Abstract
Purpose
Atelectasis (AT), or collapsed lung, is frequently associated with central lung tumors. We investigated the variation of atelectasis volumes during radiation therapy and analyzed the effect of AT volume changes on the reproducibility of the primary tumor (PT) position.
Methods and materials
Twelve patients with lung cancer who had AT and 10 patients without AT underwent repeated 4-dimensional fan beam computed tomography (CT) scans during radiation therapy per protocols that were approved by the institutional review board. Interfraction volume changes of AT and PT were correlated with PT displacements relative to bony anatomy using both a bounding box (BB) method and change in center of mass (COM). Linear regression modeling was used to determine whether PT and AT volume changes were independently associated with PT displacement. PT displacement was compared between patients with and without AT.
Results
The mean initial AT volume on the planning CT was 189 cm3 (37-513 cm3), and the mean PT volume was 93 cm3 (12-176 cm3). During radiation therapy, AT and PT volumes decreased on average 136.7 cm3 (20-369 cm3) for AT and 40 cm3 (−7 to 131 cm3) for PT. Eighty-three percent of patients with AT had at least one unidirectional PT shift that was greater than 0.5 cm outside of the initial BB during treatment. In patients with AT, the maximum PT COM shift was ≥0.5 cm in all patients and >1 cm in 58% of patients (0.5-2.4 cm). Changes in PT and AT volumes were independently associated with PT displacement (P < .01), and the correlation was smaller with COM (R2 = 0.58) compared with the BB method (R2 = 0.80). The median root mean squared PT displacement with the BB method was significantly less for patients without AT (0.45 cm) compared with those with AT (0.8cm, P = .002).
Conclusions
Changes in AT and PT volumes during radiation treatment were significantly associated with PT displacements that often exceeded standard setup margins. Repeated 3-dimensional imaging is recommended in patients with AT to evaluate for PT displacements during treatment.
Summary.
This study analyzed 12 patients with atelectasis and 10 patients without atelectasis who underwent repeat 4-dimensional fan beam computed tomography during radiation therapy. Patients with atelectasis had significantly greater tumor displacements than patients without atelectasis, and these tumor displacements often exceeded standard setup margins. Patients with atelectasis may benefit from repeated 3-dimensional imaging during radiation therapy and possible replanning for large tumor displacements.
Introduction
Conventional radiation therapy combined with chemotherapy remains the standard of care for patients with inoperable, locally advanced non–small cell lung cancer. Unfortunately, high local recurrence rates are observed, which are associated with poor overall survival.1 Recently, improved imaging techniques2, 3 and the inclusion of respiratory motion management4, 5, 6 have allowed for more accurate target definition, which is expected to improve tumor control.
Routinely, treatment planning is performed prior to therapy and adds population-based safety margins to account for systematic and random errors during daily patient setup.6, 7, 8, 9 Pathoanatomic conditions such as atelectasis (AT) can occur in up to 25% of patients with locally advanced lung cancer and may increase tumor position variability.10, 11, 12, 13, 14 There is a paucity of data that explore interfractional tumor position and shape changes that might be associated with AT. Available data suggest that AT can cause changes in tumor position over the course of radiation therapy that is not normally taken into account with standard setup margins.5 Therefore, repeated imaging during therapy and adaptive replanning have been suggested to adjust for density changes and tumor displacements that are associated with atelectatic changes.12 However, the frequency and magnitude of tumor position changes in patients with AT have not been well defined.
The purpose of this study was to investigate geometric PT changes that are associated with AT. In particular, we evaluated how changes in AT and PT volumes are related to PT displacement during treatment. Additionally, given the frequently observed anisotropic PT regression and associated displacements during radiation therapy, consistent anatomic landmarks (bronchi near the tumor and AT) were selected to better estimate true tissue displacement as a result of atelectatic change that is independent from potential PT volume regression. PT displacement in patients with and without AT was compared.
Methods and materials
Twenty-two patients with lung cancer stage Ib to IIIb who were previously enrolled in prospective image acquisition studies that included repeated 4-dimensional fan beam computed tomography (CT) scans were reviewed in accordance with an institutional review board–approved protocol. The study groups consisted of 12 patients who were treated on the basis of prior protocols and who were found to have AT. The control group consisted of 10 consecutive patients who were enrolled in prior imaging studies and who did not have AT. All patients were treated with fractionated radiation therapy at standard fractionation, which was combined with concurrent chemotherapy in the majority of patients (Table 1).
Table 1.
Patient characteristics
| Patient Group | With Atelectasis | Without Atelectasis |
|---|---|---|
| Patients | 12 | 10 |
| Age (mean), y | 59 | 63 |
| Sex | ||
| Male | 10 | 5 |
| Female | 2 | 5 |
| Race | ||
| Caucasian | 9 | 7 |
| African American | 3 | 3 |
| AT and PT tumor size | ||
| AT volume (mean), cm3 | 189 | 0 |
| PT volume (mean), cm3 | 93 | 42 |
| Radiation Technique | ||
| 3-dimensional | 8 | 4 |
| IMRT | 4 | 6 |
| Dose (range), Gy | 54-70 | 62-70.2 |
| Tumor Stage | ||
| T1-2 | 3 | 7 |
| T3-4 | 9 | 3 |
| Nodal Stage | ||
| N0-1 | 3 | 2 |
| N2-3 | 9 | 8 |
| Received Chemotherapy | 10 | 10 |
AT, atelectasis; IMRT, intensity modulated radiation therapy; PT, primary tumor.
Imaging acquisition
CT scans were prospectively obtained as respiration-correlated 4-dimensional fan-beam CT scans (Brilliance Big Bore, Philips Medical Systems) with 10 breathing phases (0%-90%) and phase-based sorting with 3-mm slices in the treatment position. Patients underwent a median of 4 scans (range 2-7) during the course of radiation therapy. A total of 97 4-dimensional fan beam CT scans were used in this study.
Contouring
All scans were rigidly registered on the basis of the bony anatomy of the spine to the initial planning scan with a commercial registration and contouring package (MIM Maestro v.6.2, Cleveland, OH). PT and AT (in the study group only) were contoured during a single respiratory phase, which remained consistent for every repeated scan. PT and AT were contoured on the initial planning scan with both the lung and soft tissue windows and on all repeated scans by referencing the initial planning scan to ensure consistency. When available, diagnostic CT scans with contrast and positron emission tomography (PET)-CT scans were used during contouring to allow for differentiation between PT and AT. Bronchi were contoured in selected patients with AT who had a centrally located tumor near the bronchi to serve as a consistent anatomic landmark as the PT regressed during treatment. The mainstem bronchi were defined as starting at the carina and stopping at the division in secondary bronchi. Secondary bronchi were defined as starting at the secondary carina and stopping at the division of tertiary bronchi. All PT, AT, and bronchus contours were peer reviewed between repeated scans for accuracy and reproducibility.
Data analysis
To evaluate the geometric changes that are associated with AT, center of mass (COM) of the PT and bronchi were recorded for each scan in lateral (LR), anterior-posterior (AP), and superior-inferior (SI) directions. In addition, the volume changes of PT and AT over time were examined. The calculation of change in COM can be affected by asymmetric change in the tumor (Fig. 1). The bounding box (BB) method only measures tumor shifts, which are outside of the initial tumor volume and likely the most clinically relevant shifts. Therefore, we also employed a BB method15 for comparison. We also measured displacement of consistent anatomic landmarks (bronchi near the tumor), which are supposedly unaffected by tumor regression.
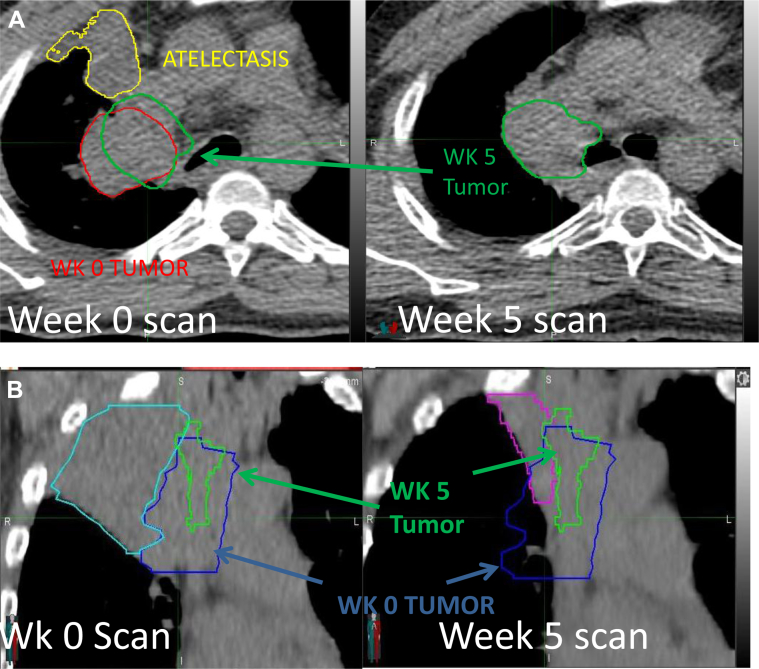
Fig. 1.
A, Example of atelectasis volume change and primary tumor position change. The tumor at week 0 is in red, at week 5 in green, and at atelectasis in yellow. The tumor at week 5 is transferred to the week 0 scan after fusion of week 0 and week 5 scans to the spine to illustrate tumor movement. B, Example of atelectasis volume change and primary tumor position change. Initial atelectasis is in cyan, initial tumor in blue, tumor at week 5 in green, and atelectasis at week 5 in purple. The scan at week 0 shows atelectasis and the initial tumor position. The scan at week 5 shows the resolution of atelectasis and the tumor position at week 5. For color images, please access the electronic version of this figure.
Changes in individual LR, AP, and SI COM directions and the total 3-dimensional vector change for PT and bronchus COMs were recorded for all scans relative to the initial planning scan. For the BB approach, the six borders (left, right, anterior, posterior, superior, and inferior) that define the minimum rectilinear box that bounds the entire structure were recorded. Changes in the BB border positions compared with the initial planning scan were calculated in the LR, AP, and SI directions. The root mean square (RMS) of the change over all directions was calculated to approximate the total change in bounding position compared with the initial planning scan. Patients with AT were compared with patients without AT to determine whether AT increased PT movement.
Statistics
The associations between PT and AT parameters, such as volume reduction and PT position changes, were analyzed using a linear regression model or Mann–Whitney U test. Significance was assumed at P < .05. A multivariate model with independent variables for changes in PT and AT volumes was used to determine an association with PT displacement using both the COM and BB methods.
Results
Change in PT position in patients with AT
Figure 2a shows the frequency and magnitude of PT COM shifts relative to the initial COM position during the course of treatment with the most common shift being between 1 and 1.5 cm. Upon examination of the maximum shift during the course of radiation therapy for each individual patient, the maximum shift of COM was ≥0.5 cm in all patients and >1 cm in 58% of patients (range, 0.5-2.4 cm).
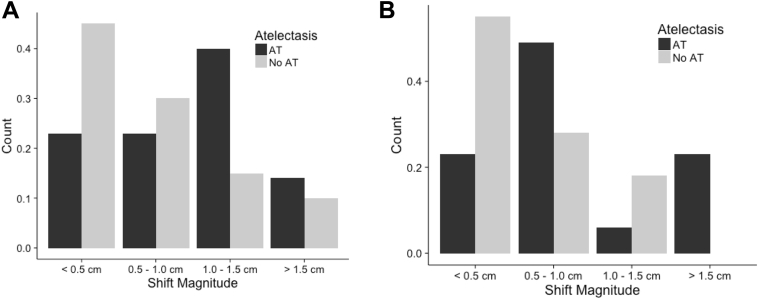
Fig. 2.
Total vector shift with the center of mass (COM) method or root mean squared (RMS) shift with the bounding box (BB) method for patients with or without atelectasis. A, The frequency of the magnitude of the shift with the COM method. B, The RMS shift with the BB method in patients with or without atelectasis.
When using the BB method to examine the change in PT position, we found that for 59 of 210 (28%) measured shifts the PT was outside of the initial BB position. Most patients (83%) had at least one unidirectional shift of the PT >0.5 cm outside of the initial BB during the treatment course. At any measured time point, the probability of a shift >5 mm outside of the BB was 14% in the LR direction, 26% in the AP direction, and 17% in the SI direction. Figure 2B shows the RMS relative shift over all directions, which provides an estimate of the total movement with reference to the initial BB. An example of change in AT that corresponds with PT displacement is shown in Fig 1.
Change in atelectasis and PT volume over time in patients with AT
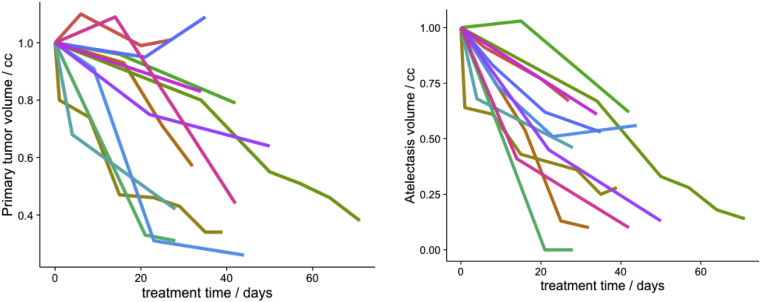
The mean (range) initial volume of AT from planning CT was 189 cm3 (37-513 cm3), and mean PT volume was 93 cm3 (12-176 cm3). Volume changes over the course of therapy are shown in Fig 3. Both PT and AT volumes appeared to change slowly over the course of treatment, but in rare cases the AT volume changed abruptly. During radiation therapy, AT and PT volumes decreased by an average of 136.7 cm3 (20-369 cm3) and 40 cm3 (-7-131 cm3), respectively. Linear regression was used to estimate the change in AT and PT per day during treatment. Median change in AT volume was 2.56 cm3/d (0.59-13.3 cm3), and PT was 0.93 cm3/d (−0.16 to 3.32 cm3).
Fig. 3.
Volume changes in atelectasis and primary tumor during radiation therapy.
A multivariate model with independent variables for PT and AT volume change showed an association with PT displacement (P < .001). The model with COM (R2 = 0.58) as the dependent variable showed less correlation compared with the model that used the BB method (R2 = 0.80).
Change in bronchus position
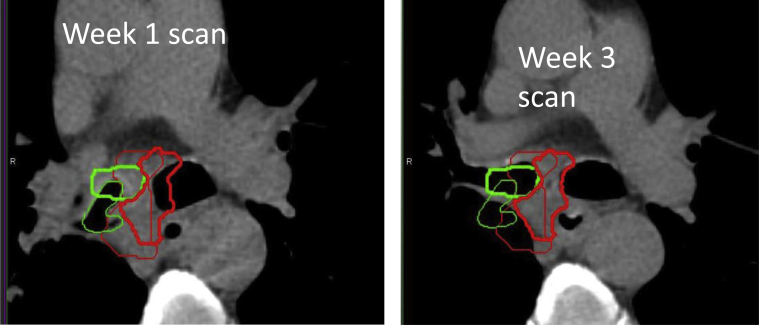
Main stem bronchi could be reproducibly contoured on repeated scans in 11 patients and secondary bronchi in 7 patients. The contoured bronchus on repeated scans was outside the initial bronchus BB in 139 of 336 measurements (41%). Maximum unidirectional shift outside the bounding box was >1 cm in 36% of patients, and the maximum observed unidirectional shift was 2.1 cm. Bronchus displacements based on BB and COM were highly correlated (R2 = 0.90 for main stem bronchi and R2 = 0.96 for secondary bronchi), implying little shape change in the bronchi during therapy. If the PT moved outside of the BB, bronchi moved in the same direction 80% of the time. An example of bronchus and PT that move concordantly is shown in Fig 4.
Fig. 4.
Example of a patient at week 1 and week 3 during radiation therapy, which illustrates the change in bronchus position. The primary tumor is shown in red (thin line week 2, thick line week 4) and bronchus is in green (thin line week 2, thick line week 4). Bronchus and the primary tumor center of mass shifted medially 0.9 cm and 1.8 cm, respectively. For color images, please access the electronic version of this figure.
Comparison group
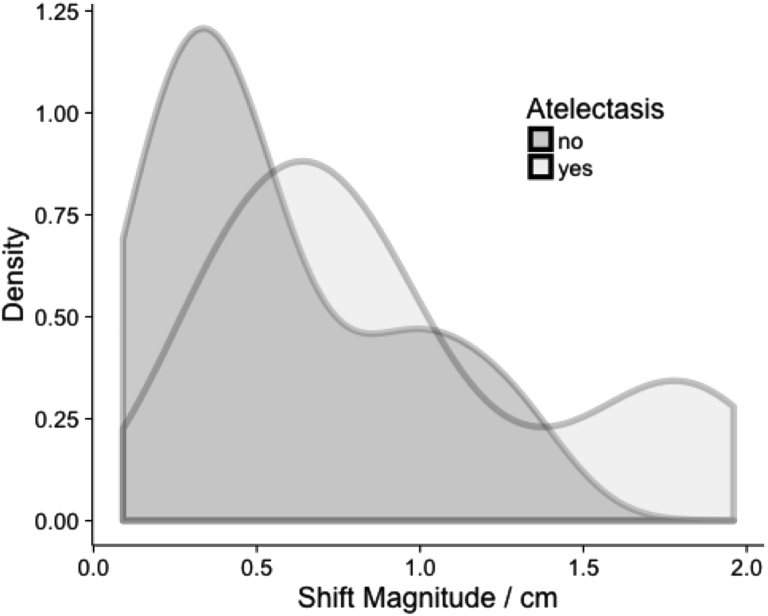
PT movement in patients with AT was compared with PT movement in patients without AT to determine whether AT increased PT movement. The initial PT was larger for patients with AT than for those without AT (median 80 cm3 vs 31 cm3, respectively), likely because patients with large PT are more likely to develop AT. A comparison of changes in COM showed a median total vector shift for patients with AT of 0.99 cm versus 0.48 cm for patients without AT (P = .01). The RMS tumor movement for the BB method was significantly less for patients without AT compared with those with AT (median 0.8 cm for patients with AT vs 0.45 cm for patients without AT; P = .0018). A probability density estimation was used to build histograms of the magnitude of shifts in the AT and non-AT groups (Fig 5). The graph shows that in patients with AT, there is a higher probability of a greater shift magnitude compared with patients without AT.
Fig. 5.
Probability density that is related to shift magnitude for patients with atelectasis compared to patients without atelectasis.
Discussion
This is the first study to our knowledge to quantitatively determine via repeated fan beam CT the change in AT volume and related geometric PT volume during radiation therapy. Previous studies have documented interfraction motion of the PT and found average PT displacements that range from 5 mm to 9 mm when measured over the course of radiation therapy.4, 5, 8, 16 AT, which frequently presents in patients with locally advanced lung cancer,11, 12, 13 has been shown to increase PT displacements during lung radiation therapy.4, 5 However, unlike these previously mentioned studies, our study used 4-dimensional fan beam CT to quantify AT changes over time and correlate the changes to PT displacement.
Additionally, we contoured both AT and PT on all repeated scans throughout the radiation therapy course and calculated the shift with both COM and BB methods. The BB method may better represent clinically significant tumor shifts of targets that undergo shape and volume changes. We found that patients with AT have increased PT displacement compared with patients without AT and that AT volume undergoes clinically significant changes during radiation therapy. Almost all patients had shifts >5 mm outside of the initial BB, with one patient experiencing a maximum shift of the PT outside the BB of 0.3 cm to the left, 1.1 cm anteriorly, and 0.9 cm superiorly. All patients with AT had at least one COM shift >5 mm during the course of treatment. Patients with AT had a 77% probability of COM shift >5 mm. These shift magnitudes would likely result in inadequate target coverage with typical margins if unaccounted for by image guidance.
Determining PT movement during radiation therapy for lung cancer is difficult because of tumor regression. All previous studies that have examined change in AT and PT displacement to our knowledge used change in COM of tumor relative to spine.5, 12 This method assumes that a change in tumor COM will correspond to a shift in tumor position. However, this method is less accurate in the common situation of lung tumor volume and shape change during treatment because the tumor displacement is not rigid. Therefore, to improve accuracy, we also used the BB method and created a BB around the initial tumor. On subsequent scans, the amount the PT outside the initial BB was identified as a simple method to account for shape and volume changes.
We also evaluated COM change for comparison and found examples in which the COM method either overestimated or underestimated PT displacement. Both the COM and BB methods found that PT and AT volume changes were independently associated with PT displacement. However, multivariate modeling (including volume change for both PT and AT) found that the correlation of PT displacement was lower with COM compared with the BB method, which implies that the BB method may be a more accurate means to assess PT motion.
An example of an asymmetric response by a tumor that resulted in a change in COM and overestimation of tumor motion is shown in Fig 1B (patient 2). The tumor responded asymmetrically and moved toward the mediastinum. Qualitatively, the tumor appears to be mostly encompassed within the initial primary tumor and moves slightly superiorly. When we assessed this movement with the BB method, we found a 3-mm shift outside the initial BB in the superior direction, which correlates qualitatively with the image. However, when the COM method assessed the change, it showed a 1.5-cm shift in the superior direction, which is qualitatively incorrect.
Conversely, COM can also underestimate clinically relevant tumor shifts. Figure 1A shows an obviously clinically significant shift of tumor volume in the anterior direction. Using the BB method to assess the shift, we see that the tumor has moved outside the initial BB anteriorly by 1.1 cm. We find that the COM method underestimated the anterior shift (0.5 cm). Additionally, tumor regression during treatment will likely bias the COM method to underestimate the PT shift. Thus, the BB method may be a superior method to quantify clinically relevant PT shifts.
The PT often regresses during therapy, and it can be difficult to determine whether all lung sections that were initially affected by the tumor remain in the radiation field. This becomes especially important for patients with AT because they are likely to experience large shifts as the AT resolves during therapy, but the primary tumor may experience significant regression. In these cases, the bronchus near the tumor may serve as a good surrogate during patient setup if the PT is difficult to identify and decouple the effects of anatomical shift and regression. Figure 4 shows a scan from a patient with a subcarinal mass in which the bronchus and PT move in the same direction. On the cone beam CT scan, bronchi may be easier to visualize that the actual PT. However, further study is needed to determine whether bronchi can be a useful surrogate for tumor motion. Although there was 80% concordance of movement between bronchi and the tumor, 20% of the time the bronchus moved in the direction opposite to the PT.
Several studies have investigated whether changes in AT lead to underdosage of tumor volume. In an attempt to separate the dosimetric effects due to changes in lung density from the resolution of AT and positional uncertainty, Grams et al17 performed a simulation study of 20 patients wherein tumor and AT were replaced with lung equivalent tissue to determine whether there was a significant dosimetric change that would require replanning. The authors found that V95% of the planning target volume changed only by 0.1%. However, when they simulated positional changes by shifting the isocenter by up to 5 mm in the LR, AP, and SI directions, they found changes in the V95% of the planning target volume of up to 4%. Thus, the positional changes that were caused by changes in AT volume seem more important for target dose coverage compared with changes in lung density. Persoon et al18 used a 3-dimensional portal dose measurement system to identify dose changes in 5 patients with atelectasis. This study reported that in all cases in which dosimetric changes were observed, change in atelectasis also resulted in a shift of the tumor volume.
Moller et al12 confirmed this observation in their study of 24 patients with AT to determine the benefit of adaptive replanning. This study identified shifts >5 mm relative to the spine and assessed whether these shifts would require replanning. The study also assessed for replanning if there was a significant change in AT volume. Using cone beam CT, the authors found that 70% of patients with AT would require replanning to ensure good dosimetric coverage of the target volume. However, in patients with PT shifts <5 mm, replanning was not helpful, which implies that the geometrical shifts that were caused by AT were primarily responsible for changes in dosimetric target coverage rather than changes in the lung density that surrounds the target volume. On the basis of these observations, we assume that the large PT displacements that were observed in our study would have likely caused tumor underdosage if they had not been taken into account.
In a study on the impact of respiratory motion versus anatomic changes over the course of radiation therapy, Schmidt et al19 found that anatomic changes affected dosimetry more than changes in respiratory motion and that these changes cannot be reasonably accounted for by increased margins. Adaptive therapy has been proposed to reduce the target volume over the course of treatment to ensure better target coverage and decrease normal tissue toxicity.12, 20, 21, 22 Given the anatomic changes that are associated with AT, these patients likely need specifically designed image guidance and adaptive protocols to manage tumor coverage over the course of their treatment.
Conclusion
Patients with AT have significantly greater PT displacement than patients without AT. AT and PT volume changes during radiation treatment were associated with tumor displacements that exceed standard setup margins. The use of bronchi that are close to the PT to assess the impact of AT change on PT shift without the confounding issue of PT regression needs further study before bronchi can be used as a surrogate for the PT. Repeated 3-dimensional imaging is recommended in patients with AT to evaluate for PT displacements during treatment and image guidance strategies such as a traffic light protocol11 should be considered to manage these patients.
Footnotes
Sources of support: This work was partially supported by the National Cancer Institute of the National Institutes of Health under award number R01CA166119. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: Virginia Commonwealth University receives research support from Philips Medical Systems and Varian Medical Systems. Drs. Weiss and Hugo have a licensing agreement with Varian Medical Systems.
References
- 1.Machtay M., Paulus R., Moughan J. Defining local-regional control and its importance in locally advanced non–small cell lung carcinoma. J Thorac Oncol. 2012;7:716–722. doi: 10.1097/JTO.0b013e3182429682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang W., Fan M., Liu B. Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non–small cell lung cancer treated with concurrent chemoradiotherapy. J Nucl Med. 2014;55:1584–1590. doi: 10.2967/jnumed.114.142919. [DOI] [PubMed] [Google Scholar]
- 3.Huang W., Liu B., Fan M. The early predictive value of a decrease of metabolic tumor volume in repeated (18)F-FDG PET/CT for recurrence of locally advanced non–small cell lung cancer with concurrent radiochemotherapy. Eur J Radiol. 2015;84:482–488. doi: 10.1016/j.ejrad.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Glide-Hurst C.K., Gopan E., Hugo G.D. Anatomic and pathologic variability during radiotherapy for a hybrid active breath-hold gating technique. Int J Radiat Oncol Biol Phys. 2010;77:910–917. doi: 10.1016/j.ijrobp.2009.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss E., Robertson S.P., Mukhopadhyay N. Tumor, lymph node, and lymph node-to-tumor displacements over a radiotherapy series: Analysis of interfraction and intrafraction variations using active breathing control (abc) in lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:e639–e645. doi: 10.1016/j.ijrobp.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugo G.D., Yan D., Liang J. Population and patient-specific target margins for 4D adaptive radiotherapy to account for intra- and inter-fraction variation in lung tumour position. Phys Med Biol. 2007;52:257–274. doi: 10.1088/0031-9155/52/1/017. [DOI] [PubMed] [Google Scholar]
- 7.Britton K.R., Starkschall G., Tucker S.L. Assessment of gross tumor volume regression and motion changes during radiotherapy for non–small-cell lung cancer as measured by four-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2007;68:1036–1046. doi: 10.1016/j.ijrobp.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Hugo G., Vargas C., Liang J. Changes in the respiratory pattern during radiotherapy for cancer in the lung. Radiother Oncol. 2006;78:326–331. doi: 10.1016/j.radonc.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Jan N., Balik S., Hugo G.D. Interfraction displacement of primary tumor and involved lymph nodes relative to anatomic landmarks in image guided radiation therapy of locally advanced lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:210–215. doi: 10.1016/j.ijrobp.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson K., Nymann J., Baumann P. Retrospective cohort study of bronchial doses and radiation-induced atelectasis after stereotactic body radiation therapy of lung tumors located close to the bronchial tree. Int J Radiat Oncol Biol Phys. 2013;87:590–595. doi: 10.1016/j.ijrobp.2013.06.2055. [DOI] [PubMed] [Google Scholar]
- 11.Kwint M., Conijn S., Schaake E. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol. 2014;113:392–397. doi: 10.1016/j.radonc.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Moller D.S., Khalil A.A., Knap M.M., Hoffman L. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol. 2014;110:517–522. doi: 10.1016/j.radonc.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Vaaler A.K., Forrester J.M., Lesar M., Edison M., Venzon D., Johnson B.E. Obstructive atelectasis in patients with small cell lung cancer. Incidence and response to treatment. Chest. 1997;111:115–120. doi: 10.1378/chest.111.1.115. [DOI] [PubMed] [Google Scholar]
- 14.Wurstbauer K., Deutschmann H., Kopp P. Nonresected non-small-cell lung cancer in stages I through IIIB: Accelerated, twice-daily, high-dose radiotherapy–a prospective phase I/II trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2010;77:1345–1351. doi: 10.1016/j.ijrobp.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 15.Robertson S.P., Weiss E., Hugo G.D. Localization Accuracy from Automatic and Semi-Automatic Rigid Registration of Locally-Advanced Lung Cancer Targets during Image-Guided Radiation Therapy. Med Phys. 2012;39:330-341. 2012;39:330–341. doi: 10.1118/1.3671929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Elmpt W., Ollers M., van Herwijnen H. Volume or position changes of primary lung tumors during (chemo-)radiotherapy cannot be used as a surrogate for mediastinal lymph node changes: The case for optimal mediastinal lymph node imaging during radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:89–95. doi: 10.1016/j.ijrobp.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 17.Grams M.P., Fong de Los Santos L.E., Brown L.C. Separating the dosimetric consequences of changing tumor anatomy from positional uncertainty for conventionally fractionated lung cancer patients. Pract Radiat Oncol. 2014;4:455–465. doi: 10.1016/j.prro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Persoon L.C., Egelmeer A.G., Ollers M.C., Nijsten S.M., Troost E.G., Verhaegen F. First clinical results of adaptive radiotherapy based on 3d portal dosimetry for lung cancer patients with atelectasis treated with volumetric-modulated arc therapy (vmat) Acta Oncol. 2013;52:1484–1489. doi: 10.3109/0284186X.2013.813642. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M.L., Hoffmann L., Kandi M., Moller D.S., Poulson P.R. Dosimetric impact of respiratory motion, interfraction baseline shifts, and anatomical changes in radiotherapy of non–small cell lung cancer. Acta Oncol. 2013;52:1490–1496. doi: 10.3109/0284186X.2013.815798. [DOI] [PubMed] [Google Scholar]
- 20.Guckenberger M., Kavanagh A., Partridge M. Combining advanced radiotherapy technologies to maximize safety and tumor control probability in stage III non–small cell lung cancer. Strahlenther Onkol. 2012;188:894–900. doi: 10.1007/s00066-012-0161-9. [DOI] [PubMed] [Google Scholar]
- 21.Mehta M., Scrimger R., Mackie R., Paliwal B., Chappell R., Fowler J. A new approach to dose escalation in non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;49:23–33. doi: 10.1016/s0360-3016(00)01374-2. [DOI] [PubMed] [Google Scholar]
- 22.Weiss E., Fatyga M., Wu Y. Dose escalation for locally advanced lung cancer using adaptive radiation therapy with simultaneous integrated volume-adapted boost. Int J Radiat Oncol Biol Phys. 2013;86:414–419. doi: 10.1016/j.ijrobp.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]