Abstract
Purpose
To report a single-institutional experience using magnetic resonance imaging (MRI) guided radiation therapy for the reirradiation of recurrent and second cancers of the head and neck.
Methods and materials
Between October 2014 and August 2016, 13 consecutive patients with recurrent or new primary cancers of the head and neck that occurred in a previously irradiated field were prospectively enrolled in an institutional registry trial to investigate the feasibility and efficacy of MRI guided radiation therapy using a 0.35-T MRI scanner with a cobalt-60 radiation therapy source called the ViewRay system (ViewRay Inc., Cleveland, OH). Eligibility criteria included biopsy-proven evidence of recurrent or new primary squamous cell carcinoma of the head and neck, measurable disease, and previous radiation to >60 Gy. MRI guided reirradiation was delivered either using intensity modulated radiation therapy with conventional fractionation to a median dose of 66 Gy or stereotactic body radiation therapy (SBRT) using 7 to 8 Gy fractions on nonconsecutive days to a median dose of 40 Gy. Two patients (17%) received concurrent chemotherapy.
Results
The 1- and 2-year estimates of in-field control were 72% and 72%, respectively. A total of 227 daily MRI scans were obtained to guide reirradiation. The 2-year estimates of overall survival and progression-free survival were 53% and 59%, respectively. There were no treatment-related fatalities or hospitalizations. Complications included skin desquamation, odynophagia, otitis externa, keratitis and/or conjunctivitis, and 1 case of aspiration pneumonia.
Conclusions
Our preliminary findings show that reirradiation with MRI guided radiation therapy results in effective disease control with relatively low morbidity for patients with recurrent and second primary cancers of the head and neck. The superior soft tissue resolution of the MRI scans that were used for planning and delivery has the potential to improve the therapeutic ratio.
Summary.
Magnetic resonance imaging (MRI) guided intensity modulated radiation therapy is a relatively novel technique that has the potential to improve the therapeutic ratio for tumors of the head and neck because of its superior ability to visualize soft tissue compared with computed tomography–based image guided platforms that have historically been used. This study reports our preliminary experience with the use of MRI guided reirradiation for patients with recurrent head neck tumors.
Introduction
The role of reirradiation in the treatment of recurrent and new primary cancers of the head and neck is controversial. Although reirradiation was historically avoided because of concerns of serious complications, reports have increasingly demonstrated that reirradiation is feasible and effective for carefully selected patients with a variety of techniques and fractionation schedules.1, 2, 3 More recently, the incorporation of advanced technologies such as intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT) have led to more promising treatment results with reirradiation. However, because the treatment plans generated by these highly conformal techniques are characterized by steep dose gradients, the precise targeting and delivery of radiation therapy on a per-fraction basis is paramount to maximize the dose to the tumor and minimize the dose to neighboring critical structures.
Magnetic resonance imaging (MRI) guided radiation therapy is a relatively novel technique that has the potential to improve the therapeutic ratio because of its superior soft tissue resolution compared with computed tomography (CT)–based onboard imaging platforms that have traditionally been used for image guided radiation therapy (IGRT). With improved visualization of the head and neck structures and the ability to acquire images during treatment delivery, this technology has been proposed as a means to overcome potential uncertainties in setup and account for both intra- and interfraction error. Additionally, this enhanced precision allows for the employment of planning target volume (PTV) margins that are smaller than those conventionally used for radiation planning, which leads to decreased normal tissue exposure.
Although MRI guided radiation therapy continues to generate excitement, few studies have investigated actual clinical outcomes. We report our initial experience with patients who were treated for recurrent and new primary malignancies of the head and neck and underwent reirradiation.
Methods and materials
Patients
Between February 2015 and August 2016, 13 consecutive patients with recurrent or new primary cancers of the head and neck that occurred in a previously irradiated field were prospectively enrolled in an institutional registry trial to investigate the feasibility and efficacy of MRI guided radiation therapy. Institutional review board approval was obtained before the activation of this trial. Patients who were enrolled in the study had biopsy-proven evidence of recurrent or new primary cancer of the head and neck, measurable disease, squamous cell carcinoma histology, and had previously received a dose of >60 Gy for their initial cancer. All patients had been previously treated with definitive therapy for their initial disease, including surgical resection with postoperative radiation therapy in 7 patients (64%) and external beam radiation therapy with or without chemotherapy in an additional 6 patients (46%). The median total radiation dose that was previously administered to the site of reirradiation was 66 Gy (range, 60-70 Gy). Six patients (46%) had previously received concurrent chemotherapy.
All patients were presented to a multidisciplinary head and neck cancer tumor conference before initiation of reirradiation and were deemed to be unfit for salvage surgery. Axial imaging with either CT or MRI of the head and neck with intravenous contrast and positron emission tomography was performed in all patients as part of their work-up before reirradiation. No patient had evidence of distant metastasis at the time of registration.
Patient immobilization and simulation
At simulation and before daily treatment, the patient's head, neck, and shoulders were immobilized using a perforated, thermoplastic mask with the occiput supported on a modified Timo cushion (S-type, Med-Tec, Orange City, IA) mounted on a plastic board (ViewRay Inc., Cleveland, OH), which allowed indexing of patient positioning and facilitated MRI imaging coil placement. The bottom receiver surface coil fitted into a specially designed T-shaped notch on the Timo cushion so that it was flush to the surface of the cushion, which allowed for reproducible placement of the coil for each treatment. The coils were placed from the top of the head to above the patient's chest and covered the length of the expected PTV. The receiver surface coils had approximately 1% beam attenuation, which was uniformly accounted for in the treatment planning system during subsequent optimization and dose calculation. Patients were fitted with a customized mouthpiece to immobilize the oral cavity. All patients underwent simulation with intravenous contrast initially on a large bore, 16-slice CT scanner (Siemens SOMATOM Definition AS, Siemens Healthcare, Inc., Los Angeles, CA) with acquisition of contiguous 3 mm slice thickness. The MRI scan was then obtained on the ViewRay system (ViewRay, Inc., Oakwood Village, OH) incorporating the 0.35 T MRI and obtaining anatomic sections with 1.5 mm thickness without contrast. The physical characteristics of the ViewRay system have previously been described.4 Both imaging datasets, which encompassed the patient from apex of the skull to below the clavicles, were then transferred to a specialized contouring workstation, MIM (MIMvista Corporation, Cleveland, OH). Notably, all contouring and subsequent treatment planning were performed on the MRI dataset.
Treatment planning and dose specification
The gross tumor volume (GTV) was specified as the gross extent of recurrent or new primary tumor as demonstrated by pretreatment imaging. The GTV was expanded by 0 to 0.2 cm in all directions (mean, 0.15 cm) to create a PTV to account for internal motion and set-up error. Elective nodal areas were not treated.
The target and avoidance structure contours together with the MRI and CT datasets were transferred to the ViewRay treatment planning system using the DICOM protocol. The system used a convex nonlinear programing model for dose optimization for a group of selected IMRT beams, and the optimization was controlled by the user by setting dose optimization parameters for the target and each organ at risk. Dose calculation was performed with a grid resolution of 3 mm using a Monte Carlo–based algorithm that tracks the interactions of particles with the various components of the treatment delivery system and patient with the consideration of the MRI magnetic field.
Treatments were delivered using a step-and-shoot technique with ViewRay's 3 cobalt sources, which lie 120 degrees apart and each of which is independently collimated by 30 pairs of doubly focused multileaf collimator leaves that project up to 1.05 cm at the isocenter. The 3 sources rotate in concert; therefore, a beam group was defined as a group of up to 3 beams positioned at a particular gantry angle. Generally, 5 to 7 gantry groups totaling 15 to 21 beams were selected for planning. Electron density information for dose calculation was obtained by fusing the CT scans with the planning MRI dataset and transferring electron density from the CT to the MRI. Metal artifacts were manually contoured with a bulk density assignment of 1 g/cm3. Treatment plans were designed to deliver 100% of the prescription dose to 95% of the corresponding PTV while sparing neighboring critical structures.
The choice of prescription dose to the PTV was at the discretion of the treating physician. The planned reirradiation treatment consisted of SBRT for 7 patients and IMRT for 6 patients. For SBRT, the planned dose ranged from 35 to 40 Gy (median, 40 Gy) in 5 fractions, delivered on nonconsecutive days. For the latter, the planned dose ranged from 60 to 66 Gy (median, 66 Gy) at 2 Gy per fraction daily, using a continuous, nonsplit course schedule in all patients except 1 who had ethmoid sinus cancer that involved the orbits who was treated to 64.8 Gy at 1.2 Gy twice daily. All patients had dosimetry from their previous courses reviewed and were generally assumed to have received the maximal allowable spinal cord and brainstem doses (45 Gy and 50 Gy, respectively). Figures 1 and 2 illustrate typical IMRT and SBRT plans used for reirradiation.
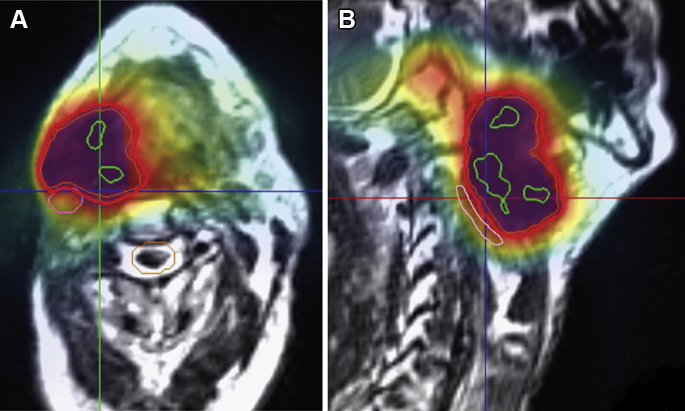
Figure 1.
Case illustration of intensity modulated radiation therapy (IMRT): A 74-year-old male patient with recurrent oropharynx cancer presenting as a large right neck mass approximately 1 year after previously completing definitive chemoradiation to a dose of 70 Gy with concurrent cisplatin for ipsilateral p16-negative tonsil cancer. The patient was reirradiated to a dose of 66 Gy in 33 fractions over the course of 6 weeks with magnetic resonance image guided IMRT. The axial (A) and sagittal (B) sections of the IMRT treatment plan are displayed. The thick red line represents the prescription dose that encompasses the planning target volume (blue color wash), which comprised the gross tumor volume plus a 0.1 cm margin circumferentially. The green lines show the 105% hotspots. Notably, an effort was made to spare the ipsilateral uninvolved carotid artery (in pink) and spinal cord (in orange).
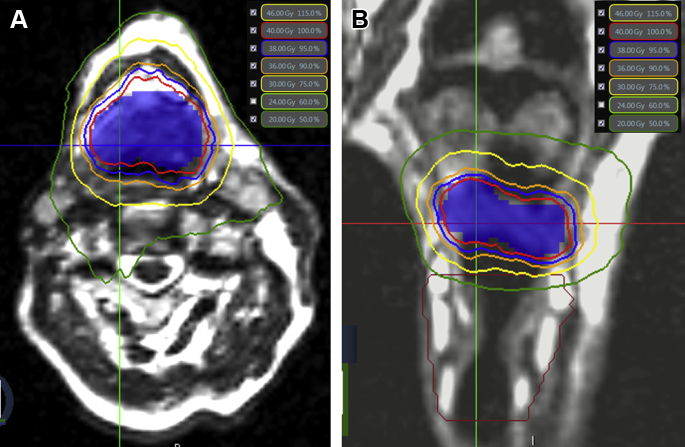
Figure 2.
Case illustration of stereotactic body radiation therapy (SBRT): A 63-year-old male patient with a new primary cancer that involved the central base of the tongue after previously receiving radiation therapy to the mucosal axis with concurrent cisplatin approximately 10 years earlier for an unknown primary cancer. The patient was reirradiated to a dose of 40 Gy in 5 fractions on nonconsecutive days with magnetic resonance image guided SBRT. The axial (A) and coronal (B) sections of the SBRT treatment plan are displayed. The red line represents the prescription dose that encompasses the planning target volume, which comprised the gross tumor volume plus a 0.1 cm margin circumferentially (blue color wash). The blue, orange, yellow, and green lines represent the 95%, 90%, 75%, and 50% isodose lines, respectively. Notably, an effort was made to avoid dose spillage to the neighboring larynx (in maroon contour).
MRI guidance
A daily setup of 0.35 T MRIs was acquired at 1.5 mm isotropic spatial resolution and 172 second acquisition time using a balanced steady-state free precession sequence (TrueFISP sequence) before each treatment delivery. After the MRI slices were reconstructed, they were fused with the treatment planning MRI that had been previously acquired with the ViewRay system using an automated imaging registration software provided by ViewRay and with the soft tissue presets focusing on the PTV. Manual adjustments were performed as needed. During each fraction, an attending physician reviewed the fused image in the sagittal, coronal, and axial planes using the fusion split screen display and signed off to approve the treatment (Fig 3). After image fusion was satisfactorily accomplished, the couch was repositioned for treatment delivery. Adaptive replanning, either online or offline, was not routinely performed during reirradiation.
Figure 3.
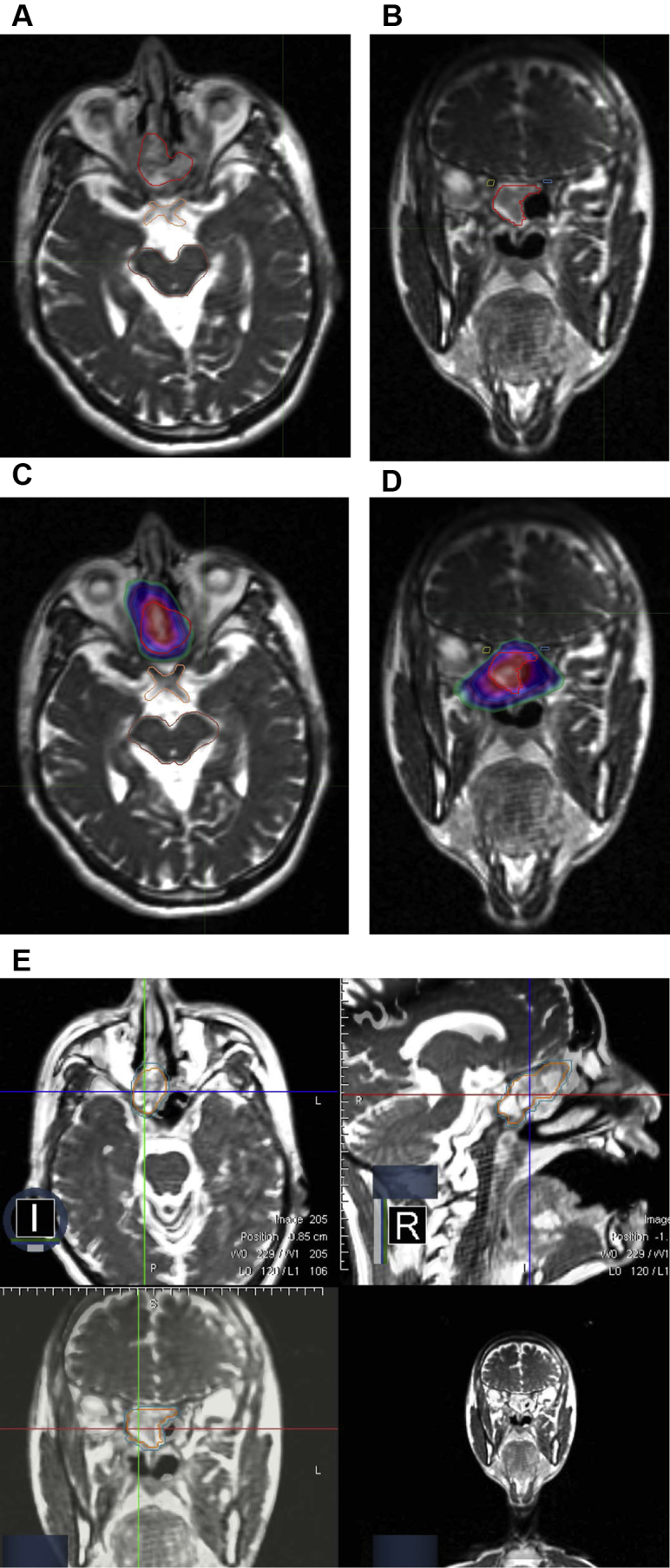
Case illustration of stereotactic body radiation therapy (SBRT): A 40-year-old male patient with recurrent nasopharynx cancer that involved the skull base. (A) Axial and (B) coronal sections of the magnetic resonance imaging scans that were obtained at the time of simulation for treatment planning shows the proximity of the gross tumor (red) to critical organs including the optic chiasm (orange), brainstem (brown), and optic nerves (blue and yellow). The patient was treated with a regimen of 40 Gy in 5 fractions to the gross tumor volume. Because of the proximity to normal tissue, no planning target volume expansion was used. The conformal intensity modulated radiation therapy plan (C and D) illustrates the 40 Gy isodose cloud in red color wash, with the white, blue, purple, and green representing the 105%, 95%, 90%, and 70%, isodose distributions, respectively. (E) The fusion split screen display on the ViewRay dashboard for a patient who underwent reirradiation with stereotactic body radiation therapy for recurrent nasopharyngeal cancer. The gross tumor volume (orange) and planning target volume (blue) are clearly demarcated on the magnetic resonance imaging sections. Note the high spatial resolution that enables precise visualization of the recurrent tumor in the right nasopharynx in proximity to the skull base in the axial, coronal, and sagittal planes.
A total of 227 MRI scans were obtained as part of the image guided process to assist with daily set-up. Cine TrueFISP MRI sequences were acquired at 4 frames per second on a sagittal plane through the target center in the field of view during each treatment fraction to monitor the intrafractional motion. To quantify tumor motion, anatomic landmark tracking points were placed around the target boundary with an additional point placed at the mandible for reference. Two patients had serial diffusion-weighted imaging sequences that were obtained at weekly intervals, the results of which have previously been reported.5
Follow-up and statistical analysis
In-field recurrence was defined as radiographic and/or clinical progression of recurrent disease in the salvaged site treated by reirradiation. Regional failure was recorded separately if there was evidence of an enlarging cervical or supraclavicular mass that was distinct from the primary site. A baseline posttreatment positron emission tomography/CT scan was typically obtained approximately 3 months after completion of treatment.
The response to reirradiation was determined using the Response Evaluation Criteria In Solid Tumors criteria.6 Actuarial rates of overall survival, in-field control, and progression-free survival were calculated using the Kaplan-Meier method and comparisons among groups were performed with 2-sided log-rank tests. Acute and late normal tissue effects were graded at each follow-up visit according to the National Cancer Institute's Common Toxicity Criteria.7 All P values reported were 2-sided with P < .05 used to denote statistical significance.
Results
Patient and treatment characteristics
Of the 13 patients enrolled on the protocol, 1 patient withdrew consent shortly after registration and was treated elsewhere. Table 1 outlines the clinical and disease characteristics of the remaining 12 evaluable patients who comprised the study population, all of whom completed the planned MRI guided radiation therapy. The median time interval from the last day of radiation therapy for initial disease to the first day of reirradiation was 10 months (range, 5-143 months), with 6 patients (50%) and 4 patients (33%) who underwent salvage therapy more than 1 year and 5 years after initial treatment, respectively. Seven patients were reirradiated to mucosal sites and 5 patients to nodal regions. The median tumor size was 3 cm (range, 1-9 cm). Two patients were irradiated to separate foci within the same PTV. The median age was 62 years (range, 50-78 years).
Table 1.
Clinical and disease characteristics
| Characteristic | N | % |
|---|---|---|
| Age at recurrence, y | ||
| 40-50 | 3 | 25 |
| 50-60 | 3 | 25 |
| 60-70 | 3 | 25 |
| >70 | 3 | 25 |
| Primary site | ||
| Oropharynx | 5 | 42 |
| Nasopharynx | 4 | 33 |
| Oral cavity | 2 | 17 |
| Paranasal sinus | 1 | 8 |
| HPV status | ||
| Positive | 4 | 33 |
| Negative | 2 | 17 |
| Unknown | 6 | 50 |
| Smoking history | ||
| None | 4 | 33 |
| Yes, <10 packs/year | 1 | 8 |
| Yes, 10-40 packs/year | 3 | 25 |
| Yes, >40 packs/year | 4 | 33 |
| Sex | ||
| Male | 9 | 75 |
| Female | 3 | 25 |
| Karnofsky performance status | ||
| 90 | 3 | 25 |
| 80 | 5 | 42 |
| 70 | 4 | 33 |
| Ethnicity | ||
| White | 8 | 67 |
| Asian | 4 | 33 |
| Radiation therapy technique | ||
| SBRT | 6 | 50 |
| IMRT | 6 | 50 |
| Dose per fraction | ||
| 2 Gy | 6 | 50 |
| >2 Gy | 6 | 50 |
| Time from initial course of radiation therapy | ||
| <1 y | 6 | 50 |
| >1 y | 6 | 50 |
| Concurrent chemotherapy | ||
| Yes | 2 | 17 |
| No | 10 | 83 |
HPV, human papillomavirus; IMRT, intensity modulated radiation therapy; SBRT, stereotactic body radiation therapy.
Disease control
Four patients had died at the time of this analysis. Overall survival for the entire patient population at 1 and 2 years was 61% and 53%, respectively. Of the 12 patients who underwent reirradiation, 6 (50%) had a complete response and 4 (33%) had a partial response. A total of 2 patients (17%) ultimately experienced tumor recurrence and/or progression at the reirradiated site at 4 months and 10 months, respectively, after completion of treatment. Another patient developed dermal metastasis in the vicinity of the irradiated volume approximately 8 months after completion of reirradiation. These in-field recurrences were the only known sites of disease at the time of failure.
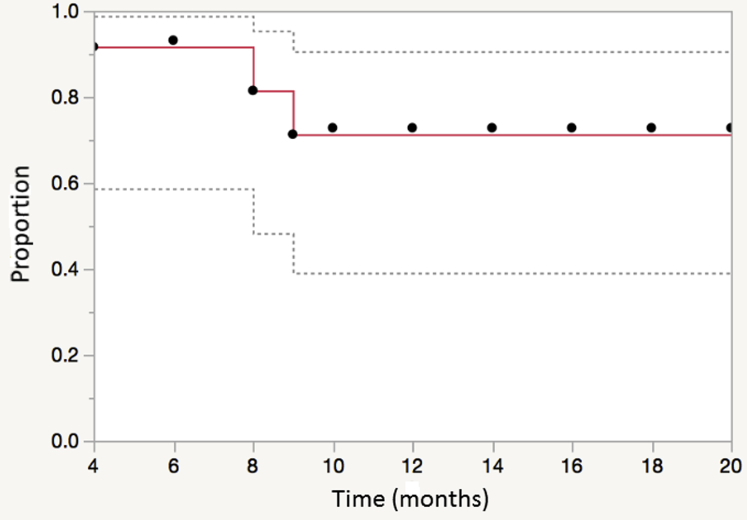
One patient developed distant metastasis in the bilateral lungs approximately 13 months after completion of reirradiation. No regional failures were observed. The 1- and 2-year estimates of in-field control were 72% and 72%, respectively. In-field control after irradiation is illustrated in Figure 4. The 1- and 2-year estimates of progression-free survival were 65% and 59%, respectively.
Figure 4.
Local (in-field) control for patients treated with reirradiation for recurrent and new primary head and neck cancer using magnetic resonance image guided intensity modulated radiation therapy.
Complications
There were no fatalities or hospitalizations related to reirradiation. One patient developed sepsis from gastric perforation due to gastrostomy-tube placement after the second fraction of SBRT and subsequently died. The incidence of grade 3+ toxicity among the cohort of patients treated with reirradiation was 42%. Table 2 lists all observed grade 3+ acute toxicities. Three patients (25%) required gastrostomy tube placement midway through their radiation treatment courses for decreased alimentary intake due to odynophagia and/or mucositis.
Table 2.
Acute toxicities (grade 3+)
| Toxicity | N | % |
|---|---|---|
| Skin desquamation | 5 | 42 |
| Odynophagia/dysphagia | 4 | 33 |
| Mucositis | 4 | 33 |
| Keratitis/conjunctivitis | 1 | 8 |
Fibrosis of the neck was the most commonly reported late toxicity, but this was present to varying degrees even before the initiation of reirradiation for most patients. One patient was diagnosed with aspiration pneumonia, which was thought to be secondary to epiglottic dysmotility, approximately 3 months after completion of reirradiation and was managed as an outpatient with antibiotics. The proportion of patients who were gastrostomy-tube dependent at 6 months and 1 year was 17% and 0%, respectively.
Discussion
The present study on the feasibility and efficacy of MRI guided radiation therapy is the first to our knowledge to demonstrate the usefulness of this technology as a complement to IMRT and SBRT in the management of recurrent and new primary cancers of the head and neck. In view of concerns with regard to the potential overdosing of critical structures in a previously irradiated field, this technology provides assurance that what was targeted at the time of simulation was what was indeed treated with each fraction. Additionally, the superior soft tissue contrast of MRI enabled the use of reduced PTV margins, which decreased the amount of normal tissue that was exposed to irradiation, a particularly important achievement in the scenario of tumors that arise within previously irradiated regions. Clearly, integrating such technology with reirradiation has the potential to improve the therapeutic ratio.
Despite advances in the multidisciplinary management of head and neck cancer, a significant proportion of patients develop local-regional recurrence or new primary cancers in a previously irradiated field after completion of high-dose therapy. Therapeutic options are limited for patients with unresectable disease or those who are at unacceptably high risk for perioperative complications. Although chemotherapy alone is traditionally considered in this setting, response rates are poor and nearly all patients die of local-regional progression within months.8
The enthusiasm for salvage therapy with reirradiation has traditionally been limited by concerns of toxicity. In a study of 169 patients, De Crevoisier et al demonstrated that full-dose reirradiation of head and neck sites resulted in high rates of late toxicity, including mucosal necrosis in 21% of patients, osteoradionecrosis in 8%, and 5 deaths due to carotid hemorrhage.9 Although the results of more recent studies that analyzed reirradiation using altered fractionation and/or more conformal techniques have been more promising, the role of reirradiation after previous full-course radiation is still considered investigational by many.1, 2, 3 However, a growing body of data suggests that the use of IMRT and SBRT has significantly improved clinical outcomes in the setting of reirradiation.
Although these advanced technologies have been heralded for their ability to deliver elegantly conformal treatment plans to complex target volumes, this technology is critically based on an inherent assumption that the region of interest lies in the exact same position with each fraction as at the time of simulation. The ability to deliver a high dose to tumor while achieving substantially sharp dose fall-off gradients between targets and surrounding normal tissue is of unquestionable importance, but precise and accurate targeting on a consistent basis is equally germane to achieve optimal results. In the setting of high-dose reirradiation, a failure to reasonably account for issues such as interfraction motion and setup uncertainty could potentially be the difference between cure and complication. Indeed, Chen et al demonstrated that setup errors as detected by online imaging via traditional CT-based IGRT methods were significant and thus raised questions with regard to the suitability of such a delivery platform for reirradiation.10
However, an MRI-based IGRT and/or IMRT system is unique in many ways. First, as demonstrated in the present study, the high-quality resolution of the images allows for precise determination of target volumes and essentially eliminates any interfraction error. Another advantage relates to the reduction in interference from dental artifact that is associated with MRI scans. As importantly, the ability to acquire images continuously in real-time eliminates any intrafraction error, which is particularly relevant in cases in which treatments are longer, such as during SBRT.
Potential drawbacks to this technology, however, do exist and continue to be investigated. For example, the cobalt-60 source is known to provide beams with lower output, less penetration, larger penumbra, and higher surface doses than a linear accelerator. Second, the lack of electron density information and image distortion that leads to the geometric inaccuracies inherent to MRI scans precludes accurate dose calculation. Additionally, the low 0.35-T field strength and lack of contrast enhancement could have detracted from delineation.
Despite these limitations, others have shown that the IMRT plans obtained from such technology compare favorably in conformality to those obtained from traditional IMRT systems.11 Similarly, it has been suggested that magnetic susceptibility–induced geometric distortion may not be clinically relevant and that quality assurance benchmarks can be reasonably achieved with MRI guided radiation therapy.12, 13
Although we did not include the outcomes of patients who were reirradiated without MRI guidance in the present series and are thus unable to determine with any certainty the gains that are associated with this technology, the results of our study are nonetheless encouraging. The 1-year local control rate of 71%, for example, compares favorably with reported rates of local control in the literature, which range from 25% to 85%.1, 2, 3 Patient selection criteria and heterogeneity with respect to disease characteristics likely contributed to the wide variation in published rates of disease control. It must also be noted that our experience comprises a relatively small subset of patients who were selected for reirradiation on the basis of multiple patient- and disease-specific parameters.
In view of the aggressive fractionation regimens used in our series, the relatively low rate of observed complications is particularly reassuring. In contrast, the incidence of toxicities such as osteoradionecrosis, carotid artery hemorrhage, and fistula formation has been estimated to be higher among historical control subjects.1, 2, 3 In one of the largest series to date, Salama et al reported 19 treatment-related deaths, including 9 patients who died during treatment among 115 patients who underwent reirradiation with a variety of fractionation regimens.14 In our opinion, the low incidence of complications relates to MRI's ability to delineate and visualize critical organs such as the carotid artery and central nervous system more clearly than CT.
Given the uncertainty involved when critical structures have already been irradiated to the brink of commonly accepted tolerance levels, the ability to accurately determine doses to these organs is important. Because of the devastating complications that can be associated with overdosing these tissues, the additional certainty that MRI provides with respect to the spatial orientation of critical organs before and during the delivery of radiation provides an additional and unprecedented level of safety. This assurance is what in some cases allowed for the use of PTV margins as small as 0 mm. Indeed, the improved localization and targeting of tumor at the treatment console at the time of radiation delivery allowed us to eliminate margins that are typically used to account for inter- and intrafraction motion and represents one of the most prominent advantages of this technology. Whether this practice contributed to the improved toxicity profile observed in the present series is strictly speculative, but these margins do lead to decreased dose exposure to normal tissue.15 Notably, the fact that no marginal misses occurred with this practice lends additional credence to this approach. Furthermore, it is important to recognize that all the observed complications cannot be clearly attributed to reirradiation because virtually all patients who were enrolled had side effects from prior radiation.
Notably, the literature continues to expand and suggests that the use of advanced technologies can improve the therapeutic ratio, namely by reducing potential toxicity, in the setting of reirradiation. In the largest series of patients treated by reirradiation using IMRT, Takiar et al reported a 2-year incidence of grade 3+ toxicity of 32%, which is consistent with the current findings.1 Curtis et al similarly showed that although dermatitis and mucositis were relatively common among the 81 patients who were reirradiated in their study, 77 of whom received IMRT, the incidence of serious toxicities was low.16 Studies that analyzed the use of proton therapy in the reirradiation setting have also shown promise, with seemingly improved rates of local control accompanied by decreased toxicity.17, 18
The relatively small sample size did not allow us to comment on the relative value of IMRT versus SBRT and/or the role of concurrent chemotherapy, but we were nonetheless able to demonstrate the feasibility of reirradiation using an MRI guided approach.
Our results demonstrate that although overall survival after recurrence or persistence of head and neck cancer after definitive therapy is relatively limited, effective disease control with acceptable side effects can be achieved in appropriately selected patients using MRI guided reirradiation. The intent of this study was not to draw any definitive conclusions but to highlight the capacity of a relatively novel technology to perform reirradiation to the head and neck. Because MRI offers significantly improved soft tissue resolution of the head and neck, the integration of such technology with reirradiation has the potential to improve the therapeutic ratio. On the basis of our early experiences with MRI guided radiation therapy, all patients who undergo reirradiation to the head and neck at our institution are treated with this approach. Future investigation will center on the addition of functional imaging sequences such as diffusion weighting and the incorporation of novel radiosensitizers.
Footnotes
Conflicts of interest: The authors indicate no disclosure of potential conflicts of interest. MLS serves on the scientific advisory board for Viewray, Inc. and YY and MC have received honorariums from Viewray, Inc.
References
- 1.Takiar V., Garden A.S., Ma D. Reirradiation of head and neck cancers with intensity modulated radiation therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016;95:1117–1131. doi: 10.1016/j.ijrobp.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Lee N., Chan K., Bekelman J.E. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:731–740. doi: 10.1016/j.ijrobp.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall W.M., Mendenhall C.M., Malyapa R.S., Palta J.R., Mendenhall N.P. Re-irradiation of head and neck carcinoma. Am J Clin Oncol. 2008;31:393–398. doi: 10.1097/COC.0b013e3181637398. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y., Rankine L., Green O.L. Characterization of the onboard imaging unit for the first clinical magnetic resonance image guided radiation therapy system. Med Phys. 2015;42:5828–5837. doi: 10.1118/1.4930249. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Cao M., Sheng K. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys. 2016;43:1369–1373. doi: 10.1118/1.4942381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumors: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Available at: Accessed January 3, 2016.
- 8.Gibson M.K., Li Y., Murphy B. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): An intergroup trial of the Eastern Cooperative Group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 9.De Crevoisier R., Domenge C., Wibault P. Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16:3556–3562. doi: 10.1200/JCO.1998.16.11.3556. [DOI] [PubMed] [Google Scholar]
- 10.Chen A.M., Farwell D.G., Luu Q., Cheng S., Donald P.J., Purdy J.A. Prospective trial of high-dose reirradiation using daily image guidance with intensity-modulated radiotherapy for recurrent and second primary head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:669–676. doi: 10.1016/j.ijrobp.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Wooten H.O., Green O., Yang M. Quality of intensity modulated radiation therapy treatment plans using a ⁶⁰Co magnetic resonance image guidance radiation therapy system. Int J Radiat Oncol Biol Phys. 2015;92:771–778. doi: 10.1016/j.ijrobp.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 12.Li H.H., Rodriquez V.L., Green O.L. Patient-specific quality assurance for the delivery of (60)Co intensity modulated radiation therapy subject to a 0.35-T lateral magnetic field. Int J Radiat Oncol Biol Phys. 2015;91:65–72. doi: 10.1016/j.ijrobp.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weygand J., Fuller C.D., Ibbot G.S. Spatial precision in magnetic resonance imaging-guided radiation therapy: the role of geometric distortion. Int J Radiat Oncol Biol Phys. 2016;95:1304–1316. doi: 10.1016/j.ijrobp.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Salama J.K., Vokes E.E., Chmura S.J. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:382–391. doi: 10.1016/j.ijrobp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen A.M., Farwell D.G., Luu Q., Donald P.J., Perks J., Purdy J.A. Evaluation of the planning target volume in the treatment of head and neck cancer with intensity-modulated radiotherapy: What is the appropriate expansion margin in the setting of daily image guidance? Int J Radiat Oncol Biol Phys. 2011;81:943–949. doi: 10.1016/j.ijrobp.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Curtis K.K., Ross H.J., Garrett A.L. Outcomes of patients with loco-regionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol. 2016;11:55. doi: 10.1186/s13014-016-0630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan J., Sio T.T., Nguyen T.P. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016;96:30–41. doi: 10.1016/j.ijrobp.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 18.McDonald M.W., Zolali-Meybodi O., Lehnert S.J. Reirradiation of recurrent and second primary head and neck cancer with proton therapy. Int J Radiat Oncol Biol Phys. 2016;96:808–819. doi: 10.1016/j.ijrobp.2016.07.037. [DOI] [PubMed] [Google Scholar]