Abstract
Purpose
To investigate the long-term effects of vertebral-body-sparing proton craniospinal irradiation (CSI) on the spine of young patients with medulloblastoma.
Methods and materials
Six children between the ages of 3 and 5 years with medulloblastoma were treated with vertebral-body-sparing proton CSI after maximal safe resection. Radiation therapy was delivered in the supine position with posterior beams targeting the craniospinal axis, and the proton beam was stopped anterior to the thecal sac. Patients were treated with a dose of either 23.4 Gy or 36 Gy to the craniospinal axis followed by a boost to the posterior fossa and any metastatic lesions. Chemotherapy varied by protocol. Radiographic effects on the spine were evaluated with serial imaging, either with magnetic resonance imaging scans or plain film using Cobb angle calculations, the presence of thoracic lordosis, lumbar vertebral body-to-disc height ratios, and anterior-posterior height ratios. Clinical outcomes were evaluated by patient/family interview and medical chart review.
Results
Overall survival and disease free survival were 83% (5/6) at follow-up. Median clinical and radiographic follow-up were 13.6 years and 12.3 years, respectively. Two patients were clinically diagnosed with scoliosis and treated conservatively. At the time of follow-up, no patients had experienced chronic back pain or required spine surgery. No patients were identified to have thoracic lordosis. Diminished growth of the posterior portions of vertebral bodies was identified in all patients, with an average posterior to anterior ratio of 0.88, which was accompanied by compensatory hypertrophy of the posterior intervertebral discs.
Conclusion
Vertebral-body-sparing CSI with proton beam did not appear to cause increased severe spinal abnormalities in patients treated at our institution. This approach could be considered in future clinical trials in an effort to reduce toxicity and the risk of secondary malignancy and to improve adult height.
Introduction
Summary.
This retrospective review evaluated the long-term consequences of vertebral-body-sparing proton craniospinal irradiation on young children with medulloblastoma. Clinical endpoints that were measured included chronic back pain, diagnosis of scoliosis, and the need for spine surgery. Radiographic endpoints were measured, including scoliosis and changes in vertebral body height, as well as bone marrow changes in the vertebral body. With this limited cohort, we found no severe spinal toxicities from this treatment technique and excellent disease control.
Medulloblastoma is the second most common pediatric malignancy of the central nervous system1 and often requires treatment of the entire craniospinal axis because of its propensity to spread through cerebrospinal fluid. The current treatment regimen for children ages 3 years and older includes maximal safe resection followed by craniospinal irradiation (CSI) using photons with concurrent and adjuvant chemotherapy. In the case of infantile medulloblastoma, which requires a diagnosis before 3 years of age, bridging chemotherapy is often administered after surgical resection until the patient is old enough to receive CSI.
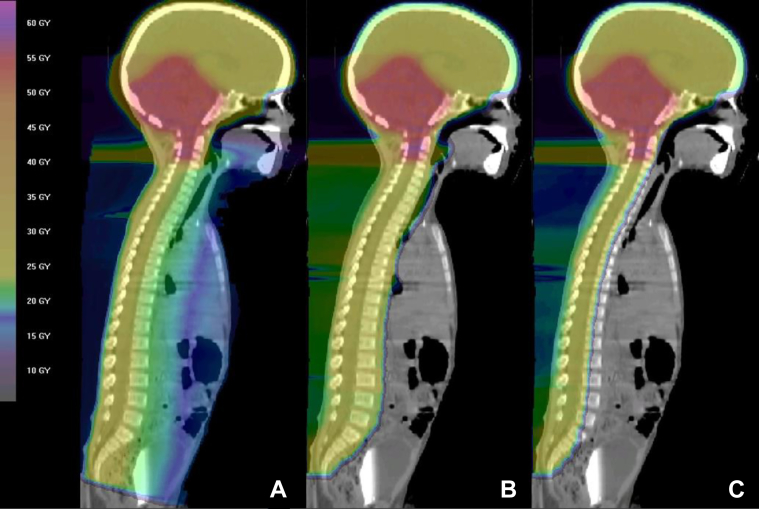
Long-term disease control rates now approach 80% for standard-risk patients and 60% to 70% for high-risk patients.2 Five-year overall survival rates for infantile medulloblastoma range between 45% and 65%.2, 3 However, with improving survival rates, there is a growing population of long-term survivors who are at risk for late toxicities. Conventional photon radiation for the spine is accompanied by sizeable exit doses through midline cervical, thoracic, abdominal, and pelvic organs (Fig 1A), which have the potential to cause both acute and chronic toxicities.4, 5
Figure 1.
Three midsagittal images of (A) a photon plan, (B) a proton plan including the entire vertebral body, and (C) a vertebral-body-sparing proton plan of a patient from the study cohort.
The inherent dose delivery properties of protons have been shown to be dosimetrically superior to photons in numerous settings, with lower integral dose and improved dose conformity.6, 7, 8, 9, 10 For CSI, protons enable dramatic dose sparing to structures beyond the spinal target volume,11, 12, 13, 14, 15, 16, 17 thereby reducing many acute toxicities, such as nausea, vomiting, weight loss, and esophagitis.18 Protons also have further potential to spare distal structures, such as bone marrow, should the distal edge be tailored to do so. This may reduce the severity of hematologic toxicities including leukopenia, thrombocytopenia, and anemia, which are commonly encountered in patients receiving chemotherapy for medulloblastoma.
Although there is some precedent for treating pediatric medulloblastoma with proton CSI, it has primarily been in the context of treating both the thecal sac and the entire vertebral body (Fig 1B).12 This strategy effectively undermines any capacity that protons have of preserving bone marrow, spine growth, and adult height.19, 20, 21, 22, 23, 24 The rationale for treating the entire vertebral body is based on evidence that shows that vertebral body irradiation with lateral dose asymmetry causes lateral vertebral body wedging and scoliosis.25, 26 However, there has been a severe lack of evidence thus far to demonstrate whether posterior-anterior dose asymmetry also causes clinically relevant skeletal abnormalities. At our institution, a cohort of pediatric patients was treated with proton CSI intended to spare the anterior vertebral body. In this report, we focus primarily on the development and severity of long-term skeletal complications to determine the suitability of this treatment technique.
Methods and materials
Six children between 3 and 5 years of age with medulloblastoma were treated with vertebral-body-sparing CSI at our institution between 2001 and 2007. All patients were enrolled in the institutional registry for proton research before treatment, and institutional review board approval was obtained to retrospectively review this registry. Each patient received maximal safe resection after initial diagnosis. Patients who were diagnosed before 3 years of age were treated with chemotherapy until they were 3 years old, at which time radiation was administered. Radiation was delivered under general anesthesia and with the patient in the supine position.
Vertebral-body-sparing proton CSI was delivered first, followed by a proton boost to the posterior fossa (Fig 1C). A dose of either 23.4 or 36 Gy was delivered to the entire craniospinal axis at 1.8 Gy per fraction, after which the posterior fossa was boosted with protons to a total dose of 54 Gy. The CSI dose and chemotherapy varied according to patient risk stratification and/or per study protocol. Additional radiation was delivered depending on the extent of disease (Table 1).
Table 1.
Patient and radiation treatment characteristics
| Patient A | Patient B | Patient C | Patient D | Patient E | Patient Fa | |
|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||
| Age at Diagnosis (y) | 2.1 | 2.3 | 2.2 | 4.5 | 3.0 | 4.2 |
| Age at RT (y) | 3.7 | 3.5 | 3.8 | 5.1 | 3.1 | 5.2 |
| Risk Level | High Risk | High Risk | High Risk | High Risk | High Risk | High Risk |
| Metastasis Stage | M 3 | M 0 | M 3 | M 1 | M 0 | M 3 |
| RT Characteristics | ||||||
| Proton CSI Dose (Gy) | 36.0 | 23.4 | 36.0 | 36.0 | 23.4 | 36.0 |
| PF Boost (Gy) | 18.0 | 30.6 | 18.0 | 18.0 | 30.6 | 18.0 |
| Days to Complete CSI | 44 | 47 | 51 | 45 | 52 | 47 |
| Additional Radiation | SRS to 4 additional intracranial lesions | None | 9.0 Gy proton boost to 4 spine metastases | None | None | Whole spine boost to 39.6 Gy |
| Other factors | ||||||
| Chemotherapy | Bridging CT (CCG-9921) | Bridging CT (COG-9934) | Bridging CT, Concurrent CT | Adjuvant CT, Concurrent CT | Adjuvant CT, Concurrent CT, Consolidative CT | Adjuvant CT, Consolidative CT (Head Start II) |
| Stem Cell Transplant | No | No | Yes (after bridging CT and prior to RT) | Yes (after adjuvant CT and prior to RT) | No | Yes (after adjuvant CT and prior to RT) |
RT, radiation therapy; CSI, craniospinal irradiation; PF, posterior fossa; SRS, stereotactic radiation surgery; CT, chemotherapy; CCG, Children's Cancer Group; COG, Children's Oncology Group.
Deceased, not analyzed radiographically.
The study included patients with medulloblastoma small enough to be treated to the whole brain without intracranial match lines—only young patients whose crania were of appropriate size were considered. Field size limitation at that time was 18 cm in diameter, which meant that only young children who were 5 years old or younger could be treated with this method. Treatment of the whole brain was achieved with lateral fields.
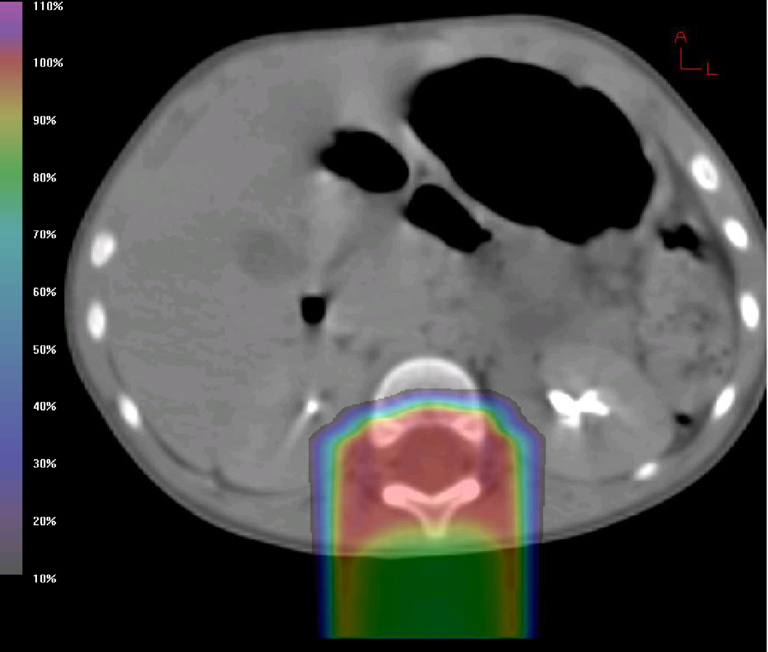
The spine was treated with 3 matched posteroanterior fields that were designed to stop the beam just anterior to the thecal sac (Fig 2) with match lines that were moved every 9 Gy throughout treatment. A lateral margin of 3 mm was applied, and daily kV imaging was used to ensure patient position. A 3 mm range uncertainty was applied to account for the distal edge of the proton beam, and a 3.5% density uncertainty was also included in the beam range to account for uncertainty in the water-equivalent path length as calculated from the planning computed tomography scan. The posterior fossa boost was achieved with 3 alternating fields: posteroanterior, right posterior oblique, and left posterior oblique fields, with the beam stopping proximal to the cochlea via the use of distal blocking.
Figure 2.
Isodose distribution in the axial plane at the L1 level for vertebral-body-sparing proton craniospinal irradiation.
The long-term effect of vertebral-body-sparing radiation on the spine was evaluated with serial magnetic resonance imaging (MRI) scans and/or plain film. Imaging of the spine was analyzed for scoliosis by an institutional radiologist using the greatest measured Cobb angle, as described by Malfair et al,27 with either MRI multiplanar reconstruction or available plain film radiographs. In short, the Cobb angle method involves capturing the maximum angle of curvature in a scoliotic spine by extrapolating 2 straight lines from the most rostral and caudal vertebrae in a single curvature. Scoliotic spines generally have 2 curvatures: a thoracic curvature in one direction and a lumbar curvature in the opposite direction. Lordosis was evaluated by measuring the presence or absence of thoracic lordosis. Additional measurements in the thoracolumbar spine were used to characterize vertebral body changes, including posterior-to-anterior ratios (PARs) of vertebral bodies, defined as the ratio of posterior vertebral body height to anterior vertebral body height, as well as intervertebral disc height. Moreover, the preservation of active bone marrow within the vertebral column was measured using T1-weighted MRI scans, which show hyperintensity of fat-infiltrated (inactive) bone marrow. The fraction of the vertebral body that contains active marrow was calculated by measuring the width of the hypointense portion divided by the total vertebral body diameter.
Long-term clinical outcomes were evaluated further by standardized patient/family interviews and medical chart reviews. Outcomes included ambulatory status, back pain, clinical diagnosis of scoliosis, and disease status. Patient height was evaluated using standardized growth charts, and acute effects were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0. Given the small number of patients reviewed, no statistical analysis was performed.
Results
The median age at diagnosis was 2.6 years, and the median age at the time of radiation therapy was 3.8 years. Patient and treatment characteristics are summarized in Table 1. Disease free survival and overall survival were 83% (5/6) at the time of follow-up. All 5 survivors were analyzed, with a median age of 16.3 years at the time of analysis. The median clinical and radiographic follow-up for the 5 pediatric patients were 13.6 and 12.3 years, respectively. The sixth patient, whose pathology test results showed desmoplastic medulloblastoma, experienced bony metastases outside the radiation field in the right shoulder 3 years after radiation therapy and could not be analyzed radiographically.
Spine outcomes were evaluated on the basis of the presence of scoliosis (Table 2), chronic back pain, need for corrective spine surgery, PARs, and intervertebral disc height. Two of 5 patients (40%) had scoliosis at the time of follow-up. One patient was clinically diagnosed with scoliosis and had a maximum Cobb angle of 19.3° in the lumbar spine. The other patient with scoliosis was first clinically diagnosed at 7 years of age (3.3 years after radiation therapy) and radiographically found to have a maximum Cobb angle of 36.2° at the time of analysis. The remaining 3 patients all had Cobb angles that were less than 10° and did not have clinical evidence of scoliosis.
Table 2.
Radiographic follow-up and scoliosis
| Variable | Patient A | Patient B | Patient C | Patient D | Patient E | Median |
|---|---|---|---|---|---|---|
| Clinical follow-up (y) | 15.8 | 14.8 | 13.6 | 11.8 | 8.7 | 13.6 |
| Radiographic follow-up (y) | 7.1 | 14.1 | 13.1 | 12.3 | 3.7 | 12.3 |
| Scoliosis | Yes | Yes | No | No | No | – |
| Maximum Cobb angle (degrees) | 36.2° | 19.3° | 3.9° | 9.7° | 9.7° | – |
No patients reported chronic back pain or a history of corrective spine surgery. Both patients with scoliosis used walkers to ambulate. However, 1 patient started using the walker after a stroke that resulted in chronic weakness on the left side and had been able to walk without assistance before the stroke. Two of 5 patients were able to walk without assistance. The fifth patient used a wheelchair because of severe epilepsy but was able to walk without assistance.
Changes in vertebral body growth were quantified by methods described previously and depicted in Figure 3. The average PAR was 0.88 (range, 0.73-1.00) for all vertebral bodies measured. The cervical, thoracic, and lumbar spine averaged 0.90, 0.92, and 0.82, respectively, which indicates a degree of wedging at all levels of the spine. Furthermore, we identified an increase in the fraction of posterior spine length occupied by discs in all 4 patients analyzed, a median relative difference of +32.1% (range, 28.3%-64.8%) when compared with the anterior spine. When analyzed by CSI dose (23.4 Gy vs 36.0 Gy), we found that the patients receiving 23.4 Gy had an average posterior disc height difference of +9.9%, but those receiving 36.0 Gy had a difference of +15.9% when compared with the anterior disc. The fifth patient was not analyzed because this patient's spine status was followed by plain film only, which limited the ability to measure the intervertebral disc.
Figure 3.
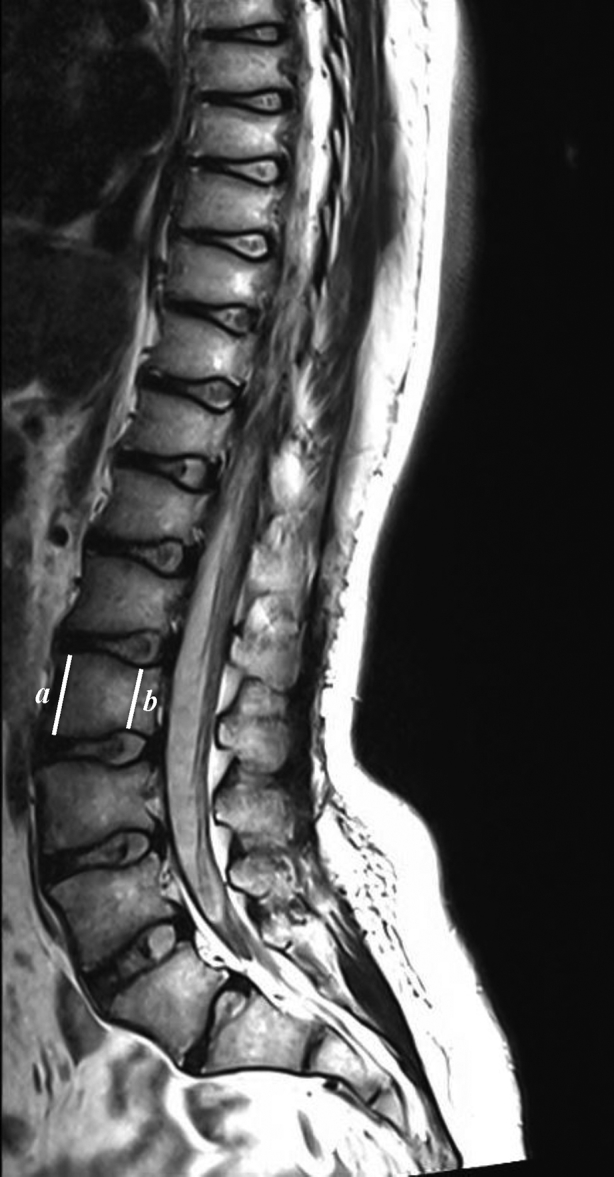
This T1-weighted magnetic resonance imaging scan of a patient 11 years after vertebral-body-sparing proton craniospinal irradiation exhibits posterior vertebral body wedging and posterior intervertebral disc hypertrophy. The image also demonstrates fatty infiltration of the posterior vertebral body and conservation of the thoracic and lumbar curvature.
Pertaining to marrow preservation, 4 of 5 patients were analyzed for active marrow preservation using the methods described previously. Each of the 4 patients had at least 7 vertebral bodies analyzed in the thoracolumbar spine, with a maximum of 9 vertebral bodies analyzed. The median spared percentage across all analyzed patients analyzed was 46% (range, 39%-53%).
All patients' heights at the time of analysis were below the 10th percentile in height. All patients were initiated on growth hormone replacement therapy; however, details on its use and duration were unavailable.
Data on acute gastrointestinal, skin, and hematologic toxicities are shown in Table 3. One patient had grade 2 esophagitis. All patients experienced a grade 2 or higher hematologic toxicity during treatment. Three of 4 patients who received concurrent chemotherapy required a blood transfusion during radiation therapy (patients C, D, and E), but the 2 patients who underwent radiation therapy without concurrent chemotherapy did not require a blood transfusion (patients A and B). No patients required a mid-treatment delay because of toxicity during radiation therapy, and the median treatment duration was 47 days (range, 44-52 days).
Table 3.
Acute toxicity profile
| Category | Patient A | Patient B | Patient C | Patient D | Patient E | Patient F |
|---|---|---|---|---|---|---|
| Skin | Grade 1 | Grade 1 | Grade 2 | Grade 2 | Grade 1 | Grade 1 |
| Gastrointestinal | Grade 2 (esophagitis) | Grade 1 (nausea/vomiting) | None | Grade 2 (oropharynx) | Grade 1 (nausea/vomiting) | None |
| Hematologic | ||||||
| Neutropenia | N/A | Grade 2 | Grade 4 | Grade 3 | Grade 3 | Grade 2 |
| Leukopenia | Grade 2 | Grade 1 | Grade 3 | Grade 4 | Grade 4 | Grade 3 |
| Thrombocytopenia | None | None | Grade 1 | Grade 1 | Grade 1 | Grade 1 |
| Anemia | None | Grade 2 | Grade 3 | Grade 2 | Grade 2 | Grade 1 |
| Other | None | None | Grade 1 (fatigue) | None | None | None |
| pRBC transfusion during RT | None | None | Week 6 of 8 | Week 5 of 7 | Week 4 of 8 | None |
pRBC, packed red blood cells; RT, radiation therapy.
Discussion
To the best of our knowledge, this case series is the first analysis of late side effects to the spine in young pediatric patients treated with vertebral-body-sparing proton CSI. The initial rationale for this treatment method was to reduce bone marrow toxicity and allow increased tolerance to chemotherapy. Our analysis focused on long-term effects on the spine, specifically the rates of scoliosis and lordosis, which are traditionally cited as reasons to include the entire vertebral body for proton CSI in young children.28
We observed scoliosis in 2 of 5 patients at the time of follow-up with a median radiographic follow-up of 12.3 years. In our review of the available literature, scoliosis is common in children who are treated with radiation that involves the spine. A 2015 report by Paulino et al cited a radiographic scoliosis rate of 45% (10/22) in a cohort of young patients with medulloblastoma treated with photon CSI that spanned the width of the vertebral body.29 Before this study, the majority of the available data were collected from the treatment of Wilms tumor and neuroblastoma. These studies also indicated that long-term scoliosis rates are 40% to 50% or higher, despite the inclusion of the entire vertebral body.30, 31, 32
To better understand the specific effect of radiation on the spine, our investigation also involved measuring radiographic changes to the structure of the spine. Asymmetrical growth of vertebral bodies was noted in all patients, with an average PAR of 0.88 across all measured vertebral bodies. The PARs varied based on the location of the vertebral body. For example, the lumbar vertebrae exhibited smaller PARs (average, 0.82) but were also spared anterior irradiation to a larger degree because of their size (Fig 3). The distal end of the Bragg Peak was sharp, which produced an abrupt, step pattern in the bone. Greater vertebral body sparing by decreasing the proton range would cause less posterior vertebral body deformity and further limit the impact on the vertebral body but potentially decrease the target coverage. Although these radiographic changes were identified in all patients analyzed, excessive or reversed spine curvatures such as thoracic lordosis were not identified in any patient. This finding was apparently due to enlargement of vertebral discs posteriorly, which appeared to fill the space in response to the growth of the nonirradiated vertebral body.
Multifactorial contributors to the pathogenesis of scoliosis should also be considered. Radiation to the whole brain causes hormonal dysfunction and is currently unavoidable with CSI of any modality. Secondly, cerebellar damage related to tumor or surgical resection must also be considered. Investigating the correlation between posterior fossa syndrome and scoliosis may be helpful.
Lastly, chemotherapy can also cause various long-term toxicities, which are not well understood. In this cohort, both patients with scoliosis had confounding factors that potentially contributed to their scoliosis. One of these patients suffered a stroke, which resulted in permanent weakness on the left side. The other patient had chronic lower extremity weakness after resection of the primary tumor and primarily used a walker for ambulation. Scoliosis was noted very shortly after this patient completed radiation therapy, implicating factors other than radiation therapy as the cause.
This cohort is represented by very young patients, and no patient was over the age of 5 years when treated with radiation therapy. This population is known to experience the most severe chronic toxicities related to radiation. Radiation is currently avoided in patients younger than 3 years of age in an effort to reduce treatment morbidity. We speculate that older patients treated with vertebral-body-sparing radiation would have significantly less asymmetric bone growth.
Fatty infiltration of bone marrow on MRI scans is known to signal decreased cellularity and inactive marrow after irradiation.33 Fatty infiltration was identified by MRI in each vertebral body that was irradiated and appeared to be permanent, without gross evidence of hematopoietic repopulation over time. This was despite its proximity to active marrow within the same vertebral body. We reported an average of 46% sparing in the thoracolumbar spine, measured across 31 vertebral bodies in 4 patients with MRI scans of the thoracolumbar spine. The assumption is that this bone marrow sparing can decrease hematologic toxicity. The cervical spine had minimal anterior sparing because of its small size, which was encompassed by the distal margin of uncertainty of the proton beam. As discussed earlier, advances in immobilization, daily imaging and positioning, and understanding of the uncertainty of the distal end proton beam could allow for further bone marrow sparing.
The irradiated vertebral column ultimately fails to retain significant growth potential, but the spared portions are able to grow. Sparing most of the vertebral body could enhance torso growth and allow patients to achieve heights closer to their natural adult height. Although none of our patients exceeded the 10th percentile in height, we concede that it is difficult to compare this result in so few patients without using a matched cohort of photon CSI patients and accurate documentation of growth hormone data. This is an area that should be further investigated.
Acute toxicity was discussed in detail in our first publication on this cohort.34 The cohort has since doubled in number of patients, and after a repeat analysis, a total of 1 of 6 patients (17%) experienced esophagitis. Yock et al cited esophageal toxicity rates (all grades) of 35% in patients treated with standard proton CSI. We expect upper gastrointestinal toxicity to be greatly improved with vertebral-body-sparing treatment because the dose to the esophagus is dramatically reduced. Recently published analyses from Yock et al and Eaton et al have shown decreased toxicity with similar survival rates in patients treated with proton CSI compared with photon CSI.35, 36 Conclusions with regard to hematologic toxicity are difficult to ascertain without a matched cohort and because patients received different chemotherapies, which contributed to decreased blood counts. However, in this very young group of high-risk patients, every patient completed treatment without a delay caused by acute toxicity.
Conclusions
Vertebral-body-sparing proton CSI did not appear to cause increased severe spinal abnormalities in our small cohort when compared with historical data. Radiographically, this treatment allowed for bone marrow sparing and vertebral body growth outside of the radiation field while achieving excellent disease control. Clinically, these patients did not experience chronic pain or require surgical intervention. Additional studies should be done to confirm the safety and advantages of this approach.
References
- 1.Rickert C.H., Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new who classification. Childs Nerv Syst. 2001;17:503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 2.Halperin E.C., Constine L.S., Tarbell N.J., Kun L.E. Tumors of the posterior fossa and the spinal canal. In: Pine J.W. Jr., editor. Pediatric Radiation Oncology. 5th. ed. Lippincott, Williams and Wilkins; Philadelphia, PA: 2011. pp. 53–66. [Google Scholar]
- 3.Packer R.J., Zhou T., Holmes E., Vezina G., Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: Results of Children's Oncology Group trial a9961. Neuro Oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone P.A., McMullen K.P., Buchsbaum J.C., Douglas J.G., Helft P. Pediatric CSI: Are protons the only ethical approach? Int J Radiat Oncol Biol Phys. 2013;87:228–230. doi: 10.1016/j.ijrobp.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Wolden S.L. Protons for craniospinal radiation: Are clinical data important? Int J Radiat Oncol Biol Phys. 2013;87:231–232. doi: 10.1016/j.ijrobp.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Bonnett D.E. Current developments in proton therapy: A review. Phys Med Biol. 1993;38:1371–1392. doi: 10.1088/0031-9155/38/10/001. [DOI] [PubMed] [Google Scholar]
- 7.Levin W.P., Kooy H., Loeffler J.S., DeLaney T.F. Proton beam therapy. Br J Cancer. 2005;93:849–854. doi: 10.1038/sj.bjc.6602754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen D.R., Bruland O.S., Frykholm G., Norderhaug I.N. Proton therapy - a systematic review of clinical effectiveness. Radiother Oncol. 2007;83:123–132. doi: 10.1016/j.radonc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Schulz-Ertner D., Jakel O., Schlegel W. Radiation therapy with charged particles. Semin Radiat Oncol. 2006;16:249–259. doi: 10.1016/j.semradonc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Schulz-Ertner D., Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25:953–964. doi: 10.1200/JCO.2006.09.7816. [DOI] [PubMed] [Google Scholar]
- 11.Howell R.M., Giebeler A., Koontz-Raisig W. Comparison of therapeutic dosimetric data from passively scattered proton and photon craniospinal irradiations for medulloblastoma. Radiat Oncol. 2012;7:116. doi: 10.1186/1748-717X-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krejcarek S.C., Grant P.E., Henson J.W., Tarbell N.J., Yock T.I. Physiologic and radiographic evidence of the distal edge of the proton beam in craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2007;68:646–649. doi: 10.1016/j.ijrobp.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu X., Bjork-Eriksson T., Nill S. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol. 2005;44:554–562. doi: 10.1080/02841860500218819. [DOI] [PubMed] [Google Scholar]
- 14.Slater J.D. Clinical applications of proton radiation treatment at Loma Linda University: Review of a fifteen-year experience. Technol Cancer Res Treat. 2006;5:81–89. doi: 10.1177/153303460600500202. [DOI] [PubMed] [Google Scholar]
- 15.Slater J.M., Archambeau J.O., Miller D.W., Notarus M.I., Preston W., Slater J.D. The proton treatment center at Loma Linda University Medical Center: Rationale for and description of its development. Int J Radiat Oncol Biol Phys. 1992;22:383–389. doi: 10.1016/0360-3016(92)90058-p. [DOI] [PubMed] [Google Scholar]
- 16.St Clair W.H., Adams J.A., Bues M. Advantage of protons compared to conventional x-ray or imrt in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 17.Yock T.I., Tarbell N.J. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. [DOI] [PubMed] [Google Scholar]
- 18.Brown A.P., Barney C.L., Grosshans D.R. Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys. 2013;86:277–284. doi: 10.1016/j.ijrobp.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang E.L., Allen P., Wu C., Ater J., Kuttesch J., Maor M.H. Acute toxicity and treatment interruption related to electron and photon craniospinal irradiation in pediatric patients treated at the University of Texas MD Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2002;52:1008–1016. doi: 10.1016/s0360-3016(01)02717-1. [DOI] [PubMed] [Google Scholar]
- 20.Cumberlin R.L., Luk K.H., Wara W.M., Sheline G.E., Wilson C.B. Medulloblastoma: Treatment results and effect on normal tissues. Cancer. 1979;43:1014–1020. doi: 10.1002/1097-0142(197903)43:3<1014::aid-cncr2820430334>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Marks L.B., Cuthbertson D., Friedman H.S. Hematologic toxicity during craniospinal irradiation: The impact of prior chemotherapy. Med Pediatr Oncol. 1995;25:45–51. doi: 10.1002/mpo.2950250110. [DOI] [PubMed] [Google Scholar]
- 22.Kiltie A.E., Lashford L.S., Gattamaneni H.R. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28:348–354. doi: 10.1002/(sici)1096-911x(199705)28:5<348::aid-mpo4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Packer R.J., Gajjar A., Vezina G. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 24.Xu W., Janss A., Moshang T. Adult height and adult sitting height in childhood medulloblastoma survivors. J Clin Endocrinol Metab. 2003;88:4677–4681. doi: 10.1210/jc.2003-030619. [DOI] [PubMed] [Google Scholar]
- 25.Arkin A.M., Pack G.T., Ransohoff N.S., Simon N. Radiation-induced scoliosis; a case report. J Bone Joint Surg Am. 1950;32A:401–404. [PubMed] [Google Scholar]
- 26.Arkin A.M., Simon N. Radiation scoliosis; an experimental study. J Bone Joint Surg Am. 1950;32A:396–401. [PubMed] [Google Scholar]
- 27.Malfair D., Flemming A.K., Dvorak M.F. Radiographic evaluation of scoliosis: Review. AJR Am J Roentgenol. 2010;194:S8–S22. doi: 10.2214/AJR.07.7145. [DOI] [PubMed] [Google Scholar]
- 28.Neuhauser E.B., Wittenborg M.H., Berman C.Z., Cohen J. Irradiation effects of roentgen therapy on the growing spine. Radiology. 1952;59:637–650. doi: 10.1148/59.5.637. [DOI] [PubMed] [Google Scholar]
- 29.Paulino A.C., Suzawa H., Dreyer Z., Bryant R., Okcu M.F., Chintagumpala M. Scoliosis in children receiving craniospinal irradiation for medulloblastoma. Pediatric Blood & Cancer. 2015;62:S209. [Google Scholar]
- 30.Makipernaa A., Heikkila J.T., Merikanto J., Marttinen E., Siimes M.A. Spinal deformity induced by radiotherapy for solid tumours in childhood: A long-term follow up study. Eur J Pediatr. 1993;152:197–200. doi: 10.1007/BF01956143. [DOI] [PubMed] [Google Scholar]
- 31.Mayfield J.K., Riseborough E.J., Jaffe N., Nehme M.E. Spinal deformity in children treated for neuroblastoma. J Bone Joint Surg Am. 1981;63:183–193. [PubMed] [Google Scholar]
- 32.Paulino A.C., Wen B.C., Brown C.K. Late effects in children treated with radiation therapy for Wilms' tumor. Int J Radiat Oncol Biol Phys. 2000;46:1239–1246. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 33.MacEwan I.J., Glembotski N.E., D'Lima D. Proton density water fraction as a biomarker of bone marrow cellularity: Validation in ex vivo spine specimens. Magn Reson Imaging. 2014;32:1097–1101. doi: 10.1016/j.mri.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Yuh G.E., Loredo L.N., Yonemoto L.T. Reducing toxicity from craniospinal irradiation: Using proton beams to treat medulloblastoma in young children. Cancer J. 2004;10:386–390. doi: 10.1097/00130404-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Yock T.I., Yeap B.Y., Ebb D.H. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016;17:287–298. doi: 10.1016/S1470-2045(15)00167-9. [DOI] [PubMed] [Google Scholar]
- 36.Eaton B.R., Esiashvili N., Kim S. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: A comparison of disease control and overall survival. Int J Radiat Oncol Biol Phys. 2016;94:133–138. doi: 10.1016/j.ijrobp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]