Abstract
Purpose
Locally recurrent pancreatic cancer after prior radiotherapy is a therapeutic challenge with limited treatment options. This study examines the safety and efficacy of stereotactic body radiation therapy (SBRT) for locally recurrent pancreatic adenocarcinoma after prior conventional fractionation radiotherapy (CRT).
Methods and materials
Outcomes from all patients treated with SBRT for locally recurrent pancreatic adenocarcinoma after prior CRT at our institution were reviewed. A total of 23 patients were identified. Prior CRT median dose was 50.4 Gy (range, 30-60 Gy). Twelve patients (52%) had previously undergone surgery and received CRT as neo- or adjuvant treatment. Nine patients (39.1%) were reirradiated with SBRT with a dose of 25 Gy in a single fraction, and 14 patients (60.8%) received a 5-fraction SBRT schedule with a median dose of 25 Gy (range, 20-33 Gy) in 5 fractions (1-5 fractions).
Results
Median follow-up time was 28 months (range, 9-77 months). The median planning target volume was 46 cm3 (range, 14-89 cm3). Median overall survival from diagnosis and from reirradiation were 27.5 months (range, 10-77 months) and 8.5 months (range, 1 month to not reached) respectively. The cumulative incidence of local failures at the last follow-up was 19%. For the 4 patients who presented with local failure, one was treated with a single fraction of 25 Gy, and the other 3 were treated with 25 Gy in 5 fractions. Three patients presented regional failure, with a cumulative incidence of 14%, all with concurrent distant progression. The cumulative incidence of distant progression was 64% at last follow-up. After reirradiation, 6 patients (26.1%) developed a grade 2 or 3 gastrointestinal toxicity, 4 of them occurring among patients treated with a single-fraction SBRT regimen.
Conclusions
Our report shows that SBRT for reirradiation of locally recurrent pancreas adenocarcinoma is a feasible option with good local control and acceptable toxicity rates, especially with a multifraction schedule.
Summary.
This is an institutional retrospective analysis of the use of stereotactic body radiation therapy (SBRT) for the treatment of locally recurrent pancreatic adenocarcinoma after prior conventionally fractionated chemoradiation. We demonstrate that SBRT in this setting is safe and provides good local control and symptom palliation. We recommend its use with a multifraction regimen.
Introduction
Pancreatic cancer is a devastating disease for which survival rates have not significantly improved in the last 20 years. Although it is only the 10th most common cancer diagnosis, it is the fourth leading cause of cancer-related death.1 Surgery gives the best chance of cure for these patients, but unfortunately, less than 20% of cases are deemed resectable at the time of diagnosis.2 Patients with locally advanced pancreatic cancer are usually treated initially with induction chemotherapy. The results of adding external beam radiation have been mixed.3, 4, 5, 6 Despite aggressive combined modality approaches, the clinical outcomes of pancreatic cancer remain dismal.
Over the last decade, multiple studies have shown the benefits of stereotactic body radiation therapy (SBRT), also referred to as stereotactic ablative radiation therapy, which commonly reaches local control rates superior to 80% in 2 years.7, 8, 9, 10, 11, 12, 13, 14, 15 Koong et al were the first to report the results of a prospective phase 1 dose escalating study of SBRT in locally advanced pancreatic cancer. Until death or at last follow-up, no local failures were observed at a dose of 25 Gy in a single fraction, despite a median survival of only 8 months.7 More recently, fractionated SBRT regimens combined with chemotherapy have demonstrated similarly successful local control but with lower toxicity.6, 16 Even so, survival has not improved, mainly because of the early onset of systemic disease progression.
Although distant progression of the disease remains the major obstacle in pancreatic cancer, local-regional control of the primary tumor is also an important factor to consider. Nearly a third of patients treated with chemotherapy or chemoradiation will experience local-regional progression.17 Uncontrolled local-regional disease often leads to pain and obstruction, and treatment options are limited in this setting. SBRT has become the treatment of choice at our institution because of its ability to deliver high doses of radiation while maximally sparing adjacent normal tissue within a shortened 1-week course.
In the present study, we aim to report the safety and efficacy of SBRT for the treatment of locally recurrent pancreatic adenocarcinoma after prior chemoradiation therapy.
Methods and materials
Data collection and study population
After receiving institutional review board approval, we retrospectively analyzed the records from all patients with pancreatic adenocarcinoma treated with SBRT after local-regional recurrence after prior conventionally fractionated chemoradiation therapy (CRT) from June 2002 to December 2015. Data regarding demographics, tumor characteristics, treatment, toxicity, and disease progression were collected for analysis. Patients were excluded if they had evidence of metastatic disease at the time of reirradiation or if follow-up data were unavailable or inadequately documented.
SBRT treatments
If not present from the prior treatment, 3 to 5 gold fiducial seeds were implanted for target localization and accuracy of setup. Fiducials are typically implanted endoscopically through ultrasound guidance. For treatment simulation, patients were immobilized in the supine position with the arms above the head, using a custom-formed binary foam mold (Alpha Cradle, Smither Products Inc., North Canton, OH).
All SBRT radiation treatments were performed with respiratory motion management to minimize normal tissue irradiation. A 4-dimensional computed tomography (CT) scan along with a dual-phase contrast-enhanced CT scan with pancreatic protocol was used for treatment simulation. A positron emission tomography (PET)-CT scan was also obtained for all patients. Treatment plans were created using Eclipse (Varian Medical Systems, Palo Alto, CA) or Multi-Plan (Accuray, Sunnyvale, CA). The gross tumor volume was contoured on the arterial CT scans with the aid of the PET images and then adjusted on each of the selected 4-dimensional CT phases to account for tumor motion and create the internal target volume. An additional 2- to 3-mm expansion was created to generate the planning target volume.
Our institutional protocols for the delivery of pancreas SBRT changed during the timeframe of this retrospective analysis. Initially, patients were treated with a single fraction by CyberKnife (Accuray), using respiratory tracking. Currently, we deliver SBRT with a 5-fraction regimen. The dose constraints for stomach, bowel and duodenum are maximum point dose <30 Gy, V25 ≤1 cm3, V20 ≤3 cm3, and V15 ≤5 cm3, respectively.
Toxicity and disease progression
Patients were followed at 3- to 6-month intervals with clinic visits for physical examination and toxicity assessment. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Event, Version 4.18 The highest grade toxicity experienced by each patient during or after the treatment course was recorded. Patients were censored for toxicity upon evidence of local progression if the toxicity was thought to be caused by the tumor.
Disease progression was assessed at each visit by CT imaging using the Response Evaluation Criteria in Solid Tumors (RECIST), Version 1.119 and/or by fluorodeoxyglucose-PET using the PET Response Evaluation Criteria in Solid Tumors (PERCIST), Version 1.0.20
Statistical analysis
Patient characteristics, including demographic, clinical, and treatment-related data, were summarized using means, medians, and ranges, as appropriate. The toxicities were coded and analyzed as categorical variables. The Kaplan-Meier method was used to estimate overall survival (OS) and progression-free survival. Cumulative incidences of local, regional, and distant progression were estimated, with death treated as a competing risk. Binary outcomes were analyzed in logistical regression models. Proportions were tested with χ2 tests or Fisher's exact tests. All tests that were performed were two-sided with an alpha level of 0.05, and all analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
In total, 26 patients with pancreatic cancer who were treated with SBRT for reirradiation after local disease recurrence or progression following prior CRT were identified, but 3 were excluded for showing evidence of systemic disease at the time of reirradiation. Among the remaining 23 patients, 12 had local recurrence after a pancreatectomy with prior neoadjuvant or adjuvant CRT. The median prior radiation therapy dose was 50.4 Gy (range, 30-60 Gy) delivered in 28 fractions, most commonly with concurrent continuous infusion 5-fluorouracil or capecitabine. Detailed patient and prior treatment characteristics are listed in Table 1. Upon local recurrence or progression, 6 patients (26.1%) received additional chemotherapy. Five patients received gemcitabine before SBRT reirradiation and one after reirradiation, with one of the patients receiving cisplatin combined with gemcitabine.
Table 1.
Patient demographic and prior treatment characteristics
| Characteristic | No. (%) or Median [range] |
|---|---|
| No. of patients | 23 |
| Age, years | 59 [40-85] |
| Male sex | 13 (56.5) |
| Karnofsky performance status ≥80% | 22 (95.6) |
| Initial tumor location | |
| Head of pancreas | 13 (56.5) |
| Body of pancreas | 7 (30.5) |
| Uncinate process | 2 (8.7) |
| Tail of pancreas | 1 (4.3) |
| Tumor histology | |
| Ductal adenocarcinoma | 21 (91.4) |
| Mucinous non-cystic carcinoma | 1 (4.3) |
| Adeno-squamous carcinoma | 1 (4.3) |
| Initial TNM staging | |
| Tumor (T) | |
| T1 | 2 (8.7) |
| T2 | 4 (17.3) |
| T3 | 7 (30.5) |
| T4 | 10 (43.5) |
| Node (N) | |
| N0 | 15 (65.2) |
| N1 | 8 (34.8) |
| Metastases (M) | |
| M0 | 23 (100) |
| M1 | 0 (0) |
| Initial resectable disease, yes | 12 (52.2) |
| Surgery characteristics | |
| Whipple procedure | 8 (66.7) |
| Distal pancreatectomy | 4 (33.3) |
| Vessel reconstruction, yes | 2 (16.7) |
| Negative margins, yes | 8 (66.7) |
| Perineural invasion, yes | 2 (16.7) |
| Lymphovascular invasion, yes | 2 (16.7) |
| Prior radiation therapy regimen (conventional fractionation) | |
| Neoadjuvant to surgery | 2 (8.7) |
| Adjuvant to surgery | 10 (43.5) |
| Definitive | 11 (47.8) |
| Total dose, Gy | 50.4 [30-60] |
| No. of fractions | 28 [10-30] |
| Prior chemotherapy regimen∗ | |
| Gemcitabine based | 22 (95.6) |
| Cisplatin based | 6 (26.1) |
| Immunotherapy | 3 (13) |
| Chemotherapy concurrent with prior radiation therapy | |
| Capecitabine | 8 (34.8) |
| Infusional 5-Fluorouracil | 8 (34.8) |
| Gemcitabine | 3 (13) |
| Other | 4 (17.4) |
| CA19-9 at recurrence, U/mL | 201 [1.0-3098.9] |
Regimens are not mutually exclusive.
Reirradiation characteristics
Patients were reirradiated with SBRT after a median of 13 months (range, 2-32 months) after the end of the prior CRT regimen. Nine patients (39%) treated with SBRT for local tumor recurrence received a single fraction of 25 Gy delivered with the CyberKnife treatment machine. The remaining 14 patients (61%) received multifraction SBRT. The most commonly used multifraction SBRT reirradiation schedule was 25 Gy delivered in 5 consecutive daily fractions. The target volumes for reirradiation did not include elective nodes (Table 2).
Table 2.
SBRT reirradiation treatment characteristics and gastrointestinal toxicity occurrence
| Patient No. | Time from prior CRT (mo) | Prior resection | Prior RT |
(Reirradiation) SBRT |
GI toxicity∗ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (Gy) | No. of fractions | Recurrence Treated Location | Treatment machine | Dose (Gy) | No. of fractions | PTV (cm3) | Acute (Grade) | Late (Grade) | Type of toxicity | |||
| 1 | 21 | No | 51 | 28 | Pancreatic tail | CyberKnife | 25 | 1 | 21.5 | - | - | - |
| 2 | 3 | No | 50.4 | 28 | Pancreatic head | CyberKnife | 25 | 1 | 20.0 | - | - | - |
| 3 | 4 | No | 60 | 30 | Pancreatic head | CyberKnife | 25 | 1 | 34.0 | 2 | - | Gastric ulcer |
| 4 | 13 | Yes | 50.4 | 28 | Pancreatic head | CyberKnife | 25 | 1 | 28.3 | - | - | - |
| 5 | 3 | No | 54 | 28 | Pancreatic head | CyberKnife | 25 | 1 | 47.0 | 3 | - | Gastric fistula |
| 6 | 3 | No | 50.4 | 28 | Pancreatic head | CyberKnife | 25 | 1 | 18.8 | - | 2 | Gastric ulcer |
| 7 | 8 | No | 45 | 25 | Pancreatic head | CyberKnife | 25 | 1 | 36.0 | - | - | - |
| 8 | 10 | No | 50.4 | 28 | Pancreatic head | CyberKnife | 12.5 | 1 | 55.3 | - | - | - |
| 9 | 14 | No | 45 | 25 | Pancreatic head | CyberKnife | 25 | 1 | 37.4 | 2 | - | Nausea |
| 10 | 2 | Yes | 50.4 | 28 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 33.5 | - | - | - |
| 11 | 25 | Yes | 43.2 | 24 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 15.0 | - | - | - |
| 12 | 15 | Yes | 45 | 25 | Tumor bed/soft tissue | CyberKnife | 25 | 5 | 55.4 | - | - | - |
| 13 | 32 | No | 45 | 20 | Pancreatic head | CyberKnife | 25 | 5 | 89.8 | - | - | - |
| 14 | 13 | Yes | 50.4 | 28 | Tumor bed/soft tissue | CyberKnife | 25 | 5 | 54.7 | - | - | - |
| 15 | 15 | Yes | 50.4 | 28 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 47.3 | - | - | - |
| 16 | 11 | No | 50.4 | 28 | Pancreatic head | CyberKnife | 20 | 5 | 76.2 | - | - | - |
| 17 | 25 | Yes | 50.4 | 28 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 81.3 | - | - | - |
| 18 | 19 | Yes | 30 | 10 | Tumor bed/soft tissue | Std Linac | 27.5 | 5 | 64.3 | - | - | - |
| 19 | 3 | Yes | 45 | 25 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 46.1 | 3 | - | Gastric hemorrhage |
| 20 | 9 | No | 50.4 | 28 | Pancreatic body | Std Linac | 25 | 5 | 60.5 | 2 | - | Abdominal pain |
| 21 | 21 | Yes | 45 | 25 | Tumor bed/soft tissue | Std Linac | 25 | 5 | 14.9 | - | - | - |
| 22 | 23 | Yes | 45 | 25 | Tumor bed/soft tissue | Std Linac | 33 | 5 | 46.7 | - | - | - |
| 23 | 10 | Yes | 50.4 | 28 | Regional lymph nodes | Std Linac | 25 | 5 | 29.4 | - | - | - |
SBRT, stereotactic body radiation therapy; CRT, conventional fractionated radiation therapy; RT, radiation therapy; PTV, planning target volume; GI, gastrointestinal.
Grade 2 or higher according to the National Cancer Institute Common Terminology Criteria for Adverse Event, Version 4.18
Survival outcomes
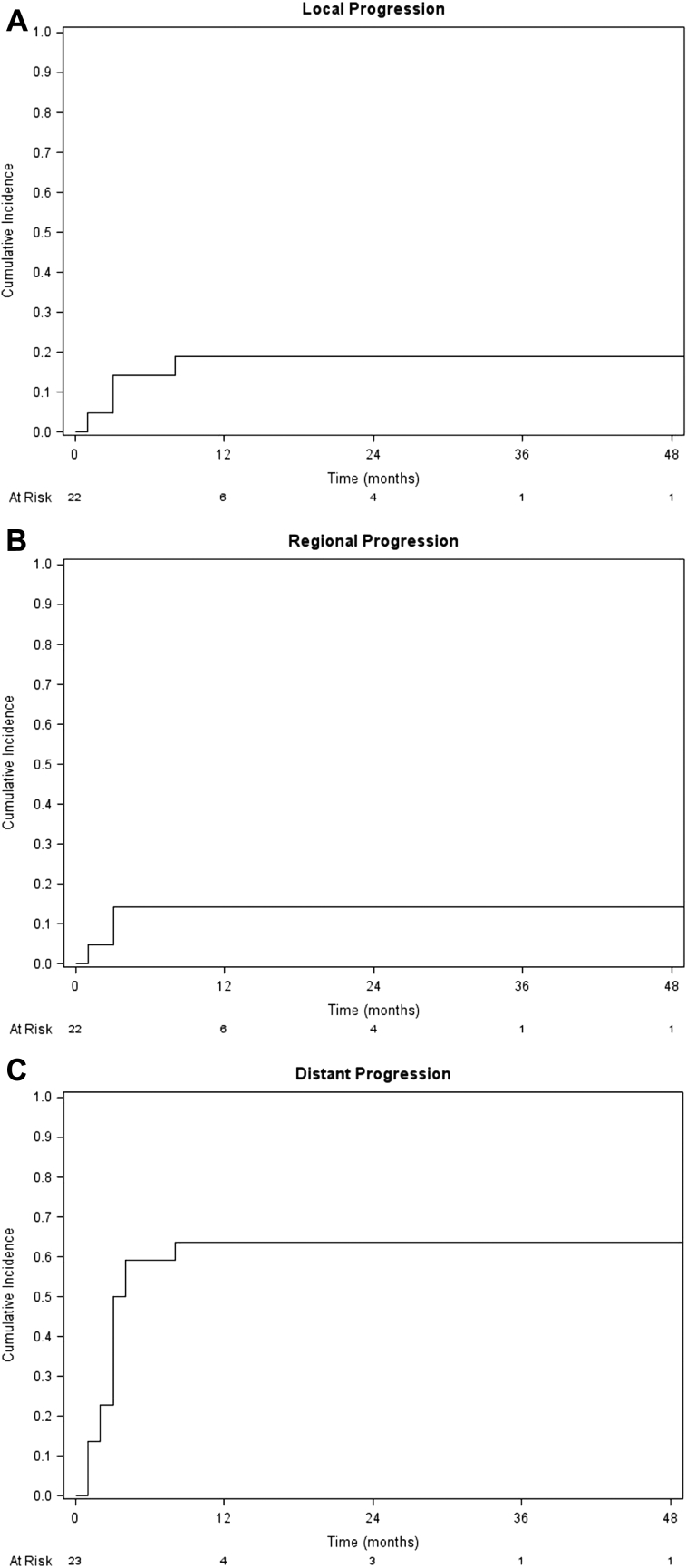
The median follow-up time was 28 months (range, 9-77 months) for all patients and 47 months (range, 41-77 months) for surviving patients. Local failure (LF) occurred in 4 patients. The cumulative incidence of LF at the last follow-up was 19%, and the freedom from local progression was 86.4% at 6 months and 81% at 1 year after reirradiation. Of these 4 patients, one was treated with a single fraction of 25 Gy and the other 3 were treated with 25 Gy in 5 fractions. In 2 of these 4 patients, LF occurred concurrently with distant disease progression. Only 3 patients presented with regional failure with a cumulative incidence of 14%, all with concurrent distant progression. Systemic progression rates were high, with a cumulative incidence of 64% at last follow-up, and with an incidence of 50% at 3 months after reirradiation (Fig 1).
Figure 1.
Cumulative incidences of (A) local progression, (B) regional progression, and (C) distant progression after stereotactic body radiation therapy reirradiation for entire group of patients, adjusted for the competing risk of death.
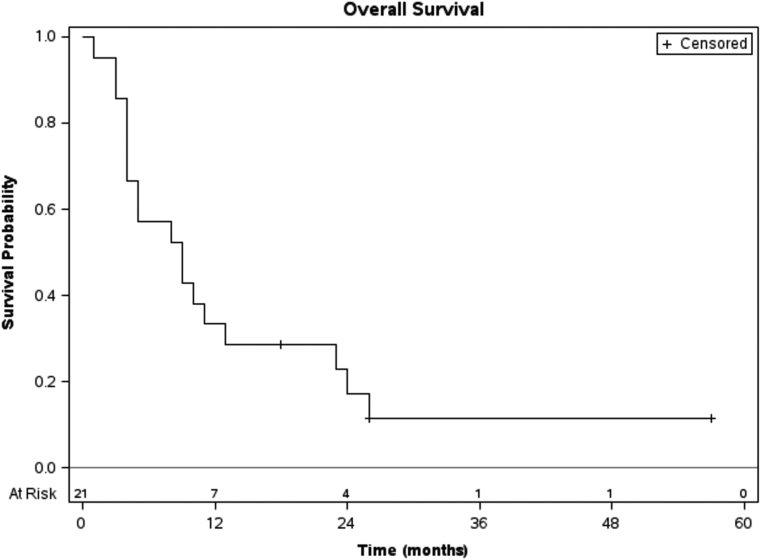
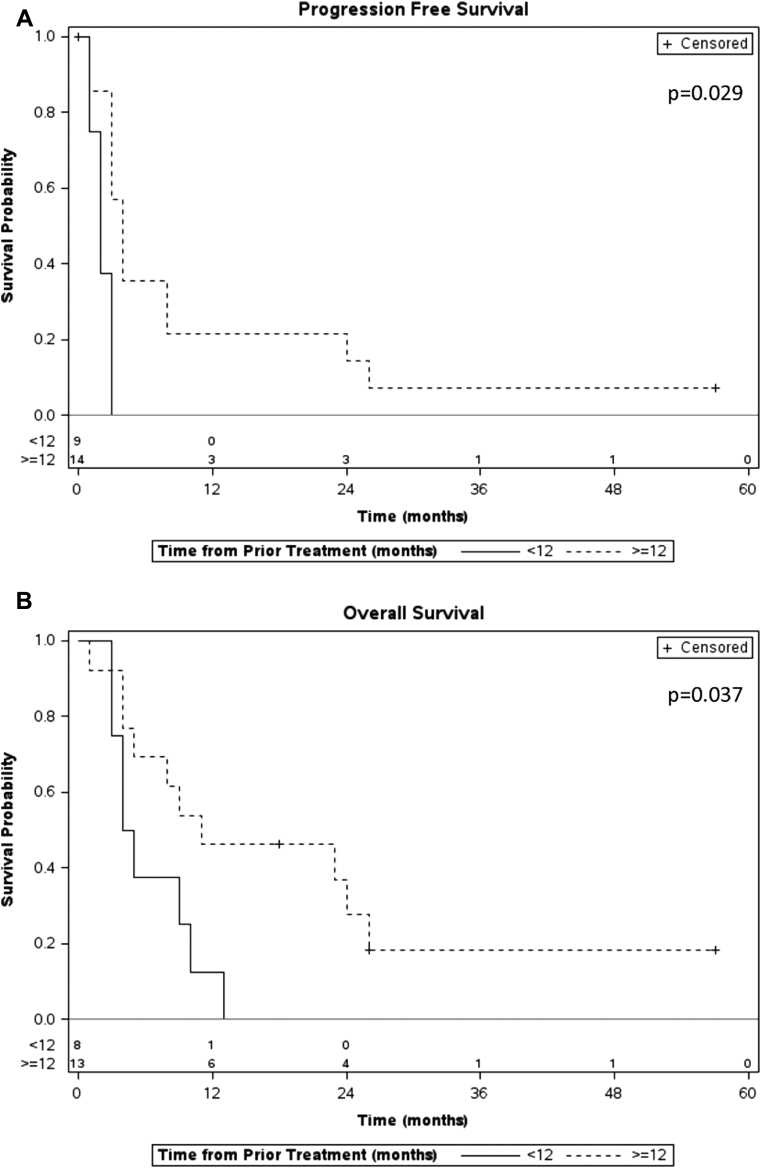
The median OS time was 27.5 months (range, 10-77 months) from initial treatment and 8.5 months from the time of reirradiation with only 3 patients surviving at the last follow-up (Fig 2). Patients who recurred or progressed 12 months or more after the initial treatment had a significantly higher OS rate (median, 10 vs 4.5 months, P = .037) and progression-free survival rate (median, 3 vs 2 months, log rank P = .029) compared with patients who recurred or progressed less than 12 months from the initial treatment (Fig 3). The group of patients who presented as resectable disease at initial diagnosis demonstrated a longer OS after local recurrence and reirradiation compared with patients who presented initially with locally advanced disease (median, 17 vs 5 months, log rank P = .032).
Figure 2.
Kaplan-Meier plot for overall survival, measured from the date of reirradiation for the entire group including number of patients at risk over time.
Figure 3.
Kaplan-Meier plots for (A) progression-free survival and (B) overall survival, measured from the date of reirradiation, comparing patients who progressed/recurred less than 12 months after the initial treatment (solid line) versus 12 months or more after the initial treatment (dashed line).
Palliation of symptoms
At presentation before reirradiation, 14 patients (60.9%) reported abdominal and/or back pain (2 reported back pain exclusively), which required medication for symptom relief. After SBRT reirradiation, only 6 patients (26.1%) reported abdominal and/or back pain, which was a relative improvement in pain control of 57.1%. One patient presented with an increase in pain intensity due to local disease progression. None of the patients demonstrated signs of gastrointestinal obstruction before SBRT, but one patient developed symptoms of gastric outlet obstruction upon local tumor progression 3 months after the end of reirradiation.
Toxicity
After reirradiation, 6 patients (26.1%) developed a grade 2 or 3 gastrointestinal toxicity, 4 of which (44%) occurred among patients who were treated with a single fraction SBRT regimen and 2 in patients who received multifraction radiation (14%), but the difference was not statistically significant (P = .36). Two patients (8.7%) developed grade 3 gastrointestinal toxicity, one occurring in the single-fraction group (11%) and the other in the 5-fraction group (7%). One of these patients had a recurrent tumor that abutted the stomach wall, which was treated with 25 Gy in a single fraction, and this patient developed a gastric fistula 1 month after SBRT. The other patient treated with 25 Gy in 5 fractions was found to have a bleeding gastric ulcer after an episode of hematemesis, which was successfully controlled with an epinephrine injection plus argon plasma coagulation by upper endoscopy. There were no grade 4 or 5 gastrointestinal toxicities. All gastric toxicities were identified in patients who received relatively high doses of radiation therapy (54 Gy and 60 Gy) at the initial treatment, before reirradiation with SBRT. Furthermore, all gastric toxicities occurred in patients who were reirradiated for a local recurrence less than 4 months after the initial radiation therapy treatment. Toxicity data are detailed in Table 2.
Discussion
The prognosis for patients with recurrent pancreatic cancer is extremely poor. However, in a subset of patients, locally recurrent tumors can result in debilitating pain and obstruction. Beyond symptoms, in an autopsy series from Johns Hopkins University, up to one-third of patients with pancreatic cancer died of predominantly local disease.21 This group of patients is most likely to benefit from intensive local therapy. More effective systemic therapies are still needed because the majority of these patients still die as a result of the development of distant metastases after treatment of the primary tumor.22, 23 In the future, as chemotherapy becomes more effective in controlling systemic disease, local recurrence after conventional chemoradiation will become increasingly more prevalent, and the impact of local control on survival will increase.
Overall, we report that SBRT after conventional chemoradiation therapy is feasible and can be performed with acceptable toxicity. In carefully selected patients, this approach may be of clinical benefit, particularly in patients with symptomatic local recurrences. Two other studies examined reirradiation with SBRT in the locally recurrent setting. The study by Wild et al demonstrated an 8.8-month median survival and a 62% 1-year rate of freedom from local progression for a small cohort of 15 patients with pancreatic cancer who were reirradiated with SBRT.24 In the current study, for the entire cohort of patients with local recurrences, we demonstrated a similar median OS of 8.5 months after SBRT and a more favorable freedom from local progression of 81% at 1 year. Interestingly, patients who recurred more than 12 months after initial treatment showed better survival outcomes. This observation may be due to inherent differences in tumor biology across the patient cohort, rendering different grades of tumor aggressiveness and response to treatment, as has been reported in the literature.25, 26, 27, 28
Distant recurrences occurred in most patients, with 50% showing evidence of systemic disease within 3 months after reirradiation. Certainly, many of these recurrences represent occult metastatic disease at the time of reirradiation, and better patient selection is needed to appropriately use SBRT, avoid unnecessary toxicity, and provide the most benefit of controlling local disease. The results from the present series may be used to start applying appropriate selection factors as we await more data. For example, patients who present with initially resectable disease or those who recur >12 months after initial therapy have better survival and may be more appropriate candidates for reirradiation.
In our series, the overall grade 3 toxicity rate was 8%, which is close to the 7% rate that was reported by Lominska et al29 and the 6% rate reported by Wild et al.24 Of note, 4 of the 6 patients who developed grade 2 or higher toxicity after reirradiation with SBRT received single-fraction SBRT, which suggests that a multifraction SBRT regimen may result in less toxicity. A similar trend was observed for the initial treatment of locally advanced disease with definitive SBRT at our institution,15 which is the reason why our treatment protocol changed from a single-fraction to a multifraction scheme during the course of this analysis. Another observation was that all objective gastrointestinal toxicities (eg, ulcers, fistulas, and bleedings caused by intestinal lining cell death and not by inflammatory mediators like for nausea or fatigue) occurred in patients who were reirradiated within 4 months after the prior radiation therapy treatment. This is of utmost importance because it reinforces the necessity of adequate time to allow normal tissue to properly repair before the delivery of a new course of radiation treatment, particularly when using hypofractionation.
The retrospective nature of the study and the relatively small sample size are limitations that make it difficult to draw definitive conclusions in this cohort of patients. Also, patients were treated heterogeneously in this study with a mix of single-fraction and multifraction SBRT as well as with different uses and types of chemotherapy.
Conclusion
Overall, our data indicate that selected patients may benefit from a multifraction SBRT reirradiation regimen in the setting of a local recurrence after conventional radiation therapy, particularly when systemic disease has been thoroughly discarded and an adequate time from the last radiation has been observed. In this setting, this strategy may provide good local tumor control and serve as a convenient and effective method of palliating local symptoms. We emphasize that attentive patient selection is crucial to ensure maximal benefit over risk of toxicity in this patient population.
Footnotes
Conflicts of interest: DTC reports support from Varian Research.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Yeo T.P., Hruban R.H., Leach S.D. Pancreatic cancer. Curr Probl Cancer. 2002;26:176–275. doi: 10.1067/mcn.2002.129579. [DOI] [PubMed] [Google Scholar]
- 3.Loehrer P.J., Sr., Feng Y., Cardenes H. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaassen D.J., MacIntyre J.M., Catton G.E., Engstrom P.F., Moertel C.G. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil – an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 5.Chauffert B., Mornex F., Bonnetain F. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 6.Herman J.M., Chang D.T., Goodman K.A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koong A.C., Le Q.T., Ho A. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer M., Roed H., Sengelov L. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Koong A.C., Christofferson E., Le Q.T. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Schellenberg D., Goodman K.A., Lee F. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Mahadevan A., Jain S., Goldstein M. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 12.Rwigema J.C., Parikh S.D., Heron D.E. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 13.Schellenberg D., Kim J., Christman-Skieller C. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Gurka M.K., Collins S.P., Slack R. Stereotactic body radiation therapy with concurrent full-dose gemcitabine for locally advanced pancreatic cancer: A pilot trial demonstrating safety. Radiat Oncol. 2013;8:44. doi: 10.1186/1748-717X-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trakul N., Koong A.C., Chang D.T. Stereotactic body radiotherapy in the treatment of pancreatic cancer. Semin Radiat Oncol. 2014;24:140–147. doi: 10.1016/j.semradonc.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Pollom E.L., Alagappan M., von Eyben R. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: Outcomes and toxicity. Int J Radiat Oncol Biol Phys. 2014;90:918–925. doi: 10.1016/j.ijrobp.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 17.Hammel P., Huguet F., van Laethem J.L. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute . U.S. Department of Health and Human Services; National Institutes of Health; National Cancer Institute; 2010. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0: May 28, 2009 (v4.03: June 14, 2010) [Google Scholar]
- 21.Iacobuzio-Donahue C.A., Fu B., Yachida S. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conroy T., Desseigne F., Ychou M. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 24.Wild A.T., Hiniker S.M., Chang D.T. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J Gastrointest Oncol. 2013;4:343–351. doi: 10.3978/j.issn.2078-6891.2013.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Xiu D., Zhan J. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco Targets Ther. 2016;9:6719–6726. doi: 10.2147/OTT.S111382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D., Shi J., Fu H. Integrinβ1 modulates tumour resistance to gemcitabine and serves as an independent prognostic factor in pancreatic adenocarcinomas. Tumour Biol. 2016;37:12315–12327. doi: 10.1007/s13277-016-5061-7. [DOI] [PubMed] [Google Scholar]
- 27.Connor AA, Denroche RE, Jang GH, et al. Association of distinct mutational signatures with correlates of increased immune activity in pancreatic ductal adenocarcinoma [e-pub ahead of print]. JAMA Oncol. doi:10.1001/jamaoncol.2016.3916, accessed November 1, 2016. [DOI] [PMC free article] [PubMed]
- 28.Chang D.T., Chapman C.H., Norton J.A. Expression of p16(INK4A) but not hypoxia markers or poly adenosine diphosphate-ribose polymerase is associated with improved survival in patients with pancreatic adenocarcinoma. Cancer. 2010;116:5179–5187. doi: 10.1002/cncr.25481. [DOI] [PubMed] [Google Scholar]
- 29.Lominska C.E., Unger K., Nasr N.M., Haddad N., Gagnon G. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol. 2012;7:74. doi: 10.1186/1748-717X-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]