Bae K., Hanks G.E. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky M.J., Fukz Z., Hunt M. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 5.Viani G.A., Stefano E.J., Afonso S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 6.Michalski J.M., Moughan J., Purdy J. A randomized trial of 79.2 Gy versus 70.2 Gy radiation therapy (RT) for localized prostate cancer [Abstract] J Clin Oncol. 2015;33(Suppl 7) abstr 4. [Google Scholar]
- 7.Shahinian V.B., Kuo Y.F., Freeman J.L., Goodwin J.S. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 8.Keating N.L., O'Malley A., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 9.Edelman S., Liauw S.L., Rossi P.J., Cooper S., Jani A.B. High-dose radiotherapy with or without androgen deprivation therapy for intermediate-risk prostate cancer: cancer control and toxicity outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1473–1479. doi: 10.1016/j.ijrobp.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Castle K.O., Hoffman K.E., Levy L.B. Is androgen deprivation therapy necessary in all intermediate-risk prostate cancer patients treated in the dose escalation era? Int J Radiat Oncol Biol Phys. 2013;85:693–699. doi: 10.1016/j.ijrobp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Valicente R.K., Bae K., Michalski J. Does hormone therapy reduce disease recurrence in prostate cancer patients receiving dose-escalated radiation therapy? An analysis of Radiation Therapy Oncology Group 94-06. Int J Radiat Oncol Biol Phys. 2011;79:1323–1329. doi: 10.1016/j.ijrobp.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Krauss D., Kestin L., Brabbins D. Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1064–1071. doi: 10.1016/j.ijrobp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Dubray B.M., Beckendorf V., Guerif S. Does short-term androgen depletion add to high-dose radiotherapy (80 Gy) in localized intermediate-risk prostate cancer? Intermediary analysis of GETUG 14 randomized trial [Abstract] J Clin Oncol. 2011;29 abstr 4521. [Google Scholar]
- 14.Nadib A., Carrier N., Vigneault E. A phase III trial of short-term androgen deprivation therapy in intermediate-risk prostate cancer treated with radiotherapy [Abstract] J Clin Oncol. 2015;33 abstr 5019. [Google Scholar]
- 15.Mohler J., Bahnson R.R., Boston B. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 16.Gadia R., Leite E.T., Gabrielli F.G. Outcomes of high-dose intensity-modulated radiotherapy alone with 1 cm planning target volume posterior margin for localized prostate cancer. Radiat Oncol. 2013;8:285. doi: 10.1186/1748-717X-8-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roach M., 3rd, Hanks G., Thames H., Jr. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Victora C.G., Huttly S.R., Fuchs S.C., Olinto M.T. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 19.Zumsteg Z.S., Spratt D.E., Pei I. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64:895–902. doi: 10.1016/j.eururo.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Khuntia D., Reddy C.A., Mahadevan A., Klein E.A., Kupelian P.A. Recurrence-free survival rates after external beam radiotherapy for patients with clinical T1-T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100:1283–1292. doi: 10.1002/cncr.20093. [DOI] [PubMed] [Google Scholar]
- 21.Zapatero A., Guerrero A., Maldonado X. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomized, controlled, phase III trial. Lancet Oncol. 2015;16:320–327. doi: 10.1016/S1470-2045(15)70045-8. [DOI] [PubMed] [Google Scholar]
- 22.Zagars G.K., Pollack A. Radiation therapy for T1 and T2 prostate cancer: Prostate-specific antigen and disease outcome. Urology. 1995;45:476–483. doi: 10.1016/S0090-4295(99)80019-3. [DOI] [PubMed] [Google Scholar]
- 23.Liebig C., Ayala G., Wilks J.A., Berger D.H., Albo D. Perineural invasion in cancer: A review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 24.Bonin S.R., Hanlon A.L., Lee W.R., Movsas B., al-Saleem T.I., Hanks G.E. Evidence of increased failure in the treatment of prostate carcinoma patients who have perineural invasion treated with three-dimensional conformal radiation therapy. Cancer. 1997;79:75–80. doi: 10.1002/(sici)1097-0142(19970101)79:1<75::aid-cncr11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]