Abstract
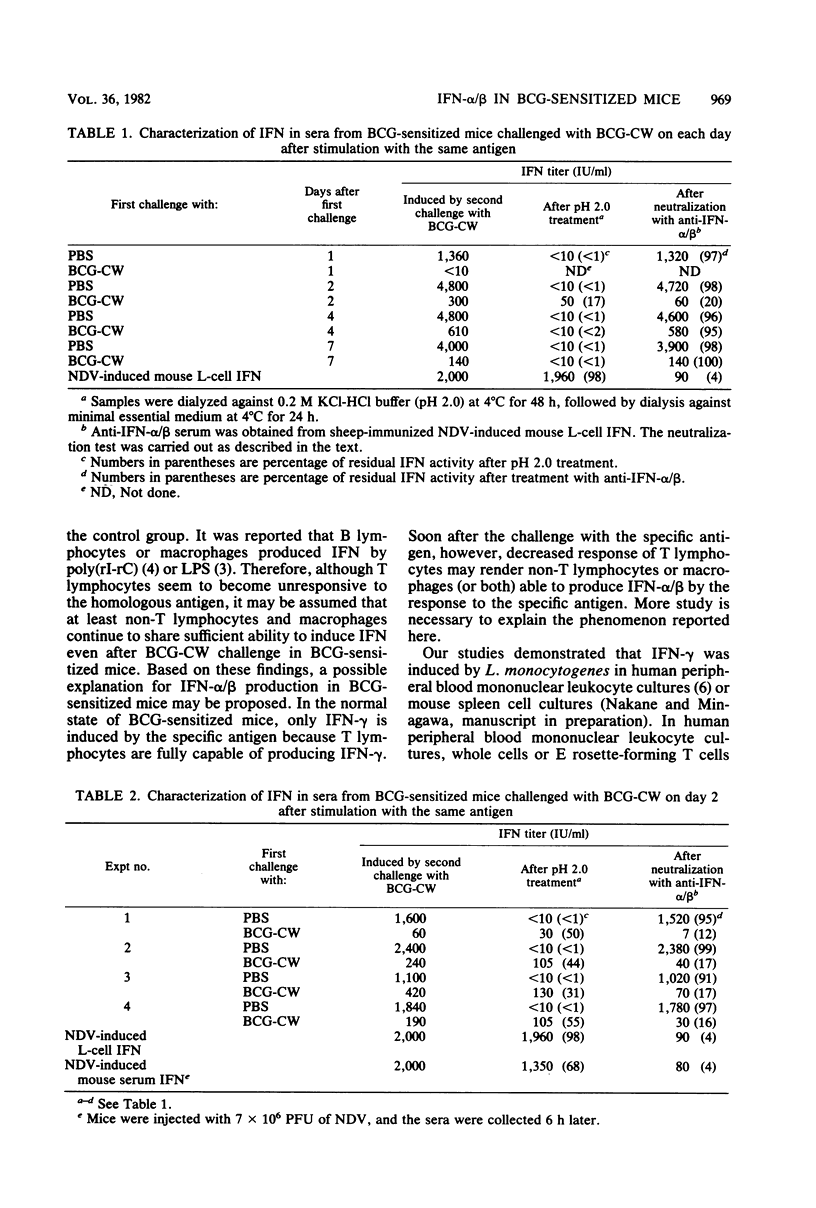
Characterization of interferon (IFN) induced by a second challenge with specific antigen was investigated during the development of hyporeactivity established after challenge of Mycobacterium bovis BCG-sensitized mice with BCG cell wall fraction (BCG-CW). When BCG-sensitized mice were challenged with BCG-CW, IFN-gamma was detected in the circulation 4 h later and rapidly disappeared. After IFN-gamma disappeared from their blood, mice became completely hyporeactive to the second challenge with BCG-CW for 1 day, and thereafter they recovered from this hyporeactive state. However, we always observed that IFN induced at first by the second challenge with BCG-CW during the hyporeactive state was type I IFN-alpha/beta, but thereafter was entirely IFN-gamma. Induction of IFN-alpha/beta by the specific antigen during restoration from the hyporeactive state in BCG-sensitized mice is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- Maehara N., Ho M., Armstrong J. A. Differences in mouse interferons according to cell source and mode of induction. Infect Immun. 1977 Sep;17(3):572–579. doi: 10.1128/iai.17.3.572-579.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Ho M. Hyporeactivity factor produced after induction of immune interferon in mice sensitized with BCG. Infect Immun. 1978 Nov;22(2):371–377. doi: 10.1128/iai.22.2.371-377.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Alternative induction of IFN-alpha and IFN-gamma by Listeria monocytogenes in human peripheral blood mononuclear leukocyte cultures. J Immunol. 1981 Jun;126(6):2139–2142. [PubMed] [Google Scholar]
- Sonnenfeld G., Mandel A. D., Merigan T. C. In vitro production and cellular origin of murine type II interferon. Immunology. 1979 Apr;36(4):883–890. [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Hyporeactivity due to infection: recognition of a transferable hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1974 Dec;10(6):1337–1342. doi: 10.1128/iai.10.6.1337-1342.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A. Hyporeactivity to interferon induction: characterization of a hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1975 Feb;11(2):294–302. doi: 10.1128/iai.11.2.294-302.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Kern E. R., Kelsey D. K., Glasgow L. A. Suppressed response to interferon inducation in mice infected with encephalomyocarditis virus, Semliki forest virus, influenza A2 virus, Herpesvirus hominis type 2, or murine cytomegalovirus. J Infect Dis. 1977 Apr;135(4):540–551. doi: 10.1093/infdis/135.4.540. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Type I and II interferons and migration inhibitory factor: production in Mycobacterium bovis BCG-infected mice desensitized with old tuberculin or lipopolysaccharide. Infect Immun. 1978 Mar;19(3):912–914. doi: 10.1128/iai.19.3.912-914.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


