Abstract
Purpose
Our goal was to determine the impact of pathologic response after neoadjuvant chemotherapy in triple negative breast cancer (TNBC) on the subsequent risk of locoregional recurrence (LRR) and disease-free survival (DFS) in the setting of adjuvant radiation therapy.
Methods and materials
This was an institutional review board–approved retrospective chart review of patients with clinical stage I-III breast cancer treated with neoadjuvant chemotherapy, local surgery (breast conservation or mastectomy), and adjuvant radiation therapy between 1997 and 2015. Medical records were reviewed for clinical stage, tumor grade and subtype, neoadjuvant chemotherapy regimen, type of surgery, pathologic stage, use of radiation therapy, date and location of recurrence, and date of death. Molecular subtypes were defined using immunohistochemistry and histologic grade. ypT0 and ypN0 were defined as no residual invasive disease in breast or nodes, respectively. LRR was defined as any failure within the breast, chest wall, or regional lymph nodes. Statistical analysis was performed; LRR and DFS rates over 30 months were determined from Kaplan-Meier plots.
Results
Ninety-four patients with TNBC were analyzed, of whom 72 received radiation therapy. This subgroup was isolated for further investigation. Median follow-up was 32.5 months in this group. The pathologic complete response (pCR) rate was 36%, and presence or absence of disease in breast and/or nodes was significantly predictive of LRR. In TNBC patients who received radiation therapy, 30-month LRR was 22% in 41 patients with ypT+ versus 0% in 31 patients with ypT0 (P = .003), 23% in 31 patients with ypN+ versus 5% in 41 patients with ypN0 (P = .016), and 20% in 46 patients with residual disease in breast or nodes versus 0% in 26 patients with pCR (P = .015). The difference in the rate of LRR between those who underwent lumpectomy versus mastectomy did not reach significance (8% vs 17%, respectively). Furthermore, patients with residual disease had a higher rate of DFS events (hazard ratio, 3.58; 95% confidence interval, 1.37-9.41; P = .006). The difference in DFS was not significantly associated with the type of surgery received.
Conclusions
Patients with TNBC treated with neoadjuvant chemotherapy who have residual disease in the breast or lymph nodes at the time of surgery have significantly higher rates of locoregional failure and lower DFS compared with those with a pCR despite the use of adjuvant radiation therapy. Strategies to intensify therapy for patients with residual disease warrant further investigation.
Summary.
The purpose of this manuscript is to describe how pathologic response after neoadjuvant chemotherapy for triple negative breast cancer affects locoregional recurrence risk and disease-free survival in the setting of adjuvant radiation. This is a single-institution, retrospective study. We conclude that presence of residual disease in either breast or nodes is associated with significantly higher rates of locoregional failure as well as lower disease-free survival despite adjuvant radiation.
Background
Breast cancer is the most common type of cancer that affects women worldwide, and about 1 in 8 women will be diagnosed with breast cancer in her lifetime. Furthermore, 1 in 5 cases of breast cancer is of the “triple negative” subtype—that is, negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2—and these cases tend to be particularly aggressive with a higher propensity for metastatic disease and local failure compared with other subtypes.1 Despite this characteristic, triple negative breast cancer (TNBC) can be chemotherapy-sensitive, particularly to anthracyclines and taxanes. We are increasingly using neoadjuvant chemotherapy (NAC) for patients with TNBC with the goal of achieving pathologic complete response (pCR) because patients who have a pCR have improved disease-free survival (DFS) compared with those with residual disease in the breast or nodes.2, 3 Previous studies have shown that NAC can achieve pCR rates in the range of 25% in this patient subset, and that if a pCR is achieved through NAC in TNBC, these patients achieve survival rates similar to patients with less aggressive subtypes of breast cancer.4
Several investigators have also shown higher rates of local recurrence in patients with TNBC.4, 5, 6 Given this, many studies have attempted to determine risk factors for locoregional recurrence (LRR) to help stratify patients and create treatment guidelines. National Surgical Adjuvant Breast and Bowel Projects 18 and 27 demonstrated that clinical tumor status and pathologic response after NAC predict risk for LRR in breast cancer patients, but did not look at molecular subtype.7 Furthermore, a retrospective study from M D Anderson Cancer Center demonstrated that there was a significant reduction in LRR with the use of radiation therapy in patients with stage III breast cancer of any subtype who received NAC.8 Several reports have shown that locoregional failure is higher for triple negative patients treated with NAC who have residual disease found at surgery9, 10, 11; however, data are scarce for locoregional failure by chemotherapy response in the setting of adjuvant radiation therapy. In this retrospective review, therefore, we analyzed outcomes of patients at the University of California San Diego (UCSD) who received NAC for TNBC, followed by surgery and radiation, to determine LRR and DFS based on pathologic response.
Methods and materials
Study design, patients, and definitions
This study was a retrospective, single-institution analysis that was approved by the institutional review board and is compliant with the Health Insurance Portability and Accountability Act. All subjects presented to the UCSD Moores Cancer Center for curative treatment of breast cancer between 1997 and 2015. The inclusion criterion of this study was women 18 years of age or older who received NAC for stage I-III breast cancer; notable exclusions included male patients, patients with ductal carcinoma in situ alone at presentation, patients with metastatic disease at presentation, and those who received neoadjuvant hormonal treatment only (ie, no chemotherapy). The UCSD cancer registry was queried to select those patients who met the inclusion criterion for the retrospective study, and their medical records were subsequently reviewed for clinical stage, tumor grade and subtype, NAC regimen, type of surgery, pathologic stage, use of radiation therapy, date and location of recurrence, and date of death. TNBC was defined as lack of any level of estrogen receptor or progesterone receptor positivity and no amplification of Her2. Only those TNBC patients who received adjuvant radiation therapy were included in this analysis. ypT0 and ypN0 were defined as no residual invasive disease in breast or nodes, respectively. LRR was defined as any first failure within the ipsilateral breast, chest wall, or regional lymph nodes alone or in combination with distant metastases.
Statistical analysis
Statistical analysis was performed using Microsoft Excel, SAS, and R. To determine if the difference in LRR rate between those with and without pCR, with and without residual breast disease, and with and without residual node disease was significant, Kaplan-Meier curves were created and log-rank tests were performed to compare the 2 curves in each instance. A similar process was done when determining 30-month DFS rate significance; a Cox hazard ratio calculation was also performed in analysis of DFS.
Results
Patient and tumor characteristics of the 72 qualifying patients included in this study are summarized in Table 1. NAC included anthracycline and taxane in the majority of patients (83%). The remainder received either an anthracycline or a taxane.
Table 1.
Patient and tumor characteristics
| Characteristic | No. (%) |
|---|---|
| Age at diagnosis (y), median (range) | 46.5 (22-72) |
| Clinical stage at diagnosis | |
| I | 3 (4) |
| II | 34 (47) |
| III | 35 (49) |
| Chemotherapy type | |
| Anthracycline alone | 8 (11) |
| Taxane alone | 4 (6) |
| Both anthracycline and taxane | 60 (83) |
| Type of surgery | |
| Breast conserving | 37 (51) |
| Mastectomy | 35 (49) |
| Postsurgical residual disease status | |
| Pathological complete response | 26 (36) |
| ypT+ and/or ypN+ | 46 (64) |
| Follow-up (mo), median (range) | 32.5 (7.9-190.5) |
Radiation therapy details
Radiation therapy to the intact breast or chest wall with or without regional nodes was at the discretion of the treating physician. The majority of patients were prescribed 50 Gy in 25 to 28 fractions to the treated area.
Locoregional control
Retrospective analysis of the patients in our study demonstrated a significant difference (P = .015) in locoregional control between patients who achieved pCR and those who had residual disease at surgery (Fig 1). None of the patients who had a complete response experienced a locoregional failure. In the patients who had residual disease, the 2-year freedom from locoregional failure was 72.5% (95% confidence interval [CI], 57.4-91.5).
Figure 1.
Kaplan-Meier curves showing significant difference in locoregional control based on response to neoadjuvant chemotherapy.
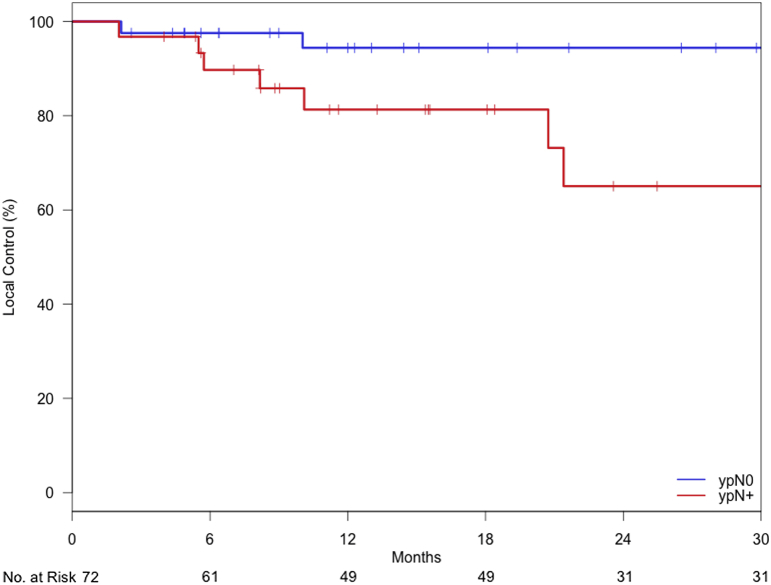
This difference remained significant (P = .003) when comparing patients who had residual breast disease versus those who had no tumor burden in the breast, regardless of node status (Fig 2). In the patients who had residual primary tumor, the 2-year freedom from locoregional failure was 67.3% (95% CI, 49.8-90.8). Figure 3 shows a similar finding of significantly improved locoregional control (P = .016) when comparisons are based on residual nodal disease alone; in the patients who had residual nodal disease, the 2-year freedom from locoregional failure was 65.0% (95% CI, 45.4-93.3).
Figure 2.
Kaplan-Meier curves showing locoregional control for patients with a complete response or residual disease within the breast.
Figure 3.
Kaplan-Meier curves for locoregional control for patients with (pN+) and without (pN0) residual disease in the axillary lymph nodes.
Of note, there was no significant difference in locoregional failure based on the type of surgery performed after NAC. Three of 37 (8.1%) patients who underwent breast conservation failed locally compared with 6 of 35 (17.1%) who underwent mastectomy (P = .25). Table 2 lists sites of LRR, including whether they were in-field. At least 78% of locoregional failures were in-field (7 of 9). Radiation therapy fields were not available for 1 patient and another had a marginal miss.
Table 2.
List of locoregional recurrence locations
| Location | No. |
|---|---|
| In-field | |
| Ipsilateral chest wall | 2 |
| Ipsilateral breast | 1 |
| Ipsilateral supraclavicular nodes | 1 |
| Ipsilateral chest wall and breast | 1 |
| Ipsilateral chest wall and supraclavicular nodes | 1 |
| Ipsilateral infraclavicular and supraclavicular nodes | 1 |
| Out-of-field | |
| Ipsilateral internal mammary nodes | 1 |
Note: 1 patient not listed here recurred in the ipsilateral axillary nodes, but whether this was in-field or out-of-field was unable to be determined because the patient's treatment records were unavailable.
DFS
Further investigation was undertaken to determine whether pCR status, aside from being associated with stronger locoregional control, was also linked to greater DFS. Kaplan-Meier survival curves were built to determine 30-month DFS rates (Fig 4). Patients with residual disease had a higher rate of DFS events (hazard ratio, 3.58; 95% CI, 1.37-9.41; P = .006). The 2-year DFS estimate in patients who had a complete response compared with those with residual disease was 82.7% (95% CI, 68.6-99.7) versus 44.0% (95% CI, 30.5-63.4). Of the 30 total DFS events, 21 were distant metastases and 9 were LRRs.
Figure 4.
Kaplan-Meier curves showing the 30 month disease free survival for patients with a complete response or residual disease following NACT.
Again, DFS was not significantly correlated with surgery type: 14 recurrences occurred in 37 patients who had breast-conserving surgery (37.8%) versus 16 recurrences in 35 patients who had mastectomies (45.7%) (P = .50).
Discussion
This report describes a retrospective analysis of locoregional failure and overall DFS outcomes in women treated with NAC for TNBC based on pathologic response at the time of surgery. If patients are found to have residual disease in the breast or axillary nodes at the time of surgery, they are at significantly higher risk of locoregional or systemic failure than those having a pCR despite the use of adjuvant radiation therapy. It is, to our knowledge, the largest report on triple negative patients with detailed radiation therapy treatment records documenting a high rate of in-field recurrences in patients with residual disease after NAC.
Le Scodan et al recently published their experience with NAC in a group of stage II and III breast cancer patients treated with mastectomy and N0 at the time of surgery. A total of 58.2% of patients had PMRT. There was no benefit to PMRT in terms of LRR-free survival or overall survival. There was a trend toward poorer survival in patients without a pCR in the breast. The strength of the report was the uniform surgical management of patients. Although they categorized estrogen receptor, progesterone receptor, and Her2 status, they did not analyze TNBC patients separately.12 Because TNBC is much more likely to recur, yet only makes up a small fraction of all breast cancers, the broader inclusion criteria in this French study could have obscured the findings presented. Jwa et al evaluated LRR in a group of 335 patients with stage II-III breast cancer treated with NAC, breast-conserving surgery, and radiation, of whom 61 had TNBC. Patients with TNBC had higher ipsilateral breast tumor recurrence rates and higher LRR rates than the non-TNBC patients, and there was a trend toward better LRR and improved ipsilateral breast tumor recurrence with a pathologic complete response similar to our study.13 Caudle et al similarly looked at response to NAC and outcome in a group of 595 patients at M D Anderson. Patients with Her2+ or TNBC biology with a poor response to NAC had worse LRR-free survival after breast-conserving therapy compared with other subtypes.10
Our current analysis adds to the existing literature on LRR in TNBC because it includes patients who have undergone both NAC and adjuvant radiation therapy, yet includes patients with any nonmetastatic stage disease. For example, even though Abdulkarim et al found that radiation therapy with breast-conserving surgery was superior to mastectomy alone for LRR in TNBC, not only did their patient population not receive NAC, but the population was limited to node-negative disease only.9 The prospective, randomized controlled Chinese trial done by Wang et al also shares similar weaknesses in that, although it demonstrates the effectiveness of radiation treatment in addition to chemotherapy over chemotherapy alone for DFS and overall survival, chemotherapy was used adjuvantly in their TNBC study.14 The study in the current literature most similar to that described here was done at Columbia University and showed results comparable to ours; namely, that if pCR is not achieved with NAC for TNBC, there is a much greater risk of LRR even if adjuvant radiation therapy is given.15 However, their study only had 36 TNBC patients and focused on comparing TNBC patient results with those of other subtypes, instead of specifically parsing out the location of residual disease in TNBC alone, as was done in our study. Our findings here therefore provide valuable insight and build upon those previously published in the literature.
Some limitations of our study should be noted. First, our study was a retrospective investigation, meaning that prospective studies should be undertaken to verify our findings. In addition, because of the strict inclusion and exclusion criteria of our study, despite surveying patients across nearly 2 decades of treatment, we were able to include only 72 patients in our analysis, and these patients had both breast-conserving surgery and mastectomy, which could obscure important findings regarding local recurrence in either group.
In conclusion, patients with TNBC who have residual disease following NAC are at high risk of both locoregional failure and death from breast cancer even when radiation therapy is given. Further treatment intensification is warranted, and consideration should be given to additional systemic therapy either by itself or concurrent with radiation therapy.
Footnotes
Conflicts of interest: None.
References
- 1.Santana-Davila R., Perez E.A. Treatment options for patients with triple-negative breast cancer. J Hematol Oncol. 2010;3:42. doi: 10.1186/1756-8722-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Minckwitz G., Untch M., Blohmer J.U. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 3.Esserman L.J., Berry D.A., DeMichele A. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 trial—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liedtke C., Mazouni C., Hess K.R. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 5.Dominici L.S., Mittendorf E.A., Wang X. Implications of constructed biologic subtype and its relationship to locoregional recurrence following mastectomy. Breast Cancer Res. 2012;14:R82. doi: 10.1186/bcr3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen P.L., Taghian A.G., Katz M.S. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and her-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 7.Mamounas E.P., Anderson S.J., Dignam J.J. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of national surgical adjuvant breast and bowel project b-18 and b-27. J Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire S.E., Gonzalez-Angulo A.M., Huang E.H. Postmastectomy radiation improves the outcome of patients with locally advanced breast cancer who achieve a pathologic complete response to neoadjuvant chemotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1004–1009. doi: 10.1016/j.ijrobp.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulkarim B.S., Cuartero J., Hanson J., Deschenes J., Lesniak D., Sabri S. Increased risk of locoregional recurrence for women with t1-2n0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2858. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caudle A.S., Yu T.K., Tucker S.L. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res. 2012;14:R83. doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarneri V., Broglio K., Kau S.W. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 12.Le Scodan R., Selz J., Stevens D. Radiotherapy for stage II and stage III breast cancer patients with negative lymph nodes after preoperative chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys. 2012;82:e1–e7. doi: 10.1016/j.ijrobp.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Jwa E., Shin K.H., Kim J.Y. Locoregional recurrence by tumor biology in breast cancer patients after preoperative chemotherapy and breast conservation treatment. Cancer Res Treat. 2016;48:1363–1372. doi: 10.4143/crt.2015.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Shi M., Ling R. Adjuvant chemotherapy and radiotherapy in triple-negative breast carcinoma: A prospective randomized controlled multi-center trial. Radiother Oncol. 2011;100:200–204. doi: 10.1016/j.radonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C., Wang S., Israel H.P. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. Springerplus. 2015;4:386. doi: 10.1186/s40064-015-1116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]