Abstract
Purpose
The use of large-field external beam reirradiation (re-RT) after pelvic radiation therapy (RT) for genitourinary (GU) cancers has not been reported. We report the results of such treatment in patients with either symptomatic GU second malignant neoplasms or locally recurrent pelvic tumors after initial RT for whom surgery or further systemic therapy was not an option.
Methods and materials
The records of 28 consecutive patients with advanced, bulky GU malignancies treated with high-dose, large-field re-RT with palliative intent between 2008 and 2014 were retrospectively reviewed. Descriptive outcome analyses focused on toxicities and symptom control, and responses were evaluated by 2 independent observers.
Results
Twenty-seven male patients (96%) were included. Median initial external beam RT dose was 64 Gy (range, 30-75.6 Gy). The median time between initial RT and re-RT was 9.5 years (range, 0.2-32 years). At the time of re-RT, there were 16 local recurrences and 12 second malignant neoplasms together comprising 16 bladder, 10 prostate, 1 ureteral, and 1 penile cancer. Indications for re-RT were pain and bleeding/hemorrhage. The median equivalent sphere diameter planning target volume for re-RT was 8.6 cm (range, 4.7-16.3 cm). Given the severity of the symptoms and the bulk of the disease at the time of re-RT, a higher dose of RT was administered. The median re-RT dose was 50 Gy (range, 27.5-66 Gy). For patients who received <60 Gy, hypofractionation of 250 cGy was used. The median cumulative dose was 113.9 Gy (range, 81.5-132.8 Gy). Re-RT was well tolerated with no Radiation Therapy Oncology Group grade 3-4 toxicities. Twenty-four patients (92%) had complete resolution of symptoms, and relief was durable in 67% of patients. The median overall survival was 5.8 months (range, 0.3-38.9 months). Of those patients who are still alive, 100% remain free of initial symptoms.
Conclusion
This small series suggests that aggressive re-RT of inoperable and symptomatic GU malignancies that is undertaken with meticulous treatment planning is well tolerated and provides excellent, durable relief without undue short-term toxicity. Validation in a larger prospective cohort is required.
Summary.
This study assesses the outcomes of 28 patients who presented with either a second malignant genitourinary malignancy or a local recurrence after radical pelvic radiation therapy for a primary malignancy. All patients had advanced, symptomatic, inoperable bulky disease that was refractory to systemic therapy and/or narcotic drugs and thus received high-dose reirradiation (re-RT) for palliation of either pain or bleeding. Re-RT was well tolerated with minor short-term toxicity and 92% control of symptoms, which was durable in 67% of patients.
Introduction
Recurrent genitourinary (GU) malignancies in a previously irradiated volume and the development of a new GU malignancy arising in the setting of prior pelvic radiation therapy (RT) present challenging problems and are generally associated with a poor prognosis. A minority of these patients are eligible for radical surgical resection. Systemic therapy is offered to patients with unresectable disease, but it is not always successful in palliating symptoms. Historically, a longstanding principle of RT has been that once definitive external beam radiation therapy (EBRT) has been administered, further RT cannot be given because it would likely exceed normal-tissue tolerances. Repeat irradiation (re-RT) has been used for GU malignancies, but to date only outcomes after brachytherapy1 or stereotactic body radiation therapy (SBRT)2, 3 have been described. Both brachytherapy and SBRT are typically limited to the treatment of smaller tumors. Unlike brain, lung, and head and neck tumors,4, 5, 6, 7, 8 for which there is considerable experience with re-RT and approved protocols that specify dose, fractionation, and field size with normal-tissue constraints,4, 5, 8 re-RT directed to larger GU targets has not been described.
The aim of this report is to present our experience with palliative high-dose external beam pelvic re-RT for local recurrences (LR) and second malignant neoplasms (SMN) in a cohort of patients with advanced GU malignancies whose tumors were too large to meet the criteria for SBRT or brachytherapy. All patients were ineligible for surgical resection of their tumors and had failed first-line, and frequently second-line, chemotherapy and/or hormone therapy. Aggressive EBRT doses were used for re-RT because prospective randomized data from 500 patients with advanced bladder cancer from Duchesne et al showed very poor control and palliation of symptoms with 21 Gy administered in 7-Gy weekly fractions or 35 Gy in 10 fractions.9
We describe our experience at the Department of Radiation Oncology at Brigham and Women's Hospital and Dana-Farber Cancer Institute with high-dose, external beam pelvic re-RT for GU malignancies, with a focus on toxicities of treatment and response (palliation of symptoms). To our knowledge, this is the first such reported experience in the available literature.
Methods and materials
Patient cohort
With the approval of the Dana-Farber/Harvard Cancer Center institutional review board, we reviewed the records of 28 sequential patients who received high-dose external beam pelvic re-RT between 2008 and 2014. Patients included in the study were aged >18 years, and all had either SMN (n = 12) or LR (n = 16) in the pelvis (Table 1). Sixty-seven percent of patients (n = 19) initially presented with prostate cancer and either had LR (n = 10) or had developed bladder cancer (n = 16) years after treatment for prostate cancer. The median initial EBRT dose was 64 Gy (range, 30-75.6 Gy; 3 doses were unknown).
Table 1.
Patient, tumor, and treatment characteristics
| Characteristics | No. of patients (%) |
|---|---|
| Patients | |
| Male | 27 (96) |
| Median age at diagnosis of first malignancy | 62 years (range, 36-82 years) |
| Primary cancer | |
| Prostate | 19 (67) |
| Bladder | 4 (14) |
| Ureteral | 2 (7) |
| Rectal | 1 (4) |
| Penile | 1 (4) |
| Large cell lymphoma | 1 (4) |
| Treatment for primary cancer | |
| Radiation alone | 13 (47) |
| Radiation plus chemotherapy | 2 (7) |
| Radiation plus surgery | 9 (32) |
| Radiation, chemotherapy, surgery | 4 (14) |
| Type of tumor at re-irradiation | |
| Local recurrence | 16 (57) |
| Second malignant neoplasm | 12 (43) |
| Site of local recurrence or second malignant neoplasm | |
| Bladder | 16 (57) |
| Prostate | 10 (38) |
| Ureteral | 1 (3) |
| Penile | 1 (3) |
At the time of referral for re-RT, all patients had locally advanced, bulky, symptomatic tumors that were no longer responsive to prior chemotherapy, hormonal therapy, and/or surgery. Patients were not eligible for further treatment other than re-RT. All patients presented with either severe pain (n = 14) that was refractory to treatment with narcotics and nerve blocks or hemorrhage/bleeding that caused a decrease in hematocrit levels (n = 14); these were the indications for re-RT. All re-RT was palliative in intent. During the informed-consent process for high-dose re-RT, potential risks of such treatment were discussed, along with the absence of other reasonable alternatives, including surgery and/or chemotherapy alone.
External beam reirradiation treatment planning
Meticulous treatment planning was undertaken for all patients to avoid excess dose to normal tissues and to correctly identify the planning targets. All patients had computed tomography (CT) and/or diagnostic magnetic resonance imaging scans taken before re-RT treatment planning. Planning CT scans were performed 45 to 60 minutes after administration of oral contrast medium. In all cases, the re-RT fields included gross tumor volume (GTV) plus an expansion for the clinical target volume (CTV) and planning target volume (PTV). Significant efforts were made to increase the precision of treatment and exclude critical normal organs with maneuvers such as 4-dimensional simulation (n = 7); customized immobilization devices (n = 28); consensus GTV determination by review of treatment planning images with a radiologist specializing in pelvic imaging (n = 28); image-guided RT delivery, including volumetric modulated arc therapy (n = 20); and 3-dimensional conformal therapy (n = 8) including 4 patients being treated anterior-posterior/posterior-anterior with subfields.
Whenever possible, patients were treated with a full bladder and empty rectum. For patients who received their first RT course at our institution, standard Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) normal-tissue metrics were used for the rectum, bladder, and small bowel.10 For the re-RT, CTV to PTV expansions were generally on the order of 5 to 7 mm, depending on the target motion and proximity to the small bowel. The small bowel was treated as an avoidance structure. Normal tissue metrics for re-RT were considered on a case-by-case basis. For tumors that did not invade or were immediately adjacent to the small bowel, bladder, or rectum, standard QUANTEC metrics were used. For tumors invading the bladder or rectum, full coverage of the tumor target was undertaken without regard for normal-tissue metrics. In the absence of established guidelines for time-dose corrections between courses for pelvic re-RT, prior radiation dose to normal tissues and target structures were carefully recorded whenever possible, as were cumulative doses (Supplement Table 1). Daily kV and cone beam CT imaging was performed for all patients.
Due to the different dose and fractionation schedules between the first and second radiation treatment, all treatment schedules were recalculated to a biologically equivalent schedule of 2 Gy fractions using the formula EQD2 = {D * [1+d/(α/β)]/[1+2/(α/β)]}, where D is total dose, d is dose per fraction, and α/β =10 for tumor and 3 for organs at risk (OAR).
For patients with a better performance status or for those whose prior RT was more recent, we protracted the radiation and used lower doses per fraction. For all other patients, we used hypofractionated regimens, generally 20 fractions over a 4-week period. Estimated cumulative doses to OARs were calculated for the first and second RT courses where possible.
Treatment response measurement
The response of re-RT was scored by assessing the reduction in patient symptoms, most commonly pain and bleeding. For those who initially presented with pain, successful palliation was defined as pain control that met one of the following criteria: (1) resulted in the discontinuation of narcotics or decreased narcotic use after re-RT; (2) achieved on the same, previously ineffective dose of narcotic; or (3) achieved with no use of narcotics either before or after re-RT.
Patient self-reports were graded and patients were followed by both a medical oncologist and a radiation oncologist. All efficacy outcomes and toxicities reported in this study were also evaluated and confirmed by 2 trained data managers and by an independent medical oncologist (LCH), an outside radiation oncologist (JAE), and the treating radiation oncologist.
Toxicity analysis
Toxicities of re-RT were abstracted by a review of medical records (physician and nursing reports) and, for bowel and bladder toxicities, from the patient-reported outcome measures (PROMs) questionnaire or validated Talcott survey completed during each treatment and follow-up visit11 or from the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. Other toxicities, such as skin reaction or vaginitis, were also recorded and were graded according to the Radiation Therapy Oncology Group toxicity scale or CTCAE v4.03. Acute toxicities were defined as those that occurred within 90 days of completion of re-RT. Grade 3 and higher toxicities were differentiated from grade 1 and 2. The duration of toxicity was measured as the interval between the development of toxicity and the first report of cessation of toxicity or the most recent visit if toxicity was still in evidence. Anemia was defined on the basis of hemoglobin levels before the initiation of and after re-RT. Adverse events were classified by time of onset as before treatment (ie, not treatment related), during treatment, or after treatment to differentiate the potential effects of radiation treatment from symptoms related to disease. Descriptive analyses focused on toxicities and symptom control of patients who received re-RT.
Statistical analysis
Stata/MP 13.1 (StataCorp, College Station, TX) was used for all statistical analyses. The Kaplan-Meier method was used to model the time to death.12 Time to death was defined as the number of months between the end of re-RT and the date of death.
Results
As shown in Table 2, the median time between initial RT and re-RT was 9.5 years (range, 0.2-32 years). The median re-RT dose was 50 Gy (range, 27.5-66 Gy), and the median EQD2 was 52.1 Gy (range, 28.6-66 Gy). For patients who received <60 Gy, the median daily re-RT dose was 250 cGy (range, 150 twice daily to 700 cGy). The median cumulative dose to the target was 113.9 Gy in EQD2 (range, 81.5-132.8 Gy; 3 doses were unknown). Estimated cumulative doses to the OAR from both initial RT and re-RT are provided in Supplement Table 1.
Table 2.
Treatment sites and radiation details by patient
| Patient | First malignancy site | First RT total dose (Gy) | SMN or LR site | Re-RT total dose/fractionation (Gy) | Re-RT target | ESD of re-RT target volume (cm) | Interval between treatments (y) |
|---|---|---|---|---|---|---|---|
| 1 | Prostate | N/A | Prostate | 55/2.5 | Prostate, bilateral seminal vesicle | 7.6 | 11.25 |
| 2 | Lymphoma | N/A | Bladder | 30/1.5 twice daily | Pelvic and retroperitoneal lymph nodes | 8.6 | 32.0 |
| 3 | Prostate | 68-72 | Bladder | 64.8/1.8 | Bladder, prostate | 9.4 | 22.0 |
| 4 | Prostate | 63 | Prostate | 55/2.5 | Prostate, bladder, rectum | N/A | 21.0 |
| 5 | Bladder | 50.4 | Bladder | 59.6/1.8 | Periureteral tissues, urethra | 6.0 | 1.25 |
| 6 | Prostate | 63 | Bladder | 46/2.0 | Bladder | 16.3 | 19.0 |
| 7 | Prostate | 63 | Bladder | 28/7.0 | Bladder | 7.4 | 17.25 |
| 8 | Bladder | 66 | Bladder | 54.05/2.35 | Bladder | 6.4 | 1.92 |
| 9 | Bladder | 54 | Bladder | 50.4/1.8 | Pelvis | 13.1 | 0.6 |
| 10 | Prostate | 63 | Prostate | 30/2.5 | Pubic symphysis, bladder | N/A | 10.0 |
| 11 | Ureteral | 64 | Ureteral | 40.5/2.7 | Lower pelvis/groin mass | 4.7 | 0.2 |
| 12 | Prostate | 75.6 | Prostate | 50/2.5 | Penis to level of prostate | 8.5 | 4.3 |
| 13 | Prostate | 144 | Prostate | 55/2.5 | Prostate, bladder | 7.1 | 8.0 |
| 14 | Prostate | 75 | Bladder | 47.5/2.5 | Pelvic mass | 8.1 | 5.6 |
| 15 | Prostate | 75.6 | Prostate | 50/2.0 | Prostate | 7.9 | 4.3 |
| 16 | Prostate | N/A | Prostate | 37.5/2.5 | Pelvic mass | 8.8 | 10.0 |
| 17 | Prostate | 70.2 | Bladder | 50/2.5 | Bladder, right distal ureter, prostate | N/A | 11.0 |
| 18 | Prostate | 45+ brachytherapy boost to 155 | Bladder | 40.5/2.7 | Sacrum, bladder, prostate | 9.1 | 11.0 |
| 19 | Rectal | 55 | Bladder | 46/2.0 | Large bladder mass invading rectum | 13.1 | 22.0 |
| 20 | Ureteral | 65 | Bladder | 52.9/unknown | Bladder, right side wall pelvic mass | 8.9 | 2.2 |
| 21 | Prostate | 45+ brachytherapy boost to 155 | Bladder | 66/2.0 | Whole bladder | 8.7 | 6.0 |
| 22 | Prostate | 72 | Prostate | 50/2.5 | Pelvis | 10.3 | 6.0 |
| 23 | Prostate | 63 | Bladder | 50/2.5 | Bladder | 8.3 | 10.0 |
| 24 | Bladder | 54 | Bladder | 27.5/2.5 | Left pelvis, upper thigh | 12.5 | 0.75 |
| 25 | Prostate | 68 | Prostate | 35/2.5 | Sacrum, bladder | 6.2 | 9.0 |
| 26 | Penile | 30 | Penile | 55/2.5 | Penis, bilateral groin, left pelvic nodes | 12.6 | 1.0 |
| 27 | Prostate | 66.6 | Prostate | 64/2.0 | Bladder | 5.9 | 14.0 |
| 28 | Prostate | 66 | Bladder | 55/2.5 | Pelvic mass | 12.7 | 11.0 |
ESD, equivalent square diameter; LR, local recurrence; N/A, not available; re-RT, reirradiation; RT, radiation therapy; SMN, second malignant neoplasm.
Seven patients received concurrent chemotherapy with re-RT. The chemotherapy regimens were carboplatin/paclitaxel (n = 2), cabazitaxel (n = 2), cisplatin (n = 1), mitomycin C/5-fluorouracil (n = 1), and gemcitabine/carboplatin (n = 1).
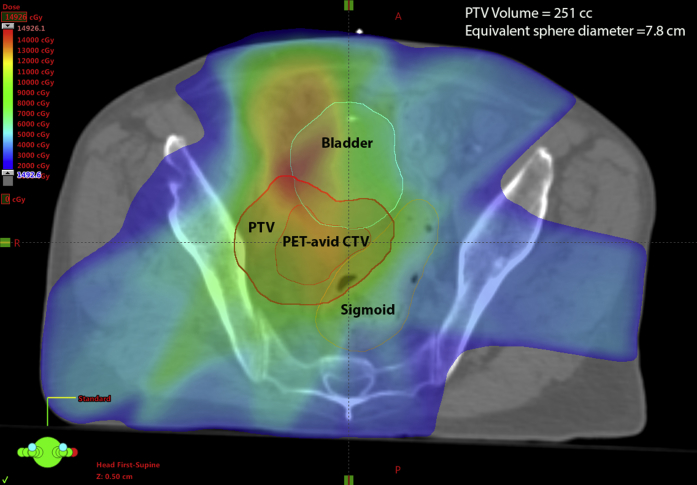
As shown in Table 2, the median equivalent sphere diameter PTV size was 8.6 cm (range, 4.7-16.3 cm) for the re-RT course. PTVs were based on GTV as seen on CT imaging at the time of simulation, with expansion for microscopic extension (CTV) with final expansion to the PTV. An example of a treated PTV is shown in Figure 1.
Figure 1.
Example of planning target volume for a patient who was treated with reirradiation.
Pelvic reirradiation response
A total of 92% of the evaluable cohort (24/26) showed a response to re-RT that ranged from improvement to complete resolution of symptoms. Only 2 patients had no improvement of initial symptoms. Two other patients were unevaluable for response to treatment and treatment-related toxicities, 1 because of unexpected, rapid, and extensive disease dissemination that required early cessation of re-RT and the other because of having received botulinum toxin injections for pain (Table 3). Among those who initially presented with pain (n = 14), pain control was achieved as follows: a decrease (n = 3) or discontinuation (n = 1) of narcotic use; use of the same, previously ineffective narcotic dose (n = 4); or no narcotic use either before or after re-RT (n = 4). The two patients who did not achieve palliation included one who received re-RT for pelvic pain, blood in his stool, and hematuria, as well as symptoms of rectal obstruction such as tenesmus, small-caliber stool passage, and mucus, which were all secondary to a prostate metastatic lesion invading the bladder and rectum. After re-RT, blood in the stool and hematuria improved, but rectal obstructive symptoms continued. The patient refused a surgical referral, and his symptoms continued to worsen. The other patient who did not achieve palliation fell 1 day after completing RT, which resulted in hospitalization, and he died within 2 weeks of admission.
Table 3.
Radiation toxicity, grading, duration, and outcome of initial symptoms
| Toxicitya | n = 28 |
|---|---|
| Urinary frequency | Total = 4 |
| Grade 2 (Talcottb) | 2 |
| Grade 3 (Talcottb) | 2 |
| Urinary irritation | Total = 1 |
| Grade 5 (Talcottc) | 1 |
| Urinary tract infection | Total = 4 |
| Grade 2 (Talcottd) | 1 |
| Grade 2 (CTCAE) | 1 |
| Grade 3 (CTCAE) | 1 |
| Unevaluable | 1 |
| Anemia | Total = 6 |
| Grade 1 (CTCAE) | 1 |
| Grade 2 (CTCAE) | 5 |
| Fatigue | Total = 15 |
| Grade 1 (CTCAE) | 10 |
| Grade 2 (CTCAE) | 5 |
| Skin | Total = 2 |
| Grade 3 (RTOG) | 2 |
| Diarrhea | Total = 1 |
| Grade 3 (Talcotte) | 1 |
| None | 4 |
| Unevaluable | 2 |
| Duration of toxicitya | n = 28 |
| ≤2 weeks | 7 |
| 3 weeks | 1 |
| >3 weeks to ≤1 month | 7 |
| 2-3 months | 4 |
| 6 months | 2 |
| Permanent | 2 |
| Unevaluable | 10 |
| Outcome of initial symptoms | n = 28 |
| Complete resolution | 24 |
| No symptom resolution | 2 |
| Unevaluable | 2 |
CTCAE, Common Toxicity Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group.
Patients can have more than one toxicity.
Talcott urinary frequencies: grade 5 = 9-12 times/day, grade 4 = 5-8 times/day. These do not meet RTOG criteria for grade 3 or 4 bladder toxicity.
Talcott urinary irritation: grade 3 = burning or pain during urination several times/day. These do not meet RTOG criteria for grade 3 or 4 bladder toxicity.
Talcott urinary tract infection: grade 2 = Burning with urination at least twice per day.
Talcott diarrhea: grade 3 = loose stool several times/day. This does not meet RTOG criteria for grade 3 or 4 lower gastrointestinal toxicity.
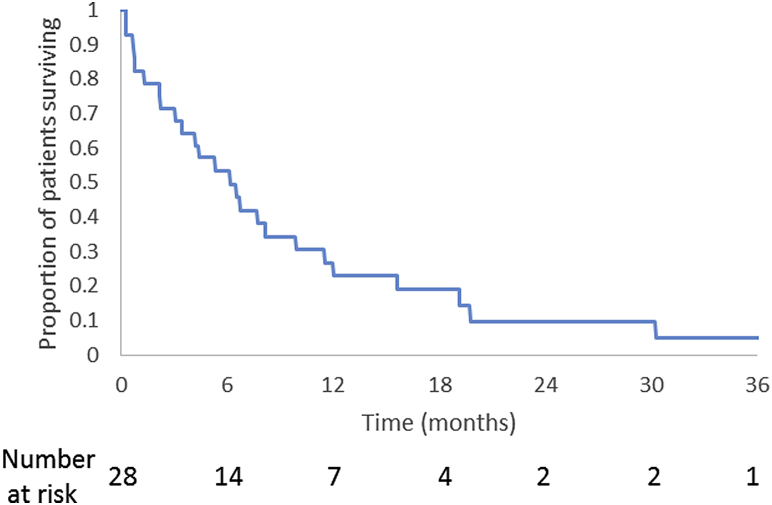
At the time of analysis, 89% of the cohort had been followed until death. The median follow-up time was 5.2 months, and the median survival between re-RT and death was 5.2 months (range, 0.2-30.2 months). The median follow-up time was 16.7 months for surviving patients. The actuarial 6-month and 1-year overall survival rates after re-RT were 53.3% and 26.7%, respectively (Fig 2). Median overall survival time was 5.8 months (range, 0.3-38.9 months) regardless of the initial primary site or histology. Death was secondary to systemic disease in all cases except the patient who died from complications of a fall. Of the 24 patients who responded to re-RT, 67% remained free from the initial symptoms prompting re-RT until the time of death, and 100% of the 3 patients who are still alive remain free from initial symptoms.
Figure 2.
Kaplan-Meier estimate for overall survival (in months, after date of reirradiation).
Pelvic reirradiation toxicity
Patient-reported outcome measures were reported for bowel and bladder morbidity and for fatigue. Re-RT was well tolerated, and most patients experienced minimal treatment-related toxicity (Table 3). The common toxicities that were recorded included fatigue (n = 15), increased urinary frequency or irritation (n = 5), anemia (n = 6), urinary tract infection that required treatment with antibiotics (n = 4), moist skin desquamation of the perineum (n = 2), diarrhea (n = 1), and vaginitis (n = 1). The patient with vaginitis required a 1-week treatment break during which the vaginitis resolved, and the treatment regimen was continued and completed. One patient had penile edema during re-RT, which responded to a course of dexamethasone.
For toxicities that could be graded, see Table 3. Most toxicities waxed and waned throughout treatment; therefore, the duration for many patients was based on the presence of toxicity at the end of re-RT and the amount of time it took to resolve after RT ended. Three of the 6 patients with anemia and the one patient with diarrhea during re-RT had received concurrent chemotherapy. Fatigue, which was the most common toxicity recorded, was confounded by factors including anemia, prior or adjuvant chemotherapy use, advanced tumor burden, and declining performance status. We saw no difference in toxicity analysis between patients who received concurrent chemotherapy (n = 7) and those who did not (n = 21).
Discussion
In this study of 28 patients with locally advanced symptomatic pelvic tumors that were no longer amenable to chemotherapy, hormonal therapy, and/or surgery, high-dose re-RT of the pelvis, delivered with meticulous treatment planning, was associated with excellent resolution of symptoms (92% of cases) with minor expected toxicities and no serious or unexpected complications. Given the absence of guidelines for re-RT after treatment of pelvic malignancies, particularly for large bulky tumors that require higher-dose EBRT, our patients were referred for re-RT late in the course of their illness. However, response was durable; most patients remained free of symptoms until death. One hundred percent of patients who are still alive remain free of the initial symptoms that warranted re-RT. These results suggest that external beam re-RT directed to larger tumors can provide safe and effective palliation in patients who are not candidates for further chemotherapy or surgery and for whom survival is expected to be short.
Although re-RT is not common, it is not a new concept. Studies that date back as early as 1962 have demonstrated that re-RT is safe as long as the treatment volume is limited and planning is done carefully.13 Re-RT is currently used in multiple disease sites, including the head and neck,4, 5 breast,7 brain,6 and lung.8 The management of recurrent or new GU malignancies after definitive pelvic RT is shifting, and there is no generally accepted optimal management strategy. Historically, external beam re-RT other than SBRT for this patient population has been avoided because of concerns about potential toxicity, tumor radio-resistance, and lack of evidence regarding efficacy.2, 3, 14, 15, 16, 17 Stereotactic radiosurgery has been reported for a few select patients with small-volume recurrent prostate cancer with apparent good outcomes. Jereczeck-Fossa et al reported on 34 patients with 38 lesions that were either isolated LR or isolated nodal recurrences after primary or salvage RT.2 Radiosurgery after meticulous treatment planning resulted in no level 4 or 5 morbidity. Local control was best for nodal recurrences; however, the small number of patients who were treated precluded statistical analysis. A separate analysis of robotic image guided re-RT of lateral pelvic recurrences in 16 patients revealed actuarial local control of 51% with excellent tolerance of treatment and no grade 3 or 4 toxicities. The median size of the tumor targets for that study was small (34.5 mm; range, 14-50 cm). That study included one patient with bladder cancer who had received prior definitive EBRT.18 We know of no other studies in the available literature.
The primary difference between these studies and our own is that our patients had very advanced disease with bulky local tumor masses at the time of referral. Despite this and the relatively high palliative RT doses that were used, our morbidity statistics compare well with those in reports on patients with smaller targets who were given lower RT doses. We were unable to address the added toxicity or efficacy of RT sensitizers such as chemotherapy because our study was too small to detect a difference between patients who received concurrent chemotherapy (n = 7) and those who did not (n = 21). Given the paucity of data on re-RT from GU malignancies, the utility of adding other agents, such as hyperthermia, to enhance the radiation effect remains unknown.
One important consideration for our patients was the balance between the number of fractions delivered, the dose per fraction, the time on treatment, our expectation for treatment efficacy, and our estimation of patient survival. Data from the study by Duchesne et al showed that both 35 Gy in 10 fractions and 7 Gy administered once weekly for 3 weeks were equally poor in controlling pelvic symptoms in patients with bladder cancer and thus were cause for concern. In that trial, half of the patients had T3 disease and only 21% had T4 disease. Symptoms were assessed at 3 months, at which time only 50% of patients had resolution of hematuria, dysuria improved in only 20% to 22%, rectal bleeding in 1% to 3%, and rectal pain in 8% to 10% 9 For this reason, we elected to use a regimen with a biological equivalent dose of no less than 50 Gy.
Study limitations
Important limitations of this study must be recognized. First, although patient-reported outcomes were reported, this was a small retrospective review without formal prospective assessment of side effects. Also, although we elected to offer higher doses than those used by Duchesne et al to increase the odds of effective palliation, no comparisons can be made because this was not a prospective study and our numbers are small.
Any retrospective study inherently introduces biases and the potential for under- or over-reporting outcomes and toxicities. Because most toxicities waxed and waned throughout the re-RT treatment, cumulative incidence plots could not be generated. There was unknown bias of patient selection for re-RT. Patients were referred to RT late in the course of their LR or SMN; therefore, the follow-up was short even though 89% of patients were followed until death. However, given the short expected survival time of these patients, late toxicity is less of a concern and improving quality of life may be the most reasonable expectation of re-RT. Finally, this short survival period made it impossible to assess a correlation between duration of symptom response and survival time.
Until guidelines can be developed, radiation oncologists must evaluate the risk-to-benefit ratio of re-RT for individual patients, including the likelihood that they will live long enough to experience late effects from the treatment. Straightforward discussion with patients must be undertaken with regard to their treatment options and the potential for toxicity.
Conclusion
In this report, 28 sequential patients with a history of definitive EBRT and bulky, symptomatic LR or new genitourinary SMN that was inoperable, refractory to systemic therapy, and causing either pain that was refractory to treatment with narcotics or bleeding/hemorrhage, were re-treated with aggressive EBRT, not SBRT, to 60 Gy or a hypofractionated dose that approached 60 Gy. Re-RT offered excellent and durable palliation of symptoms in 92% of patients and grade 1 and 2 bladder and bowel toxicities, which were all transient. Meticulous treatment planning with avoidance of sensitive normal tissues at the expense of PTVs may help explain the low morbidity, as does the short survival times for these patients who were treated palliatively. Although these data should be validated in a larger cohort of patients, they offer a possible new palliative pathway for patients who would ordinarily not have received additional RT. A future prospective study with formal quality of life assessment, treatment planning guidelines, and hypofractionated dosing is needed to validate these results.
Footnotes
Conflicts of interest: None.
Supplementary material for this article (http://dx.doi.org/10.1016/j.adro.2017.01.001) can be found at www.practicalradonc.org.
Supplementary data
References
- 1.Crehange G., Roach M., 3rd, Martin E. Salvage reirradiation for locoregional failure after radiation therapy for prostate cancer: Who, when, where and how? Cancer Radiother. 2014;18:524–534. doi: 10.1016/j.canrad.2014.07.153. [DOI] [PubMed] [Google Scholar]
- 2.Jereczek-Fossa B.A., Beltramo G., Fariselli L. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:889–897. doi: 10.1016/j.ijrobp.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Abusaris H., Hoogeman M., Nuyttens J.J. Re-irradiation: Outcome, cumulative dose and toxicity in patients retreated with stereotactic radiotherapy in the abdominal or pelvic region. Technol Cancer Res Treat. 2012;11:591–597. doi: 10.7785/tcrt.2012.500261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer U., Micke O., Schueller P., Willich N. Recurrent head and neck cancer: Retreatment of previously irradiated areas with combined chemotherapy and radiation therapy-results of a prospective study. Radiology. 2000;216:371–376. doi: 10.1148/radiology.216.2.r00au04371. [DOI] [PubMed] [Google Scholar]
- 5.Pryzant R.M., Wendt C.D., Delclos L., Peters L.J. Re-treatment of nasopharyngeal carcinoma in 53 patients. Int J Radiat Oncol Biol Phys. 1992;22:941–947. doi: 10.1016/0360-3016(92)90792-g. [DOI] [PubMed] [Google Scholar]
- 6.Veninga T., Langendijk H.A., Slotman B.J. Reirradiation of primary brain tumours: Survival, clinical response and prognostic factors. Radiother Oncol. 2001;59:127–137. doi: 10.1016/s0167-8140(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 7.van der Zee J., van der Holt B., Rietveld P.J. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer. 1999;79:483–490. doi: 10.1038/sj.bjc.6690075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gressen E.L., Werner-Wasik M., Cohn J., Topham A., Curran W.J., Jr. Thoracic reirradiation for symptomatic relief after prior radiotherapeutic management for lung cancer. Am J Clin Oncol. 2000;23:160–163. doi: 10.1097/00000421-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Duchesne G.M., Bolger J.J., Griffiths G.O. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: Results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2010;47:379–388. doi: 10.1016/s0360-3016(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 10.Marks L.B., Yorke E.D., Jackson A. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark J.A., Talcott J.A. Symptom indexes to assess outcomes of treatment for early prostate cancer. Med Care. 2001;39:1118–1130. doi: 10.1097/00005650-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Kramer S. Reirradiation: Indications–technique–results. Prog Radiat Ther. 1962;2:195–214. [PubMed] [Google Scholar]
- 14.Bosman S.J., Holman F.A., Nieuwenhuijzen G.A., Martijn H., Creemers G.J., Rutten H.J. Feasibility of reirradiation in the treatment of locally recurrent rectal cancer. Br J Surg. 2014;101:1280–1289. doi: 10.1002/bjs.9569. [DOI] [PubMed] [Google Scholar]
- 15.Valentini V., Morganti A.G., Gambacorta M.A. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–1139. doi: 10.1016/j.ijrobp.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Guren M.G., Undseth C., Rekstad B.L. Reirradiation of locally recurrent rectal cancer: A systematic review. Radiother Oncol. 2014;113:151–157. doi: 10.1016/j.radonc.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Mohiuddin M., Marks G., Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144–1150. doi: 10.1002/cncr.10799. [DOI] [PubMed] [Google Scholar]
- 18.Dewas S., Bibault J.E., Mirabel X. Robotic image-guided reirradiation of lateral pelvic recurrences: Preliminary results. Radiat Oncol. 2011;6:77. doi: 10.1186/1748-717X-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.