Abstract
Purpose
To describe the outcomes and toxicities of the largest cohort to date of patients with anal squamous cell carcinoma uniformly treated with concurrent chemoradiation using dose-painted intensity modulated radiation therapy (DP-IMRT) according to RTOG 0529.
Methods and materials
We identified 99 eligible patients with anal cancer who were treated at our institution with definitive chemoradiation using DP-IMRT between 2005 and 2015 per RTOG 0529 dosing guidelines. Primary study endpoints included event-free survival (defined as recurrence, colostomy, or death) and overall survival. Secondary endpoints were treatment duration and acute and late toxicity.
Results
At a median follow-up of 49 months (range, 2-114 months), 92% of patients had a clinical complete response. Fifteen percent underwent colostomy, including 4 pretreatment colostomies, 6 planned abdominoperineal resections (APRs), 4 salvage APRs, and 1 APR for treatment-related complications. Thirteen patients developed local recurrence, of whom 6 developed synchronous metastatic disease. The 4-year overall survival was 85.8%, and 4-year event-free survival was 75.5%. Median treatment duration was 43 days (range, 10-68 days). The overall rate of non-hematologic grade 3+ acute and grade 2+ late toxicities was 20% and 15%, respectively.
Conclusions
Long-term outcomes and tolerability were excellent In the largest cohort to date of patients with anal cancer who received DP-IMRT prescribed per RTOG 0529.
Summary.
RTOG 0529 was a phase 2 study of 52 patients with anal cancer who were prospectively treated with dose-painted intensity modulated radiation therapy. The current study of 99 patients at a single institution with a median follow-up of 49 months is the largest analysis to date of survival, recurrence, colostomy rates, and acute and late toxicity using this standardized approach. Overall, this study shows that long-term outcomes and tolerability are excellent.
Introduction
In 2016, an estimated 8000 new cases of anal cancer will be diagnosed in the United States and more than two-thirds of these patients are expected to survive at least 5 years.1 Given that the majority of patients will be cured, interventions to mitigate long-term treatment-associated toxicities are of critical importance.
Definitive radiation with concurrent 5-fluorouracil (5-FU) and mitomycin-C (MMC) is the well-established standard therapy for anal cancer.2, 3 However, due to treatment-related toxicities, various groups have reported that up to half of patients need treatment breaks to complete therapy.4, 5, 6, 7, 8 With data emerging that a longer treatment time can potentially affect outcome, improving the treatment-related toxicity profile is likely to be beneficial for patient quality of life and oncologic outcomes.9
Intensity modulated radiation therapy (IMRT) has been proposed as a method by which to spare organs at risk and decrease toxicities. Although various groups have shown promising results using this general approach, a lack of standardization initially made widespread application challenging.8, 10, 11, 12, 13 RTOG 0529 was a phase 2 study that prospectively assessed the feasibility and acute toxicities of dose-painted IMRT (DP-IMRT) with concurrent 5-FU and MMC. Although a few groups have looked at outcomes outside the clinical trial setting using this approach, these studies have been relatively small in number and with limited follow-up, making an assessment of long-term outcomes and late toxicities challenging.14, 15 Here, we present the largest analysis to date of all consecutively enrolled patients with anal cancer who received definitive chemoradiation at a single institution and were treated uniformly per RTOG 0529 with long-term follow-up.
Methods and materials
In late 2005, our institution transitioned to DP-IMRT, as prescribed by RTOG 0529, for all patients with anal cancer receiving definitive chemoradiation. With institutional review board approval, a retrospective chart review identified 107 patients with anal squamous cell carcinoma treated at our institution between September 2005 and December 2014. Eight patients were excluded for having hybrid 3-dimensional conformal-IMRT plans, metastatic disease at diagnosis, or recurrent disease after receiving initial therapy at an outside institution. As a result, data from 99 patients with anal cancer were analyzed.
Pretreatment evaluation included history and physical examinations; laboratory evaluation; imaging, including diagnostic computed tomography (CT) scans of the chest/abdomen/pelvis (with positron emission tomography in 72 patients), and colonoscopy or sigmoidoscopy. Positron emission tomography–CT was performed per physician discretion prior to 2009 but was routinely performed from November 2009 onward.
Radiation was delivered as described in RTOG 0529. For patients with T1-2N0 disease, the primary gross tumor volume was defined on the basis of physical examination, imaging, and endoscopy. The clinical target volume (CTV) was subdivided as defined in RTOG 0529 into a primary tumor CTV, involved nodal CTV, and elective nodal CTV, including the mesorectum, presacral nodes, bilateral inguinal nodes, and bilateral internal and external iliac nodes.16, 17 The planning target volume (PTV) consisted of a 7-mm expansion from the CTV except to avoid overlap with the skin and, when possible, the genitalia. Dose-painting was used to prescribe 50.4 Gy to the primary tumor PTV and 42 Gy to the elective nodal PTV over 28 fractions. For patients with T3-4 or N1-3 disease, the primary tumor and elective nodal volumes were similarly defined, with an additional involved gross tumor volume V nodal volume and corresponding CTV expansion that was created for lymph nodes that were >3 cm or prominently fluorodeoxyglucose-avid. Dose-painting was used to prescribe 54 Gy to the primary tumor PTV, 50.4 Gy to the involved nodal PTV, and 45 Gy to the elective nodal PTV. Dose constraints for organs at risk were as previously defined.17
Concurrent chemotherapy consisted of 10 mg/m2 MMC administered on days 1 and 29 with a continuous infusion of 1000 mg/m2 5-fluorouracil administered on days 1to 4 and days 29 to 32.4, 17 Appropriate dose modifications were made to account for toxicities.
Toxicities were retrospectively graded per the Common Terminology Criteria for Adverse Events Version 4.0 (on the basis of the description of toxicities by the treating physicians) with acute toxicities defined as those that occurred within 90 days of treatment and late toxicities being defined as those that occurred after 90 days. During treatment, patients were assessed weekly for acute toxicities. After completion of treatment, patients were typically scheduled for a symptom check after 2 weeks. The initial evaluation of treatment response was made 4 to 6 weeks after completion of therapy and continued monthly until clinical complete response was noted during digital rectal examination. Progression at any time prompted further evaluation with anoscopy and biopsy. Subsequent follow-up was typically every 3 months until year 2, every 6 months until year 5, and annually thereafter.
The primary study endpoints were event-free survival (EFS, defined as any recurrence, colostomy, or death) and overall survival (OS). The distributions of EFS and OS were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to evaluate associations between study variables and OS/EFS endpoints. A multivariate model was developed for EFS but not for OS due to the small number of deaths. Secondary endpoints included grade 3+ acute and 2+ late toxicity. Logistic regression models were used to evaluate the relationships between toxicity outcomes and study variables. A P-value <.05 was deemed statistically significant. Study variables evaluated included age, sex, Eastern Cooperative Oncology Group performance status, smoking, immunosuppression, body mass index, and treatment duration (<53 days vs >53 days9). Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Treatment delivery
Median follow-up was 49 months (range, 2-114 months). Clinical and treatment characteristics are described in Table 1. The median age at diagnosis was 61 years. The distribution of the disease stages was 13% for stage I, 39% for stage II, 20% for stage IIIA, and 27% for stage IIIB.
Table 1.
Patient, disease, and treatment characteristics
| Patient Characteristics | |
|---|---|
| Median Age (range) |
61 years (42-92) |
| Percent (n) |
|
| Sex | |
| Male | 31% (31) |
| Female | 69% (68) |
| ECOG Performance Status | |
| 0 | 55% (54) |
| 1 | 41% (41) |
| 2 | 3% (3) |
| 3 | 1% (1) |
| Smoking Status | |
| Never | 45% (45) |
| Prior | 31% (31) |
| Current | 23% (23) |
| Immunosuppression | |
| None | 79% (78) |
| HIV | 10% (10) |
| Prior transplant | 5% (5) |
| Other | 6% (6) |
| BMI | |
| <20 | 7% (7) |
| 20-25 | 39% (39) |
| 25-30 | 30% (30) |
| >30 | 23% (23) |
| Disease and Treatment Characteristics | |
|---|---|
| Median Elapsed Days (range) | 43 days (10-68) |
| Percent (n) |
|
| T-stage | |
| 1 | 15% (15) |
| 2 | 45% (45) |
| 3 | 28% (28) |
| 4 | 11% (11) |
| N-stage | |
| 0 | 56% (55) |
| 1 | 20% (20) |
| 2 | 12% (12) |
| 3 | 12% (12) |
| Grade | |
| 1 | 13% (13) |
| 2 | 49% (49) |
| 3 | 19% (19) |
| na | 18% (18) |
| Chemotherapy | |
| 2× 5FU/MMC | 92% (91) |
| 1× 5FU/MMC | 5% (5) |
| 5FU or Cape | 3% (3) |
| Radiation Dose | |
| 50.4 Gy | 44% (44) |
| 54 Gy | 53% (52) |
| <50.4 Gy | 3% (3) |
5FU, 5-fluorouracil; BMI, body mass index; Cape, capecitabine; ECOG, Eastern Cooperative Oncology Group; MMC, mitomycin-C; N, node; na, not available; T, tumor.
Fifty-three percent of patients received the 54/50.4/45 Gy dose-painted prescription because 39% of patients had T3-4 disease and 44% had clinical evidence of involved lymph nodes (LN). Ninety-two percent of patients received 2 cycles of 5-FU/MMC, 5% received a single cycle of 5-FU/MMC, and 3% received 5-FU or capecitabine alone because of their older age (81-91 years). The median duration of treatment was 43 days. In total, 34 patients required a treatment break with a median treatment break duration of 3 days. All treatment breaks were due to grade 3-4 neutropenia, except for 2 patients who had treatment breaks due to patient and physician preference in the context of grade 2-3 skin and gastrointestinal (GI) toxicity.
All but 3 patients completed therapy. The three patients who discontinued treatment early received 10, 16 and 25 days of radiation therapy, respectively. The patient who discontinued treatment after 10 days had a long history of lymphocytic interstitial pneumonitis and developed respiratory failure that was attributed to this underlying condition. The patient ultimately died despite high dose steroids. Although mitomycin C is known to rarely cause acute lung toxicity at higher doses, this patient's deterioration was thought to be independent of chemotherapy. The patient who discontinued therapy after 16 days died after aspiration pneumonia and renal failure, which was thought to be related to chemotherapy-associated nausea and vomiting. Disease response and late toxicities for these 2 patients were considered non-evaluable, leaving 97 evaluable patients. The patient who discontinued treatment after 25 days received 45 Gy prior to requesting that treatment be terminated for grade 3 skin and genitourinary toxicity. He subsequently recovered well and had no evidence of disease at last follow-up, 9.5 years after diagnosis.
Treatment outcomes
Among the 97 evaluable patients, 93.8% had a clinical complete response (cCR; Table 2). In total, 15 patients had a colostomy, including 4 patients who received pretreatment colostomies and 11 patients who underwent posttreatment colostomies. One of the 4 patients who underwent colostomy before chemoradiation subsequently had a cCR and was able to have surgical reanastamosis with return of stool continence. One patient was lost to follow-up after 6 months, and the remaining 2 patients developed progressive disease and subsequently died of metastatic anal cancer with a colostomy in place. For the patients who underwent posttreatment colostomy, 6 had a planned abdominoperineal resection (APR) in the context of particularly bulky T4 disease with poor bowel function before starting therapy, 4 patients had an APR for salvage or disease recurrence, and 1 patient had an APR 3 months after completing therapy in the context of persistent bowel incontinence. Of note, 1 of the 4 salvage colostomies was performed in a patient with a pathologic complete response who had some nodularity on examination, which was ultimately determined to be scar tissue.
Table 2.
Disease outcomes
| Follow-up (range) |
49 months (2-114) |
| Percent (n) |
|
| Clinical Complete Response | |
| Yes | 92% (91) |
| No | 6% (6) |
| Not evaluable | 2% (2) |
| Colostomy | 15% (15) |
| Planned | 10% (10) |
| Treatment complication | 1% (1) |
| Disease progression | 4% (4) |
| Disease Recurrence | 16% (16) |
| Locoregional only | 7% (7) |
| Locoregional + Distant | 6% (6) |
| Distant only | 3% (3) |
| Death | 14% (14) |
| Anal cancer related | 11% (11) |
| Unrelated | 3% (3) |
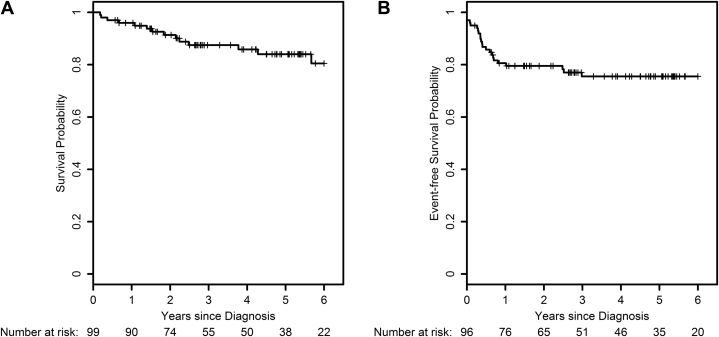
Of the 97 evaluable patients, 83.5% (n = 81) were without evidence of disease at the time of last follow-up. Nine patients developed distant metastases, with 3 being isolated distant recurrences. Seven patients developed isolated locoregional recurrences (Table 2). Of the patients who had a cCR, 3% (n = 3) developed local recurrence as the solitary site of failure. If cCR was achieved (n = 91), no patients developed distant metastatic disease as a sole site of failure. However, among the 6 patients who did not achieve a cCR, 5 subsequently developed metastatic disease. Four-year OS and EFS were 85.8% and 75.5%, respectively (Fig 1A, B). More than half of the events occurred within 6 months of diagnosis, and 79% of events occurred within 1 year.
Figure 1.
(A) Overall survival and (B) event-free survival (defined as absence of death, colostomy, or recurrence) for patients receiving dose-painted intensity modulated radiation therapy at our institution, as described in RTOG 0529.
On univariate analysis, factors associated with lower OS were more advanced T-stage, LN involvement, and a treatment duration of >53 days (Table 3). Similarly, poorer EFS was associated with more advanced T-stage and LN involvement, although there was no association with treatment duration. On multivariate analysis for EFS, only advanced T-stage remained significant (hazard ratio, 4.9; 95% confidence interval, 1.75-13.89; P = .003) although LN involvement and treatment duration were borderline significant (Table 4).
Table 3.
Univariate predictors of overall and event-free survival
| OS HR (95% CI) | P-value | EFS HR (95% CI) | P-value | |
|---|---|---|---|---|
| T3-T4 | 3.7 (1.21-11.12) | .021 | 4.6 (1.89-11.25) | .001 |
| LN+ | 5.0 (1.40-18.01) | .013 | 4.2 (1.65-10.63) | .003 |
| Female sex | 1.3 (0.44-3.92) | .620 | 1.5 (0.63-3.36) | .380 |
| Age (continuous) | 1.0 (0.97-1.06) | .560 | 1.0 (0.98-1.0) | .563 |
| BMI >30 | 0.8 (0.23-3.02) | .790 | 1.0 (0.37-2.66) | .980 |
| Current smoker | 1.3 (0.35-4.90) | .680 | 1.0 (0.33-2.84) | .956 |
| Immunosuppression | 0.9 (0.24-3.10) | .820 | 0.8 (0.27-2.37) | .692 |
| >53 days of treatment | 3.9 (1.09-13.99) | .037 | 2.8 (0.84-9.56) | .093 |
| Any grade >2 acute toxicity | 2.7 (0.94-7.87) | .070 | 1.4 (0.56-3.58) | .468 |
| Any late toxicity | 0.6 (0.22-1.82) | .400 | 0.6 (0.25-1.28) | .169 |
BMI, body mass index; CI, confidence interval; EFS, event free survival; HR, hazard ratio; LN+, lymph node involvement; OS, overall survival.
Table 4.
Multivariate predictors of event-free survival
| EFS HR (95% CI) | P-value | |
|---|---|---|
| T3-T4 | 4.9 (1.75-13.89) | .003 |
| LN+ | 2.5 (0.94-6.78) | .066 |
| >53 days of treatment | 3.9 (0.93-16.78) | .063 |
CI, confidence interval; EFS, event free survival; HR, hazard ratio; LN+, lymph node involvement.
Treatment toxicity
The cumulative acute non-hematologic grade 3+ toxicity rate was 21%. Hematologic toxicities were much more common with 63% having a grade 3+ hematologic toxicity, neutropenia being most common (Table 5). There was one grade 5 toxicity secondary to aspiration pneumonia. The cause of death of the second patient who died during treatment was attributed to underlying lymphocytic interstitial pneumonitis.
Table 5.
Toxicities
| Acute Non-hematologic Toxicity (%, n) | ||||
|---|---|---|---|---|
| Grade | Gastrointestinal | Skin | Genitourinary | Maximum Non-hematologic |
| Grade 1 | 41% (41) | 20% (20) | 16% (16) | 14% (14) |
| Grade 2 | 31% (31) | 63% (62) | 8% (8) | 62% (61) |
| Grade 3 | 8% (8) | 13% (13) | 1% (1) | 20% (20) |
| Grade 4 | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Grade 5 | 1% (1) | 0% (0) | 0% (0) | 1% (1) |
| Acute Hematologic Toxicity (Percent, n) | ||||
| Grade |
Neutropenia |
Anemia | Thrombocytopenia | Maximum Hematologic |
| Grade 1 | 6% (6) | 14% (14) | 0% (0) | 6% (6) |
| Grade 2 | 9% (9) | 27% (27) | 19% (19) | 15% (15) |
| Grade 3 | 24% (24) | 14% (14) | 16% (16) | 27% (27) |
| Grade 4 | 36% (36) | 0% (0) | 9% (9) | 36% (36) |
| Late Toxicity (Percent, n) | ||||
| Grade | Gastrointestinal | Genitourinary | Hip | Maximum Grade |
| Grade 1 | 37% (37) | 7% (7) | 0 (0) | 40% (40) |
| Grade 2 | 8% (8) | 4% (4) | 0 (0) | 12% (12) |
| Grade 3 | 2% (2) | 0% (0) | 1% (1) | 3% (3) |
| Grade 4 | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
| Grade 5 | 0% (0) | 0% (0) | 0% (0) | 0% (0) |
The most common late toxicity was grade 1 diarrhea, which was observed in 37% of patients and well managed with conservative interventions (Table 5). Fifteen percent of patients developed a grade 2+ late toxicity, including 12% of patients who developed radiation proctitis. Two patients underwent argon laser coagulation for rectal bleeding secondary to angioectasias. One patient with severe rheumatoid arthritis and multiple joint replacements who was on chronic immunosuppressive medication developed a sacral insufficiency fracture 2 years after treatment.
A body mass index >30 was found to be associated with an increased risk of grade 3 skin toxicity (odds ratio, 9.6; 95% confidence interval 2.56-36.04; P = .008). None of the other factors examined, including immunosuppression and treatment duration, were associated with either acute GI or genitourinary toxicities or late toxicities of any kind. In addition, there was no increased risk of late toxicities in patients who had higher grade acute toxicities.
Discussion
This study represents the largest cohort, to our knowledge, of patients with anal cancer who were treated uniformly per RTOG 0529 with definitive chemoradiation using DP-IMRT. Although anal cancer is a relatively rare diagnosis, because our institution is a large referral center with homogeneous practice patterns that adopted DP-IMRT per RTOG 0529 early on, we are in the unique position of having a homogenously treated patient population with a median of more than 4 years of follow-up. Our institution has a long history of rigorous regular follow-up of patients for at least 5 years, which—when combined with a retrospective review approach—provides particularly useful data with regard to outcomes such as colostomy, disease recurrence, and OS.
Although acute and late toxicities were assessed formally at weekly on-treatment visits and at regular follow-up after completion of treatment, due to the retrospective nature of the data collections, the toxicity rates reported may be an underestimation of the true toxicity. Other large retrospective studies have also been informative about the toxicity and efficacy of IMRT in the treatment of anal cancer, but these studies have not uniformly used the RTOG 0529 approach.13 In addition, given that RTOG 0529 found that 81% of treatment plans required revision, uniformity of contouring and treatment planning is critical in appropriately interpreting study endpoints. The few single-institution studies that examined outcomes in patients who received DP-IMRT per RTOG 0529 included less than half the number of patients in our current study and did not have information about late toxicities.14, 15
Although the 2-year outcomes data from RTOG 0529 were promising and revealed survival, recurrence, and colostomy rates similar to those of RTOG 9811, the current study may provide additional information about what could be expected with this treatment approach in the long term.18 RTOG 9811 and ACT II used older radiation techniques with concurrent 5-FU and MMC and found 5-year OS rates of 78.3% and 79%, respectively. With a median follow-up of 49 months, our study comparably found a 4-year OS of 85.8%.5, 6
It is not surprising that advanced T-stage and nodal involvement are significant predictors of OS and EFS. This was previously seen in many studies, including RTOG 9811 and a prior large, retrospective, multi-institutional study of patients with anal cancer receiving IMRT using a variety of treatment protocols.5, 13 The finding that treatment duration >53 days is associated with worse outcomes also has precedent in the work by Ben Josef et al,9 who showed that overall treatment duration is associated with colostomy and local failure in a post hoc analysis of RTOG 8704 and RTOG 9811. Although the statistically significant association of nodal status and treatment duration with EFS was lost on multivariable analysis, there was a trend toward significance with P-values in the .06 to .07 range, which suggests that this lack of significance may be secondary to limited power.
Overall, this study has several limitations. Although this is the largest study to date of DP-IMRT in patients with anal cancer, due to the excellent outcomes in our patient cohort, the small number of events (including colostomy, recurrence, and death) limited our analyses. Another limitation of this study is the difficulty in analyzing toxicity rates in a retrospective study. This is particularly true in the reporting of late toxicities, given variable follow-up. Also, without formal patient-reported outcomes, true toxicity rates are very possibly higher than what is documented here.
However, despite these limitations, this study may provide some general reassurance to patients undergoing definitive chemoradiation for anal cancer. Specifically, given that colostomy is a daunting prospect for patients, it is notable that if APR was not thought to be indicated on initial evaluation, only 5% of patients went on to require colostomy for incomplete response, disease recurrence, or treatment-associated toxicity. In addition, although acute toxicities during treatment were common, most were low grade and resolved after treatment completion. The lack of correlation between higher grade acute toxicity and late toxicity may give patients who are struggling with treatment hope that their long-term quality of life may still be excellent. In addition, although it is well established that human papillomavirus–mediated malignancies are more common in immunocompromised individuals, our study provides further support to the existing literature that outcomes for immunocompromised patients need not be worse in terms of OS, EFS, or treatment-associated toxicity.19
It is outside the scope of the current study to formally compare older radiation methods such as conformal 3-dimensional radiation therapy with sequential cone down to DP-IMRT, but a few general points may be worth noting. First, given that 81% of plans on RTOG0529 required modification on central review, practitioners who are new to DP-IMRT are likely to benefit from adhering to the strict contouring and planning guidelines established by RTOG to ensure that they see similarly excellent outcomes. The main advantage of DP-IMT as suggested by RTOG 0529 was the decreasing rates of grade 3+ acute GI toxicity (36% with 3-dimensional radiation therapy in RTOG 9811 vs 21% with DP-IMRT in RTOG 0529, P = .0052) and grade 3+ acute dermatologic toxicity (49% using 3-dimensional radiation therapy in RTOG 9811 vs 23% using DP-IMRT in RTOG 0529, P < .0001).17 Grade 3+ acute GI and dermatologic toxicity was lower in our study than in either of these prospective studies (8% and 13%, respectively), which may be the product of underreporting given retrospective data collection and/or aggressive symptom management. Acute 3+ hematologic toxicity rates appear similar across RTOG 9811 (62%), RTOG 0529 (58%), and our study (63%), which suggests that pelvic bone marrow is similarly suppressed by chemoradiation, regardless of radiation approach.17 Data on late toxicities is less robust, but RTOG 9811 suggested a grade 3-4 GI toxicity rate of 3%, which is perhaps comparable to the 2% late grade 3 GI toxicity rate seen in our study.5
On the basis of the promising acute toxicity profile and reassuring early outcomes from RTOG 0529, DP-IMRT has become common practice in the definitive treatment of anal cancer.17, 18 This study provides further support for the RTOG 0529 DP-IMRT treatment approach by expanding the study size, length of follow-up, and availability of late toxicity data. We have shown that this treatment protocol can be applied to a large cohort of diverse patients with anal cancer with excellent oncologic outcomes, good short-term tolerability, and low rates of long-term toxicity. As a result, radiation oncologists should feel comfortable applying this treatment approach broadly to patients with anal cancer.
Footnotes
Sources of support: Funding assistance for the study was received from the National Institutes of Health Rare Cancer Genetics Registry (4R01CA160233-05) and the Dana-Farber/Harvard Cancer Center Specialized Program in Research Excellence in Gastrointestinal Cancers (2P50CA127003).
Conflicts of interest: None.
References
- 1.NIH National Cancer Institute Cancer Stat Facts: Anal Cancer. http://seer.cancer.gov/statfacts/html/anus.html Available at: Accessed June 17, 2016.
- 2.Nigro N.D., Seydel H.G., Considine B., Vaitkevicius V.K., Leichman L., Kinzie J.J. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer. 1983;51:1826–1829. doi: 10.1002/1097-0142(19830515)51:10<1826::aid-cncr2820511012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet Lond Engl. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 4.Ajani J.A., Winter K.A., Gunderson L.L. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson L.L., Winter K.A., Ajani J.A. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–4351. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James R.D., Glynne-Jones R., Meadows H.M. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 7.Vuong T., Kopek N., Ducruet T. Conformal therapy improves the therapeutic index of patients with anal canal cancer treated with combined chemotherapy and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:1394–1400. doi: 10.1016/j.ijrobp.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Salama J.K., Mell L.K., Schomas D.A. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: A multicenter experience. J Clin Oncol. 2007;25:4581–4586. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Josef E., Moughan J., Ajani J.A. Impact of overall treatment time on survival and local control in patients with anal cancer: A pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol. 2010;28:5061–5066. doi: 10.1200/JCO.2010.29.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepek J.M., Willett C.G., Wu Q.J., Yoo S., Clough R.W., Czito B.G. Intensity-modulated radiation therapy for anal malignancies: A preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys. 2010;78:1413–1419. doi: 10.1016/j.ijrobp.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Bazan J.G., Hara W., Hsu A. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer. 2011;117:3342–3351. doi: 10.1002/cncr.25901. [DOI] [PubMed] [Google Scholar]
- 12.Han K., Cummings B.J., Lindsay P. Prospective evaluation of acute toxicity and quality of life after IMRT and concurrent chemotherapy for anal canal and perianal cancer. Int J Radiat Oncol Biol Phys. 2014;90:587–594. doi: 10.1016/j.ijrobp.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Call J.A., Prendergast B.M., Jensen L.G. Intensity-modulated radiation therapy for anal cancer: Results from a multi-institutional retrospective cohort study. Am J Clin Oncol. 2016;39:8–12. doi: 10.1097/COC.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yates A., Carroll S., Kneebone A. Implementing intensity-modulated radiotherapy with simultaneous integrated boost for anal cancer: 3-year outcomes at two Sydney institutions. Clin Oncol (R Coll Radiol) 2015;27:700–707. doi: 10.1016/j.clon.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Franco P., Arcadipane F., Ragona R. Locally advanced (T3-T4 or N+) anal cancer treated with simultaneous integrated boost radiotherapy and concurrent chemotherapy. Anticancer Res. 2016;36:2027–2032. [PubMed] [Google Scholar]
- 16.Myerson R.J., Garofalo M.C., El Naqa I. Elective clinical target volumes for conformal therapy in anorectal cancer: A radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kachnic L.A., Winter K., Myerson R.J. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kachnic L.A., Winter K.A., Myerson R.J. Two-year outcomes of RTOG 0529: A phase II evaluation of dose-painted IMRT in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. http://meetinglibrary.asco.org/content/71101-103 Available at: Accessed June 13, 2016. [DOI] [PMC free article] [PubMed]
- 19.Seo Y., Kinsella M.T., Reynolds H.L., Chipman G., Remick S.C., Kinsella T.J. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–149. [Google Scholar]