Abstract
Purpose
To report the results of a prospective study that compares small bowel doses during prone and supine pelvic intensity modulated radiation therapy.
Methods and materials
Ten patients receiving pelvic radiation therapy each had 2 intensity modulated radiation therapy plans generated: supine and prone on a belly board (PBB). Computed tomography on rails was performed weekly throughout treatment in both positions (10 scans per patient). After image fusion, doses to small bowel (SB) loops and clinical target volume were calculated for each scan. Changes between the planned and received doses were analyzed and compared between positions. The impact of bladder filling on SB dose was also assessed.
Results
Prone treatment was associated with significantly lower volumes of SB receiving ≥20 Gy. On average, prone on a belly board positioning reduced the volume of SB receiving a given dose of radiation by 28% compared with supine positioning. Target coverage throughout the treatment course was similar in both positions with an average minimum clinical target volume dose of 88% of the prescribed prone dose and 89% of the supine (P = .54). For supine treatment, SB dose was inversely correlated with bladder filling (P = .001-.013; P > .15 for prone). For 96% of treatments, the volume of SB that received a given dose deviated >10% from the plan. The deviation between the planned and delivered doses to SB did not differ significantly between the positions.
Conclusions
Prone positioning on a belly board during pelvic IMRT consistently reduces the volume of SB that receives a broad range of radiation doses. Prone IMRT is associated with interfraction dose variation to SB that is similar to that of supine positioning. These findings suggest that prone positioning with daily image guided radiation therapy is an effective method for maximizing SB sparing during pelvic IMRT.
Summary.
To evaluate whether the dosimetric advantage of prone positioning with intensity modulated radiation therapy (IMRT) is maintained throughout the entire treatment course, we conducted a prospective study in which 10 patients had prone and supine plans generated. Patients were rescanned weekly in both positions and the delivered doses were recalculated for each fraction. We conclude that prone positioning with a belly board may provide small bowel sparing that is superior to supine IMRT with interfraction dose variation similar to supine treatment.
Introduction
Gastrointestinal (GI) toxicity is the most frequently encountered complication of pelvic radiation therapy with clinically significant acute and late toxicity occurring in up to 60% and 20% of patients, respectively.1 Radiation dose to the small bowel (SB) and volume of SB irradiated are the strongest predictors of GI toxicity during pelvic radiation therapy.2 Thus, methods to reduce SB radiation exposure have the potential to decrease GI toxicity and open the possibility for target dose escalation. Prone positioning on a belly board (PBB) and intensity modulated radiation therapy (IMRT) are 2 of the most effective and frequently used techniques for reducing SB dose from pelvic radiation therapy.
PBB is a simple method for physically displacing SB away from target structures within the pelvis. Three-dimensional treatment planning studies have demonstrated that PBB significantly reduces the volume of SB receiving prescription doses.3, 4, 5, 6 Clinically, retrospective studies have shown that 3-dimensional pelvic radiation therapy with prone positioning is associated with less acute GI toxicity compared with supine controls.6, 7 On the basis of these results, PBB is routinely used at some institutions for pelvic radiation therapy. IMRT aims to decrease GI toxicity in pelvic radiation therapy by improving target dose conformality. Treatment planning comparisons have shown that IMRT is capable of significantly decreasing dose to SB in patients with rectal, gynecologic, anal, and prostate cancer.8, 9, 10, 11 In clinical practice, both retrospective12, 13 and prospective studies14 have shown that pelvic IMRT is associated with lower acute and late GI toxicity compared with 3-dimensional conformal treatment for select subsites.
The combination of both PBB and IMRT appears to offer increased SB sparing during pelvic radiation therapy. Treatment planning comparisons in patients with gynecologic, rectal, and anal cancer have demonstrated reduced SB doses with prone IMRT compared with supine IMRT or prone 3-dimensional conformal radiation therapy.7, 9, 15, 16, 17 To date, no studies using pelvic IMRT have demonstrated improved clinical outcomes with PBB over supine treatment.
Despite the potential dosimetric advantages of prone IMRT, the combination raises several concerns. Compared with supine positioning, prone treatment may be associated with both increased interfraction position variation18, 19 and greater day-to-day anatomic deformation.20 Given the increased conformality and complex beam fluences with IMRT, positioning errors and anatomic changes may result in unanticipated dose variations within SB and, potentially, target underdosage. Therefore, it is possible that the dosimetric advantage of prone IMRT seen in simulation may not be maintained through the course of treatment.
We conducted a prospective study to compare prone and supine pelvic IMRT on the basis of the reconstruction of “delivered” doses to target volumes and organs at risk. Each patient was simulated and planned for both supine and PBB. During the course of treatment, in-room computed tomography (CT) on rails was performed weekly in both positions. These datasets allowed us to use each patient as their own control and to calculate “real-world” on-treatment doses as if each patient had been treated in both positions. On the basis of the previously observed positional variations and anatomic deformations with prone positioning, we hypothesized that prone treatment would result in greater interfraction dose variation to SB than supine treatment.
Methods and materials
Ten patients receiving curative pelvic radiation therapy at the University of Utah Huntsman Cancer Hospital were enrolled in an institutional review board–approved, prospective study. Because the primary requirement for the study was to obtain weekly CT scans of each patient in both positions, inclusion criteria were intentionally broad. Disease sites were rectal (n = 4), cervical/endometrial (n = 4), and anal (n = 2). The radiation exposure from the additional CT scans was quantified and documented in the consent. All contouring and planning for the project was independent of actual patient treatment, and enrollment did not affect therapy.
Simulation
Patients were simulated on a GE LightSpeed RT 16-slice large bore CT using 2.5-mm slice thickness (GE HealthCare, Waukesha, WI). Patients were instructed to drink 700 mL of water 30 minutes before simulation. All patients were simulated both PBB and supine. The actual treatment position was at the discretion of the treating radiation oncologist; 3 patients were treated prone and 7 supine. Each patient had separate alpha cradles molded for each position (Smithers Medical Products, Canton, OH). For PBB, patients were placed face down on a 10-cm-thick polyurethane foam board with a 30-cm wide by 25-cm long rectangular aperture for the belly. This was indexed to an alpha cradle molded from below the knees up to the pelvis. An ankle pillow was used to support the patient's feet. The isocenter was marked on the skin with permanent tattoos, which in turn were marked on the belly board with room lasers. The isocenter for the patient orientation that was not treated was referred to as the research isocenter and was demarcated with indelible marker and covered with waterproof, transparent dressing.
Treatment volumes and planning
Target volumes and prescriptions were generated in accordance with Radiation Therapy Oncology Group IMRT protocols for cervical/endometrial (0418), anal (0529), and rectal cancer (0822). Within the bony pelvis, regional node clinical target volumes (CTVs) were defined by expanding vessels by 7 mm and excluding bone, muscle, and bowel loops. Inguinal and external iliac nodes were included for anal cancer plans whereas external iliac nodes were included for gynecologic cases. To minimize subjective differences between prone and supine target volumes, the CTV was first contoured on the scan that was performed in the treatment position. Next, the scan for the alternate position was registered using bony anatomy. The CTV was then copied onto the second dataset and modified if necessary. The planning target volume (PTV) was generated using a 7-mm isotropic expansion on the CTV. The peritoneal cavity was contoured from the pelvic floor inferiorly to 2 cm above the PTV. A semi-automated algorithm based on Hounsfield values was used to define hollow viscera within the peritoneal cavity, and the large bowel was manually removed from SB.
Separate 7-field IMRT plans with equidistant beam spacing were generated for each position. All plans were generated by a single planner using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA). A standardized optimization process was used for planning (Appendix e1). For all plans, 6 MV photons were used and the final dose was calculated using the analytical anisotropic algorithm for delivery on a Siemens Artiste with 160 leaf MLC (Siemens Healthcare, Erlangen, Germany). The same process was performed to generate plans in each position (Fig 1).
Figure 1.
Representative isodose distribution for rectal plan in the supine (upper panels) and prone (lower panels) positions.
Prone and supine plans for each patient were normalized for 95% of prescription dose covering 95% of the PTV. Target volumes for gynecologic and rectal cases were planned at 1.8 Gy per fraction to a total of 45 to 50.4 Gy. Anal cases were planned with a dose-painting technique using 2 target dose levels of 1.5 Gy and 1.8 Gy per fraction (Table 1).
Table 1.
Patient and initial plan characteristics
| Patient No. | Anatomic Site | Body Mass Index | Planning/Contouring Protocol | Prescription (Gy in fx) | Position | Planning Target Volume (cm3) | Clinical Target Volume min (%) | Small Bowel in Planning Target Volume (cm3) |
|---|---|---|---|---|---|---|---|---|
| 1 | Postoperative Endometrium | 24.3 | RTOG 0418 | 50.4 in 28 | Prone | 1117 | 100 | 34.7 |
| Supine | 1132 | 95 | 42.0 | |||||
| 2 | Rectum | 21.2 | RTOG 0822 | 45 in 25 | Prone | 1074 | 91 | 4.3 |
| Supine | 1158 | 93 | 40.3 | |||||
| 3 | Intact Cervix | 28.8 | RTOG 0418 | 50.4 in 28 | Prone | 1442 | 91 | 21.2 |
| Supine | 1389 | 86 | 18.1 | |||||
| 4 | Rectum | 21.8 | RTOG 0822 | 45 in 25 | Prone | 1154 | 95 | 5.0 |
| Supine | 976 | 90 | 0.6 | |||||
| 5 | Rectum | 24.9 | RTOG 0822 | 45 in 25 | Prone | 1129 | 93 | 47.0 |
| Supine | 1382 | 94 | 70.7 | |||||
| 6 | Intact Cervix | 19.7 | RTOG 0418 | 50.4 in 28 | Prone | 1122 | 83 | 87.3 |
| Supine | 1126 | 85 | 113.1 | |||||
| 7 | Postoperative Endometrium | 30.5 | RTOG 0418 | 50.4 in 28 | Prone | 1160 | 96 | 0 |
| Supine | 1152 | 96 | 12.3 | |||||
| 8 | Anus (T2, N0) | 22.8 | RTOG 0529 | 50.4/42 in 28 | Prone | 1683 | 98 | 28.0 |
| Supine | 1650 | 85 | 39.8 | |||||
| 9 | Anus (T3, N0) | 31.9 | RTOG 0529 | 54/45 in 30 | Prone | 1707 | 92 | 6.1 |
| Supine | 1777 | 94 | 29.4 | |||||
| 10 | Rectum | 30.0 | RTOG 0822 | 45 in 25 | Prone | 926 | 93 | 0.6 |
| Supine | 900 | 92 | 0.6 |
fx, fraction; RTOG, Radiation Therapy Oncology Group.
Treatment and weekly imaging
Patients were given the same bladder filling instructions for treatment as for simulation. In-room scans were performed weekly using a 40-slice Siemens CT on rails at a 2.5-mm slice thickness. One scan was performed immediately before treatment. After treatment, the patient was placed in the research orientation using the second immobilization device aligned to the research tattoos and rescanned. All patients received 5 research scans in addition to the weekly treatment scans for a total of 10 on-treatment CT scans per patient.
Each treatment CT scan was registered offline to the planning scan by a single physician using Siemens Adaptive Targeting software. The registration was primarily based on bony anatomy but took into consideration whether the PTV adequately covered all target tissue. Image guided corrections were made only via table translation and did not include rotation.
Recontouring and dose recalculations
Weekly CT scans for each patient were registered with their original simulation CT in Eclipse using bony registration and full 6 degrees of freedom corrections. CTVs and PTVs were transferred directly onto the new scans. The original patient-specific density-based algorithm was used to facilitate delineation of bowel loops, and the bladder was manually contoured (Appendix e2).
For dose recalculations, weekly CT scans were reregistered in Eclipse using the couch corrections that were obtained from the offline fusion. The original fluences were transferred to the new scans, and doses were recalculated using analytical anisotropic algorithm. Separate dose-volume histograms (DVHs) were generated for target volumes and individual loops of SB on the new scans using the original treatment prescription. SB doses were estimated for each treatment course by averaging volumes of SB receiving dose in 5-Gy dose increments among the 5 weekly scans for each patient and position.
Statistical analysis
Analyses were conducted using SPSS Version 20. Volume of SB receiving dose was recorded in 5-Gy increments from 15 Gy to 45 Gy for all plans. Simple comparisons of means with regard to prone versus supine positioning were assessed with analysis of variance. A P-value of ≤.05 was deemed to indicate statistical significance. Mixed regression analyses were used to account for the fact that data of interest were derived from repeated measures of subjects within the same visit and repeated across 5 visits for each subject.
Absolute deviation between planned dose to bowel and delivered dose was analyzed using mixed effects. At each 5-Gy increment from 15 Gy to 45 Gy, the volume of the SB that received that dose was divided by the respective volume from the planning CT scan. Any deviation between planned and delivered doses to SB of >10% was a priori designated as clinically relevant. Variability in dose to SB as a function of body mass index (BMI) was also analyzed. To assess the effect of bladder filling on SB sparing, regressions were performed at 5-Gy increments in prone and supine positions.
Results
The average BMI was 25.59 kg/m2 (standard deviation [SD], ±4.17; range, 19.7-31.9 kg/m2). Analyzable data were available for 118 of 120 CT scans (10 planning CT scans, 108 weekly CT scans). The mean PTV volume at planning was not significantly different between positions (1281.4 cm3 for prone vs 1264.2 cm3 for supine; P = .75). Average bladder volume was not significantly different between positions when analyzing all scans (178.3 for prone vs 164.0 for supine; P = .49).
Small bowel exposure
Across all scans (simulation and weekly), the average volume of SB loops contained within the PTV was significantly lower with prone positioning (28.9 cm3 prone vs 49.7 cm3 with supine positioning; P < .001). The volume of SB contained within the PTV was highly variable between the initial simulation and daily treatment in both positions (Fig 2). In general, the volume of SB within the PTV was lower at simulation than during treatment (supine average of 35.5 cm3 at simulation and 52.6 cm3 at treatment; prone average of 26.1 cm3 at simulation and 35.1 cm3 at treatment).
Figure 2.
Volume of small bowel loops contained within the planning target volume in the supine and prone positions for each patient. Scan 0 represents simulation. Note the different scale for the y-axis between patients.
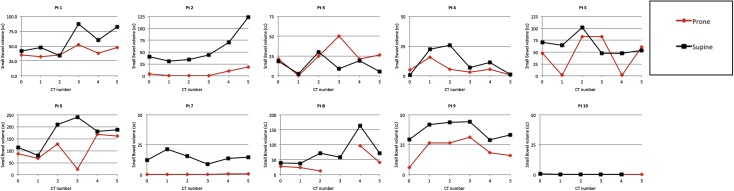
For 9 of 10 patients, prone positioning provided SB sparing superior to that of supine positioning for the initial plans (Fig 3). When the full course of treatment was evaluated, the total volume of SB that received 20 Gy to 45 Gy was significantly lower with prone positioning (Table 2, Fig 4). On average, prone positioning reduced the volume of SB receiving a given dose of radiation by 28% compared with supine positioning. Higher BMI was associated with significantly less SB receiving doses of 15 Gy to 30 Gy with prone positioning (P < .011 for each dose level).
Figure 3.
Cumulative small bowel dose estimates in the supine and prone positions for each patient. Each treatment plot represents an average of 5 scans.
Table 2.
Mean volume of small bowel (cm3) receiving doses from 15 Gy to 45 Gy in prone versus supine positions
| Volume | Prone | Supine | P-Value |
|---|---|---|---|
| V15 | 239.74 | 256.46 | .53 |
| V20 | 162.75 | 208.58 | .005 |
| V25 | 114.85 | 162.21 | <.001 |
| V30 | 82.81 | 126.07 | <.001 |
| V35 | 62.21 | 97.41 | <.001 |
| V40 | 46.46 | 76.67 | <.001 |
| V45 | 33.00 | 46.87 | .045 |
Figure 4.
Cumulative small bowel dose estimates for the entire group for simulation and all treatments (118 scans total). Error bars represent the group average of standard deviation for each patient. The P-values for intervals are indicated in Table 2.
Bladder filling
Average bladder volume did not differ significantly between treatment positions (178.3 cm3 for prone vs 163.8 cm3 for supine; P = .49). Increased bladder volume was weakly associated with lower volume of SB loops falling within the PTV (P = .012), but this was dependent on positioning. Specifically, within supine treatments, dose to SB was significantly correlated with bladder filling from V15 to V40 (P = .001-.013). For prone treatment, this relationship was not significant (P = .15-.24).
Deviation from planned dose
There were substantial deviations between planned and delivered doses to SB (>10% more or less than planned) that were observed with increasing frequency from 5 Gy up to 45 Gy. These deviations were observed in 68% of cases at 15 Gy and increased to 96% of treatments at 45 Gy. The results from χ2 analyses at each 5-Gy increment showed no significant association between positioning and clinically significant deviations (P = .05-.95). There was a significant interaction between BMI and treatment position for volumes receiving between 15 Gy to 25 Gy (P = .018-.027). These interactions showed a slight increase in deviation from the planned dose for patients with a higher BMI in the supine position. These effects were not present at doses >25 Gy. The minimum CTV dose at planning was 93.5% for prone plans and 91.6% for supine plans (P = .154). The minimum CTV coverage at the time of treatment did not differ significantly on the basis of treatment position (88% of prescription dose for prone vs 89% for supine; P = .544).
Discussion
Treatment planning comparisons have demonstrated that PBB significantly reduces the anticipated doses to SB with pelvic IMRT.5, 7, 9, 16 A systematic review of available studies conducted by Wiesendanger et al concluded that use of PBB results in a lower volume of irradiated SB compared with the supine position for both 3-dimensional conformal radiation therapy and IMRT plans.21 Despite the potential dosimetric advantages of prone positioning, use of this technique remains highly variable between centers and the clinical benefits of the combination have not been demonstrated.
To our knowledge, this study represents the only intraindividual comparison between prone and supine pelvic IMRT that addresses dosimetric differences at the time of simulation and on the day of treatment delivery. By using volumetric fan-beam CT imaging to estimate doses to SB for delivered treatment fractions, our analysis provides an accurate estimate of the expected SB dose in a range of patients who receive pelvic IMRT with pretreatment imaging. As our results demonstrate, PBB can provide additional SB sparing over supine pelvic IMRT throughout the course of treatment without observed dosimetric disadvantages.
Previous studies have demonstrated that pelvic radiation therapy in the prone position may result in greater systematic setup errors between tattoos and bony anatomy when compared with supine positioning.18, 22 Bayley et al conducted a randomized trial to compare prone and supine 3-dimensional conformal (non-IMRT) prostate cancer treatment and found increased day-to-day prostate motion with prone treatment. Given this positional variability, larger setup margins were used, which ultimately outweighed the dosimetric advantage of prone positioning.19 When analyzing the setup corrections between tattoos and bony anatomy, other authors have found less random setup error with PBB.22 However, it remains unclear whether interfraction setup errors are relevant when daily pretreatment imaging is used.6, 11, 23
Chen et al used daily megavoltage CT scans obtained from 9 patients with anal cancer who were treated supine with IMRT to estimate the target dose coverage and SB dose with and without image guided radiation therapy.23 On the basis of the degree of observed setup variations, the authors demonstrated that setup margins of >1 cm would be required to ensure adequate target coverage in the absence of pretreatment imaging. In contrast, image guided radiation therapy demonstrated consistent target coverage with 5 mm margins and was associated with significant reductions in V15 and V45. Using uniform 7 mm PTV margins, we found similar target dose coverage and SB dose variability in prone and supine treatment. Our findings support those of other authors and suggest that pretreatment imaging and rigid immobilization may negate the reproducibility concerns of prone positioning.
Interfraction small bowel dose variability
Dose recalculation studies have demonstrated significant variability between planned and delivered doses to SB.24, 25 Han et al performed weekly CT scans of patients with cervical cancer receiving pelvic IMRT in the prone position using an SB displacement device. They demonstrated significant changes in the SB dose throughout treatment, specifically that the SB dose consistently increased in later weeks of treatment.20 We observed a consistent increase in the volume of SB contained within PTV between simulation and treatment. This trended upward throughout treatment but was not as significant as in previous studies, which is possibly due to our inclusion of different disease sites. This could be a result of decreased rectal filling over time due to rectal irritation resulting from treatment.
The method of SB delineation (bowel loops vs peritoneal cavity) has been shown to affect the degree of SB sparing at the time of treatment delivery. Sanguineti et al performed dose recalculations on weekly CT scans in patients receiving whole pelvic IMRT. Separate plans were generated using either SB loops or peritoneal cavity as the primary organ at risk. They demonstrated a significantly higher interfraction variation in volume of SB receiving >45 Gy when individual loops were used for optimization.25 Our study used a peritoneal avoidance structure for optimization. Despite this approach, dose to SB loops at treatment consistently deviated from the original plan. In 3 of our patients, the SB DVH for each of the 5 subsequent scans fell more than 1 SD outside of the DVH from simulation (Fig 3; patients 7, 9, and 10). In the most extreme example, this correlated with a predicted SB V20 of 260 cm3 at simulation versus 426 cm3 on the day of treatment for a supine patient. A review of the isodose distributions suggests that these changes are more a result of the daily variability in the location of SB loops than a result of altered dose distribution within the patient. Variability of the SB volume contained within the PTV illustrates that SB position is highly inconsistent between scans (Fig 2). This is in line with the results from other authors who found that >20% of SB loops remain in the same location throughout the course of treatment.25, 26
In terms of GI toxicity, this may reduce the predictive value of dose to individual bowel loops at the time of simulation. These interfraction dose variations are likely to be of even greater clinical significance with hypofractionated regimens or stereotactic body radiation therapy, where there would be less forgiveness of daily dose variations. Ultimately, collection of daily volumetric imaging and correlation with clinical outcomes will be essential in future studies of pelvic and abdominal radiation therapy.
Positioning on belly board
Other authors have demonstrated that patient position relative to the belly board opening can greatly influence the degree of SB sparing with prone positioning.27 With optimal positioning, the lower border of the cutout lies at the level of the lumbosacral junction and displaces SB cranially (Fig 1). Although our use of rigid immobilization facilitated consistent positioning on the board, several patients were positioned high on the board. One patient (Fig 2; patient 4) was positioned with the pubic symphysis immediately at the board opening, which resulted in much of the SB falling into the pelvis. This patient had higher SB doses with PBB. High positioning on the belly board also appeared to be associated with greater interfraction variability in pelvic rotation. In contrast, pelvic rotation with prone positioning is negligible when the iliac crests are supported on the belly board.28
In aggregate, our findings are consistent with those of other authors and demonstrate the importance of optimal patient positioning and immobilization at the time of simulation for maximum bowel sparing and setup reproducibility.
The benefit of prone positioning in reducing low-intermediate doses (<50% of prescription) to SB has not been consistent across studies.5, 16, 17 Some of this variability may be due to differences in patient positioning and belly board construction. In our group, V15 was not significantly different between prone and supine positions, and 4 patients had significantly higher V15 and V20 with PBB (Fig 3; patients 1, 3, 4, and 8). Patients with high positioning on the belly board had the highest volumes of SB receiving low doses. Use of limited posterior arc delivery or static fields with only lateral and posterior fields has been advocated by some authors for prone IMRT.9 Although this could potentially improve low-intermediate SB dose in this scenario, ensuring optimal positioning at the time of simulation would likely result in greater SB sparing.
Influence of bladder volume
Increased bladder filling has been shown to decrease the SB dose during pelvic radiation therapy. Kim et al specifically evaluated the influence of bladder filling with PBB and demonstrated a significant reduction in SB dose with full versus empty bladder treatment.29 In our study, bladder volume was only weakly associated with SB dose and only in the supine position. This discrepancy may be attributable to differences in study design: Whereas Kim et al used a binary design with an initial scan performed with full bladder and a repeat scan immediately after voiding, in our study all patients were simply given standard bladder filling instructions. This resulted in significantly less variability in bladder volumes when compared with true full/empty bladder scans. Our findings indicate that PBB treatment can reduce SB dose over supine positioning when standard bladder filling instructions are used.
Study limitations
For our analysis, accumulated dose to SB for an entire treatment course was estimated from 5 weekly CT scans by recalculating the total prescription dose on the scan of the day and averaging the volumes of SB receiving a given dose. This was a pragmatic decision based on the limitations of our dataset (weekly on-treatment scans) and software constraints. The generation of true, cumulative DVHs would require daily imaging and a deformable image registration system that is capable of tracking individual segments of SB between multiple time points.
When designing this study, we arbitrarily chose a 10% deviation between planned and delivered dose to SB as “clinically significant.” In retrospect, this estimate was too stringent and may have decreased our sensitivity at detecting more significant variations in bowel dose between the 2 positions. Because contrast was not used for weekly scans and gross tumor was often not visible, each patient's target volumes were transferred from simulation scan to treatment scan without modification. Therefore, although our estimates for target dose coverage are likely valid for nodal coverage, they do not account for target deformation, which may potentially vary with treatment position. Lastly, the small number of patients within each disease site may reduce the generalizability of our results to specific disease subsites.
Conclusions
PBB can reduce the volume of small bowel receiving ≥20 Gy with pelvic IMRT. This benefit is seen at simulation and throughout the entire course of treatment. Prone IMRT is associated with interfraction dose variation to SB similar to that of supine positioning. Taken together, these findings suggest that prone positioning is an effective method for further maximizing SB sparing during pelvic IMRT. Validation in a larger series of patients and correlation with toxicity outcomes will be necessary to demonstrate the clinical benefit from prone positioning during pelvic IMRT.
Footnotes
Conflicts of interest: Dr. Tward reports personal fees from Myriad Genetics, GenomeDx, and Astellas/Medivation outside of the submitted work.
Supplementary material for this article (http://dx.doi.org/10.1016/j.adro.2017.01.005) can be found at www.practicalradonc.org.
Supplementary data
References
- 1.Creutzberg C.L., van Putten W.L., Koper P.C. The morbidity of treatment for patients with stage i endometrial cancer: Results from a randomized trial. Int J Radiat Oncol Biol Phys. 2001;51:1246–1255. doi: 10.1016/s0360-3016(01)01765-5. [DOI] [PubMed] [Google Scholar]
- 2.Kavanagh B.D., Pan C.C., Dawson L.A. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 3.Das I.J., Lanciano R.M., Movsas B., Kagawa K., Barnes S.J. Efficacy of a belly board device with CT-simulation in reducing small bowel volume within pelvic irradiation fields. Int J Radiat Oncol Biol Phys. 1997;39:67–76. doi: 10.1016/s0360-3016(97)00310-6. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh K., Padilla L.A., Murray K.P., Downs L.S., Carson L.F., Dusenbery K.E. Using a belly board device to reduce the small bowel volume within pelvic radiation fields in women with postoperatively treated cervical carcinoma. Gynecol Oncol. 2001;83:271–275. doi: 10.1006/gyno.2001.6295. [DOI] [PubMed] [Google Scholar]
- 5.Koelbl O., Richter S., Flentje M. Influence of patient positioning on dose-volume histogram and normal tissue complication probability for small bowel and bladder in patients receiving pelvic irradiation: A prospective study using a 3D planning system and a radiobiological model. Int J Radiat Oncol Biol Phys. 1999;45:1193–1198. doi: 10.1016/s0360-3016(99)00345-4. [DOI] [PubMed] [Google Scholar]
- 6.Shanahan T.G., Mehta M.P., Bertelrud K.L. Minimization of small bowel volume within treatment fields utilizing customized “belly boards”. Int J Radiat Oncol Biol Phys. 1990;19:469–476. doi: 10.1016/0360-3016(90)90559-3. [DOI] [PubMed] [Google Scholar]
- 7.Huh S.J., Kang M.K., Han Y. Small bowel displacement system-assisted intensity-modulated radiotherapy for cervical cancer. Gynecol Oncol. 2004;93:400–406. doi: 10.1016/j.ygyno.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero Urbano M.T., Henrys A.J., Adams E.J. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys. 2006;65:907–916. doi: 10.1016/j.ijrobp.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 9.Mok H., Crane C.H., Palmer M.B. Intensity modulated radiation therapy (IMRT): Differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol. 2011;6:63. doi: 10.1186/1748-717X-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nutting C.M., Convery D.J., Cosgrove V.P. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:649–656. doi: 10.1016/s0360-3016(00)00653-2. [DOI] [PubMed] [Google Scholar]
- 11.Portelance L., Chao K.S., Grigsby P.W., Bennet H., Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51:261–266. doi: 10.1016/s0360-3016(01)01664-9. [DOI] [PubMed] [Google Scholar]
- 12.Kidd E.A., Siegel B.A., Dehdashti F. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:1085–1091. doi: 10.1016/j.ijrobp.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Mundt A.J., Lujan A.E., Rotmensch J. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330–1337. doi: 10.1016/s0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi A.K., Sharma D.N., Rath G.K. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: A prospective randomized study. Int J Radiat Oncol Biol Phys. 2013;87:542–548. doi: 10.1016/j.ijrobp.2013.06.2059. [DOI] [PubMed] [Google Scholar]
- 15.Adli M., Mayr N.A., Kaiser H.S. Does prone positioning reduce small bowel dose in pelvic radiation with intensity-modulated radiotherapy for gynecologic cancer? Int J Radiat Oncol Biol Phys. 2003;57:230–238. doi: 10.1016/s0360-3016(03)00409-7. [DOI] [PubMed] [Google Scholar]
- 16.Nijkamp J., Doodeman B., Marijnen C., Vincent A., van Vliet-Vroegindeweij C. Bowel exposure in rectal cancer IMRT using prone, supine, or a belly board. Radiother Oncol. 2012;102:22–29. doi: 10.1016/j.radonc.2011.05.076. [DOI] [PubMed] [Google Scholar]
- 17.Stromberger C., Kom Y., Kawgan-Kagan M. Intensity-modulated radiotherapy in patients with cervical cancer. An intra-individual comparison of prone and supine positioning. Radiat Oncol. 2010;5:63. doi: 10.1186/1748-717X-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allal A.S., Bischof S., Nouet P. Impact of the “belly board” device on treatment reproducibility in preoperative radiotherapy for rectal cancer. Strahlenther Onkol. 2002;178:259–262. doi: 10.1007/s00066-002-0889-8. [DOI] [PubMed] [Google Scholar]
- 19.Bayley A.J., Catton C.N., Haycocks T. A randomized trial of supine vs. prone positioning in patients undergoing escalated dose conformal radiotherapy for prostate cancer. Radiother Oncol. 2004;70:37–44. doi: 10.1016/j.radonc.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Shin E.H., Huh S.J., Lee J.E., Park W. Interfractional dose variation during intensity-modulated radiation therapy for cervical cancer assessed by weekly CT evaluation. Int J Radiat Oncol Biol Phys. 2006;65:617–623. doi: 10.1016/j.ijrobp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Wiesendanger-Wittmer E.M., Sijtsema N.M., Muijs C.T., Beukema J.C. Systematic review of the role of a belly board device in radiotherapy delivery in patients with pelvic malignancies. Radiother Oncol. 2012;102:325–334. doi: 10.1016/j.radonc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Siddiqui F., Shi C., Papanikolaou N., Fuss M. Image-guidance protocol comparison: Supine and prone set-up accuracy for pelvic radiation therapy. Acta Oncol. 2008;47:1344–1350. doi: 10.1080/02841860802304564. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y.J., Suh S., Nelson R.A., Liu A., Pezner R.D., Wong J.Y. Setup variations in radiotherapy of anal cancer: Advantages of target volume reduction using image-guided radiation treatment. Int J Radiat Oncol Biol Phys. 2012;84:289–295. doi: 10.1016/j.ijrobp.2011.10.068. [DOI] [PubMed] [Google Scholar]
- 24.Kupelian P.A., Langen K.M., Zeidan O.A. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:876–882. doi: 10.1016/j.ijrobp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Sanguineti G., Little M., Endres E.J., Somani M.P., Parker B.C. Comparison of three strategies to delineate the bowel for whole pelvis imrt of prostate cancer. Radiother Oncol. 2008;88:95–101. doi: 10.1016/j.radonc.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Hysing L.B., Kvinnsland Y., Lord H., Muren L.P. Planning organ at risk volume margins for organ motion of the intestine. Radiother Oncol. 2006;80:349–354. doi: 10.1016/j.radonc.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Koelbl O., Vordermark D., Flentje M. The relationship between belly board position and patient anatomy and its influence on dose-volume histogram of small bowel for postoperative radiotherapy of rectal cancer. Radiother Oncol. 2003;67:345–349. doi: 10.1016/s0167-8140(03)00164-6. [DOI] [PubMed] [Google Scholar]
- 28.Cranmer-Sargison G., Kundapur V., Park-Somers E., Andreas J., Vachhrajani H., Sidhu N.P. Planning target volume margin evaluation and critical structure sparing for rectal cancer patients treated prone on a bellyboard. Clin Oncol (R Coll Radiol) 2013;25:e17–e22. doi: 10.1016/j.clon.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Kim T.H., Chie E.K., Kim D.Y. Comparison of the belly board device method and the distended bladder method for reducing irradiated small bowel volumes in preoperative radiotherapy of rectal cancer patients. Int J Radiat Oncol Biol Phys. 2005;62:769–775. doi: 10.1016/j.ijrobp.2004.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.