Abstract
Objective
The objective of this study was to present the treatment technique and evaluate clinical outcomes after intensity modulated radiation therapy (IMRT) for vulvar cancer.
Methods and materials
This retrospective study included 39 patients with squamous cell carcinoma of the vulva treated with IMRT from 2005 to 2015. There were 21 patients treated with postoperative IMRT, 13 with definitive IMRT, and 5 with preoperative IMRT. Tumor staging was Federation of Gynecology and Obstetrics stage I in 6, stage II in 7, stage III in 19, and stage IV in 7 patients. Concurrent chemotherapy was administered to 14 patients. Brachytherapy was delivered in 8 patients.
Results
The median follow-up was 34 months (range, 3.3-71). Median IMRT dose to patients receiving pre- or postoperative IMRT was 5040 cGy (range, 5040-6080). Median combined IMRT and brachytherapy dose to gross tumor was 7000 cGy (range, 5040-7520) in those treated with definitive RT. The 3-year locoregional control (LRC) and overall survival for those receiving postoperative RT were 89% and 67%, respectively. The 3-year LRC and overall survival for those receiving definitive IMRT were 42% and 49%, respectively. In patients receiving definitive or neoadjuvant IMRT, 69% had complete clinical response and 44% had complete pathologic response. The actuarial 3-year inguinal recurrence rate was 7%. There were no acute grade 3-4 hematological, gastrointestinal, or genitourinary toxicities. There were no late grade 3-4 gastrointestinal or genitourinary toxicities.
Conclusions
IMRT for vulvar cancer is associated with high rates of LRC in the postoperative setting and limited radiation-related toxicity. Durable LRC of disease after definitive IMRT remains challenging, and several refinements to our treatment technique are suggested.
Summary.
We present the treatment technique and outcomes from a retrospective review of 39 patients with squamous cell carcinoma of the vulva treated with intensity modulated radiation therapy (IMRT). The 3-year locoregional control was 89% in patients treated postoperatively and 42% in patients treated definitively. Complete pathologic response was observed in 44% of patients treated with definitive or neoadjuvant IMRT. There was limited acute and late toxicity.
Introduction
Radiation therapy (RT) has played an important role in the treatment of vulvar cancer in both the pre- and postoperative settings and the definitive setting.1, 2 Radiation has traditionally been administered in the supine, frog-legged position using opposed anteroposterior-posteroanterior field setup in addition to supplemental dose to the vulva or inguinal nodes as needed. Beriwal et al3 and Bloemers et al4 have shown in dosimetric studies that intensity modulated RT (IMRT) may be advantageous in the treatment of vulvar cancer because it may reduce dose to small bowel, rectum, bladder, and femurs.
The benefit of IMRT in reducing toxicity in pelvic malignancies has been demonstrated in multiple prospective phase 2 trials.5, 6 Additionally, phase 3 randomized trials comparing postoperative 3-dimensional conformal RT (3D-CRT) versus IMRT have been performed for cervical cancer in India7 and for cervical or endometrial cancer in a multinational setting.8 Preliminary data from these studies also suggest reduced acute or late toxicity with IMRT. IMRT may reduce dose to pelvic bones, which could in turn reduce hematologic toxicity for patients receiving concurrent chemotherapy.6 Beriwal et al have published extensively on the use of preoperative IMRT with concurrent chemotherapy for vulvar cancer, which resulted in a complete pathologic response rate (pCR) of 48.5% and limited treated-related toxicity.3, 9, 10 There is additional interest in the use of IMRT for vulva cancer after the publication of consensus contouring guidelines11 and National Comprehensive Cancer Network12 clinical guidelines allowing IMRT as a therapeutic option. The Gynecological Oncology Group (GOG) has opened a clinical trial (protocol 279) that prospectively investigates combined weekly gemcitabine and cisplatin chemotherapy with IMRT to 6400 cGy in patients with locally advanced vulvar cancer.13 The purpose of our study was to present our institutional experience of IMRT in the treatment of vulvar cancer with an emphasis on treatment technique, patient outcomes, and toxicity.
Methods and materials
Study population
The records of 39 patients with squamous cell carcinoma of the vulva treated with curative intent IMRT at Washington University in St. Louis School of Medicine from January 2005 to January 2015 were reviewed. All patients underwent a complete staging workup according to the International Federation of Gynecology and Obstetrics 2009 edition and American Joint Committee on Cancer, 7th edition, clinical staging criteria, including a full history and physical examination, routine laboratory evaluation, and metastatic evaluation. Patients underwent imaging with 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) (33 of 39), magnetic resonance imaging (10 of 39), and/or computed tomography (CT) (39 of 39). The data source for this study was the Washington University in St. Louis radiation oncology department's institutional review board–approved, retrospective registry of treatment data (institutional review board approval #20131149). The data were deidentified by an “honest broker” before research use.
Surgical resection
Surgical resection was performed in 26 patients, whereas 13 had a biopsy alone. A radical vulvectomy was performed in 16 patients, and 10 had a modified radical vulvectomy, hemivulvectomy, or wide local excision. Bilateral inguinal lymph node dissection was performed in 19 patients. Sentinel lymph node sampling was performed in 2 patients.
Radiation
All patients received IMRT with or without high-dose-rate (HDR) brachytherapy. Adjuvant postoperative IMRT was administered to patients with high-risk features such as surgical margins <8 to 10 mm, extracapsular extension, >1 positive lymph node, or lymphovascular space invasion.14 Definitive or neoadjuvant IMRT was performed for unresectable tumors, medically inoperable patients, or patients in which surgical resection had a high likelihood of unacceptable gastrointestinal (GI) or genitourinary (GU) toxicity. All patients underwent a treatment planning CT simulation for IMRT. Patients receiving definitive or neoadjuvant IMRT underwent FDG-PET/CT simulation. Diagnostic FDG-PET scans were fused with the planning CT to aid in treatment planning. Patients were routinely simulated in the supine position with the legs straight and were fitted with a customized immobilization device (Alpha Cradle, Smithers Medical Products, Inc, North Canton, OH) to minimize daily setup variability. Bolus on the vulva was not routinely used in the postoperative setting, but was used over the vulvar tumor and superficial or bulky lymph nodes in the definitive or preoperative setting. Treatment positioning was confirmed via daily 2-dimensional imaging as well as weekly cone beam CT.
IMRT planning
The gross tumor volume (GTV) as defined by FDG-PET and the clinical target volume (CTV), and organs at risk were contoured for treatment planning. The entire vulva was included in the vulvar CTV (CTVvulvar). The low pelvic vessels were contoured from 1 cm above the bifurcation of the common iliac artery and extended inferiorly to include the internal and external iliac vessels, the obturator space, and the inguinal region along the femoral vessels until the deep femoral artery branches and goes deep into the thigh. An isotropic 7-mm expansion was performed on the vessel contour, excluding the pelvic bones, to create the nodal CTV (CTVnodal). Of note, more recent consensus guidelines recommend contouring the CTVnodal in the inguinal region as a compartment rather than as a 7-mm isotropic expansion on the inguinal vessels; this will be discussed in detail later. The CTVnodal was edited to include FDG-avid or involved lymph nodes. The CTVvulvar and CTVnodal were combined to form the final CTV (CTVfinal) as seen in Fig 1. The planning target volume was defined as a 5-mm isotropic expansion on the CTVfinal.
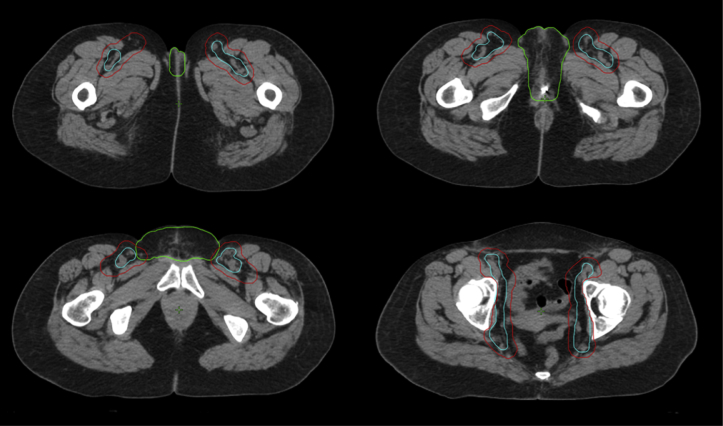
Figure 1.
Representative axial slices of vessel, nodal clinical target volume (CTV), and vulva CTV contours. Top left: inferior extent of vessel contour and vulva CTV contour. Top right: level of the partial vulvectomy site (marked by fiducial) and ischial ramus. Bottom left: level of the pubic symphysis. Bottom right: level of the femoral heads. Blue, vessel contour; green, vulva CTV; red, nodal CTV.
The IMRT prescription doses to the CTVfinal ranged from 5040 to 5120 cGy in 160 to 180 cGy per fraction. The GTV was typically treated to a total dose of 6000 to 7000 cGy via an integrated boost in 200 cGy per fraction. Some patients received a lower dose of 5040 cGy to the vulva GTV by IMRT; and additional dose was given later by brachytherapy to achieve a total dose of >6000 cGy. FDG-avid nodal disease typically received an integrated boost of 6000 to 6600 cGy in 180 to 200 cGy per fraction. The median doses and ranges for adjuvant and neoadjuvant IMRT were 5040 cGy (range, 4960-6080 cGy) and 5040 cGy (range, 5040-6000 cGy), respectively. In patients treated definitively, the median external beam dose to the vulva (not including dose from brachytherapy) was 6000 cGy (range, 5040-7000 cGy) and the median total definitive dose to the vulva including brachytherapy (8 patients with IMRT and brachytherapy; 5 patients with IMRT alone) was 7000 cGy (range, 5040-7520 cGy). The median IMRT dose to the involved FDG-avid lymph nodes was 6000 cGy (range, 5040-7000 cGy). Plans were created to limit the percentage of rectum receiving doses >40 Gy (V40) to <60%, bladder V45 Gy to <50%, bowel V40 Gy to <30%, unilateral kidney to <20 Gy, and spinal cord maximum dose to <45 Gy.
Brachytherapy
HDR iridium-192 interstitial brachytherapy was performed in 8 patients. We typically select patients with locally advanced disease, large tumor at diagnosis, or residual vulva disease after IMRT for brachytherapy consolidation. We intended to delivery brachytherapy to most patients treated with definitive IMRT because they typically fall within these criteria. Of the 5 patients treated with definitive IMRT who did not receive brachytherapy, the reasons for not performing brachytherapy consolidation included identification of distant progression before implant, death before completion of IMRT, declining performance status, patient noncompliance, and brachytherapy not indicated because of small tumor at initial diagnosis and complete response by IMRT. We used the brachytherapy method described by Dyk et al15 and placed interstitial catheters in the region of the vulvar tumor with the aid of a template. CT simulation was used in treatment planning, and a brachytherapy treatment plan with uniform loading was prescribed to cover the implant volume. The median HDR brachytherapy dose was 1200 cGy (range, 1000-2000 cGy) in 8 fractions delivered twice per day over a 4-day period. HDR brachytherapy was performed 3 weeks after completion of IMRT. The median combined dose to the vulva was 7120 cGy (range, 6240-7520 cGy) in patients who completed definitive IMRT and consolidative brachytherapy.
Chemotherapy
Concurrent chemotherapy was administered to 14 patients. The decision to include chemotherapy was made by the treating physician based on patient-specific criteria and was typically preferred for locally advanced disease. Of the patients that received chemotherapy, 13 received weekly cisplatin (40 mg/m2) while one patient received cisplatin (100 mg/m2, day 1) and 5-fluorouracil (1000 mg/m2/day, days 1-5) every 3 weeks.
Toxicity evaluation
Patients were retrospectively scored for acute and late toxicity using the Radiation Therapy Oncology Group (RTOG) toxicity criteria.16 Acute toxicities were defined as events occurring <90 days after initiation of RT, whereas late toxicities were defined as events occurring ≥90 days after the initiation of RT. Acute hematologic toxicities were retrospectively scored for those receiving chemotherapy.
Follow-up and response assessment
The patients were seen at 6 weeks, 3 months, 6 months, 12 months, and at least annually after completion of RT. Locoregional control (LRC) was defined as time until biopsy proven recurrence in the vulva or regional lymph nodes, with censoring at last follow-up or death. Overall survival (OS) was defined as time until death with censoring at last follow-up in patients still alive. Follow-up times used in the LRC and OS analysis were defined starting from the date of diagnosis. The date of diagnosis rather than date of completion of radiation was selected for the starting time to allow for a uniform comparison of patients treated with adjuvant, neoadjuvant, and definitive radiation. Complete clinical response (cCR) was defined as no evidence of disease noted on physical examination after completion of radiation. Complete pathological response (pCR) was defined as a biopsy or surgery of the vulva showing no carcinoma within 1 year of completion of radiation.
Statistical analysis
The Kaplan-Meier method was used to derive estimates of LRC and OS. The log-rank test and univariate analysis of Cox proportional hazards models were used to evaluate the impact of clinical factors and patient characteristics on LRC and OS. Multivariate analysis was not performed because of the relatively small number of patients. Patterns of recurrence and toxicity profiles were compared using the χ2 test or Fisher exact test, as appropriate. Statistical significance was considered as a P < .05. All levels of significance were 2-sided. Numerical P values are reported in tables if <.10 and marked as not significant if ≥.10.
Results
Patient characteristics
The median follow-up was 34 months (range, 3.3-71). Median age was 62 years (range, 27-93). International Federation of Gynecology and Obstetrics stage at diagnosis was stage I in 6 (15%), stage II in 7 (18%), stage III in 19 (49%), and stage IV in 7 (18%) patients. None had distant metastatic disease at diagnosis. IMRT to the vulva, pelvic lymph nodes, and inguinal lymph nodes was delivered to 35 (87%) patients and vulva-only IMRT was delivered to 4 (13%) patients. The median total duration of IMRT was 41 days. Two patients (5%) had prolonged duration of IMRT of ≥50 days; both of these patients later developed locoregional recurrence. Additional clinical and pathologic characteristics are summarized in Table 1.
Table 1.
Demographics and univariate analysis of LR control
| All patients |
LR recurrence |
LRC Cox univariate HR (95% CI) |
P | |||
|---|---|---|---|---|---|---|
| n | % (total) | n | % (row) | |||
| Age | ||||||
| <70 y | 28 | 72 | 6 | 21 | Reference | |
| ≥70 y | 11 | 28 | 3 | 27 | 2.16 (0.53-8.77) | NS |
| Grade | ||||||
| NA | 3 | |||||
| 1 | 4 | 11 | 1 | 25 | Reference | |
| 2 | 27 | 75 | 6 | 22 | 1.11 (0.13-9.43) | NS |
| 3 | 5 | 14 | 1 | 20 | 0.78 (0.05-13.08) | NS |
| FIGO stage | ||||||
| I | 6 | 15 | 0 | 0 | No events | |
| II | 7 | 18 | 2 | 29 | 1.30 (0.12-14.43) | NS |
| III | 19 | 49 | 6 | 32 | 1.74 (0.21-14.46) | NS |
| IV | 7 | 18 | 1 | 14 | Reference | |
| AJCC stage | ||||||
| T1-T2 N0 | 13 | 33 | 2 | 15 | Reference | |
| T3 N0 | 4 | 10 | 0 | 0 | No events | |
| T1-3 N1 | 10 | 26 | 2 | 20 | 1.22 (0.17-8.88) | NS |
| T1-3 N2 | 11 | 28 | 5 | 46 | 4.48 (0.82-24.47) | NS |
| T1-3 N3 or pelvic LN+ | 1 | 3 | 0 | 0 | No events | |
| Nodal status | ||||||
| Negative | 17 | 44 | 2 | 12 | Reference | |
| Positive | 22 | 56 | 7 | 32 | 3.20 (0.66-15.47) | NS |
| Treatment strategy | ||||||
| Definitive radiation | 13 | 33 | 5 | 39 | Reference | |
| Adjuvant radiation | 21 | 54 | 3 | 14 | 0.24 (0.06-0.99) | 0.05 |
| Neoadjuvant radiation | 5 | 13 | 1 | 20 | 0.41 (0.05-3.60) | NS |
| Surgical resection | ||||||
| No | 13 | 33 | 5 | 39 | Reference | |
| Yes | 26 | 67 | 4 | 15 | 0.27 (0.07-0.99) | 0.05 |
| Type of vulva surgery | ||||||
| Biopsy only | 13 | 33 | 5 | 39 | Reference | |
| WLE/HV/MRV | 10 | 26 | 1 | 10 | 0.19 (0.02-1.63) | NS |
| Radical vulvectomy | 16 | 41 | 3 | 19 | 0.31 (0.07-1.29) | NS |
| Radiation volume | ||||||
| Vulva only | 4 | 13 | 0 | 0 | No events | |
| Vulva, pelvis, inguinal | 35 | 87 | 9 | 26 | NS | |
| Radiation dose, Gy | ||||||
| ≤51.2 | 28 | 72 | 6 | 21 | Reference | |
| >51.2 | 11 | 28 | 3 | 27 | 1.32 (0.33-5.30) | NS |
| Brachytherapy | ||||||
| No | 31 | 80 | 7 | 23 | Reference | |
| Yes | 8 | 20 | 2 | 25 | 0.74 (0.15-3.61) | NS |
| Concurrent chemotherapy | ||||||
| No | 25 | 64 | 6 | 24 | Reference | |
| Yes | 14 | 36 | 3 | 21 | 0.96 (0.24-3.85) | NS |
| Tumor size | ||||||
| NA | 8 | |||||
| ≤3 cm | 18 | 58 | 2 | 11 | Reference | |
| >3 cm | 13 | 49 | 5 | 39 | 5.84 (1.09-31.41) | 0.04 |
| Surgical margin | ||||||
| NA | 15 | |||||
| ≥8 mm | 8 | 33 | 2 | 25 | Reference | |
| <8 mm | 10 | 42 | 1 | 10 | 0.54 (0.05-5.94) | NS |
| Positive | 6 | 25 | 1 | 17 | 0.58 (0.05-6.38) | NS |
| Depth of invasion | ||||||
| NA | 21 | |||||
| ≤9 mm | 13 | 72 | 3 | 23 | Reference | |
| >9 mm | 5 | 28 | 1 | 20 | 1.07 (0.11-10.58) | NS |
| Lymphovascular invasion | ||||||
| NA | 16 | |||||
| Negative | 12 | 52 | 3 | 25 | Reference | |
| Positive | 11 | 48 | 2 | 18 | 0.93 (0.15-5.56) | NS |
LR, locoregional; LVSI, lymphovascular space invasion; NA, unknown or not applicable; NS, not statistically significant; WLE/HV/MRV, wide local excision/hemivulvectomy/modified radical vulvectomy.
P-values in bold indicate statistically significant associations.
LRC after postoperative IMRT
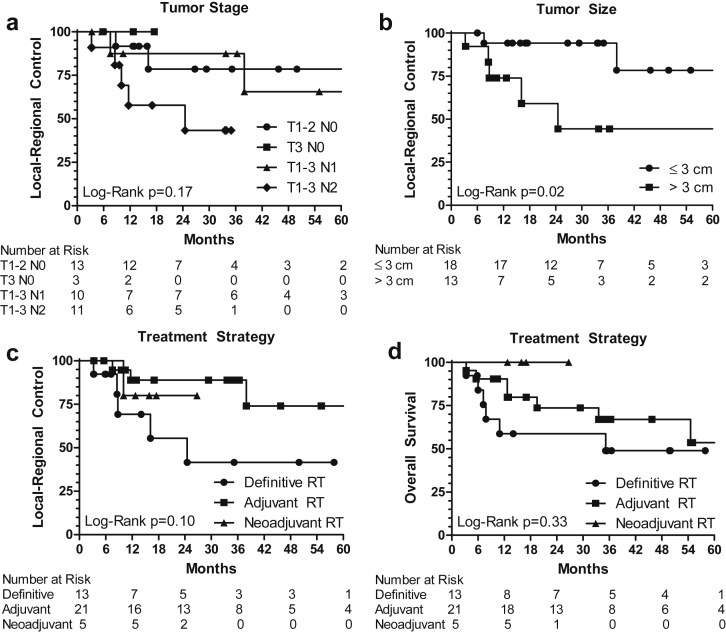
Twenty-one patients received postoperative IMRT. Locoregional recurrence occurred in 3 patients; of these, 2 were in the vulva and 1 was in an inguinal node. The median time to locoregional recurrence was 12 months (range, 7.5-38). The 3-year actuarial LRC rate was 89% for those receiving postoperative IMRT as shown in Fig 2. The crude rates of vulvar and inguinal recurrence were 10% (2 of 21) and 5% (1 of 21), and the 3-year actuarial rates of vulvar and inguinal recurrence were 5% and 6%, respectively. Of the 2 patients with vulva recurrence, the dose to the vulva was 5940 cGy in a patient with a positive surgical margin and 5120 cGy in a patient with a widely negative margin. The inguinal nodal recurrence occurred at a site of a previously resected 3-cm lymph node with extracapsular extension; the inguinal radiation dose was 5040 cGy. On Cox univariate analyses, surgical resection of the primary vulvar tumor (hazard ratio [HR], 0.27; 95% confidence interval [CI], 0.07-0.99, P = .05) was associated with improved LRC and tumor size >3 cm (HR, 5.84; 95% CI, 1.09-31.41; P = .04) were associated with worse LRC, as seen in Table 1. Additional information on patterns of recurrence is in Table 2.
Figure 2.
Kaplan-Meier curves for locoregional control and overall survival by tumor stage, tumor size, and treatment strategy. (A) Locoregional control by American Joint Committee on Cancer stage. (B) Locoregional control by tumor size. (C) Locoregional control by treatment strategy. (D) Overall survival by treatment strategy. RT, radiation therapy.
Table 2.
Patterns of recurrence
| No recurrence |
Vulva |
Inguinal |
Distant |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % (row) | n | % (row) | n | % (row) | n | % (row) | |
| All patients | 27 | 69 | 7 | 18 | 2 | 5 | 3 | 8 |
| FIGO stage | ||||||||
| I | 6 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| II | 5 | 71 | 2 | 29 | 0 | 0 | 0 | 0 |
| III | 11 | 58 | 4 | 21 | 2 | 11 | 2 | 11 |
| IV | 5 | 71 | 1 | 14 | 0 | 0 | 1 | 14 |
| AJCC stage | ||||||||
| T1-T2 N0 | 11 | 85 | 2 | 15 | 0 | 0 | 0 | 0 |
| T3 N0 | 4 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| T1-3 N1 | 6 | 60 | 2 | 20 | 0 | 0 | 1 | 10 |
| T1-3 N2 | 6 | 55 | 3 | 27 | 2 | 18 | 1 | 9 |
| T1-3 N3 or pelvic LN+ | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100 |
| Treatment strategy | ||||||||
| Definitive radiation | 7 | 54 | 4 | 31 | 1 | 8 | 2 | 15 |
| Adjuvant radiation | 16 | 76 | 2 | 10 | 1 | 5 | 1 | 5 |
| Neoadjuvant radiation | 4 | 80 | 1 | 20 | 0 | 0 | 0 | 0 |
| Brachytherapy | ||||||||
| No | 21 | 68 | 6 | 19 | 1 | 3 | 3 | 10 |
| Yes | 6 | 75 | 1 | 13 | 1 | 13 | 0 | 0 |
| Concurrent chemotherapy | ||||||||
| No | 17 | 68 | 4 | 16 | 2 | 8 | 1 | 4 |
| Yes | 10 | 71 | 3 | 21 | 0 | 0 | 2 | 14 |
FIGO, International Federation of Gynecology and Obstetrics; RTOG, Radiation Therapy Oncology Group.
LRC after definitive IMRT
Thirteen patients received definitive IMRT. Locoregional recurrence occurred in 5 patients receiving definitive IMRT; of these, 4 were in the vulva and 1 was in an inguinal lymph node. The median time to locoregional recurrence was 9 months (range, 3.2-25). The 3-year actuarial LRC rate after definitive IMRT was 42%, as shown in Fig 2. The crude rates of vulvar and inguinal recurrence were 31% (4 of 13) and 8% (1 of 13), and the 3-year actuarial rates of vulvar and inguinal recurrence were 53% and 12%, respectively. The actuarial rates are higher than the crude rates because of patients lost to follow-up or death from metastatic disease or other causes before 3 years. Two of the patients who developed recurrent vulvar disease initially received 5040 cGy by IMRT and no consolidation because of noncompliance in 1 patient and decline in performance status as well as local progression of tumor, making it not amenable to consolidative brachytherapy or surgery, in another patient. The other 2 patients with vulvar recurrence initially received 6600 cGy by IMRT for an 8-cm vulvar tumor or 5040 cGy by IMRT followed by 2000 cGy brachytherapy for a 6-cm vulvar tumor. The inguinal nodal recurrence occurred within a 4-cm FDG-avid lymph node that was treated to 6000 cGy.
Brachytherapy was delivered after IMRT in 8 patients. The cumulative dose was 5040 cGy by IMRT plus a median of 2000 cGy (range, 1200-2000 cGy) by brachytherapy in 3 patients and 6000 cGy by IMRT plus a median of 1200 cGy (range, 1000-1520 cGy) by brachytherapy in 5 patients. Among the patients who completed definitive treatment with IMRT and brachytherapy, there was 1 vulvar recurrence, and the crude and actuarial 3-year vulvar recurrence rates were 13% and 15%, respectively. None of the 5 patients who successfully completed definitive treatment of 6000 cGy by IMRT followed by brachytherapy consolidation to a total dose of 7000 cGy or higher developed vulvar recurrence at a median follow-up of 25 months (range, 8.5-60).
LRC after neoadjuvant IMRT
Five patients were treated with neoadjuvant IMRT. One vulvar recurrence occurred, and the crude and 3-year actuarial LRC rates in this group were both 80%. This patient was initially treated to a dose of 5040 cGy followed by radical vulvectomy, which showed residual tumor in the vulva and inguinal nodes in the pathology specimen. This patient developed a vulvar recurrence 10 months after treatment.
Treatment response
Clinical response and pathologic response could be assessed from available reports in 16 (89%) and 13 (72%) patients, respectively, of the 18 patients who received neoadjuvant or definitive IMRT. cCR was observed in 61% (11 of 18) of the patients overall and 69% (11 of 16) when adjusted for the number of evaluable patients. pCR was observed in 44% (8 of 18) of patients overall and 62% (8 of 13) when adjusted for the number of evaluable patients. Concurrent chemotherapy with IMRT yielded a 78% (7 of 9) rate of cCR compared with 57% (5 of 7) for IMRT alone in evaluable patients. Concurrent chemotherapy yielded a 67% (4 of 6) rate of pCR compared with 57% (4 of 7) for IMRT alone in evaluable patients. The use of concurrent chemotherapy was not statistically significant for increased odds of cCR (P = .38) or pCR (P = .73) as assessed by logistic regression compared with patients treated with IMRT alone. cCR and pCR were not significantly associated with improved LRC (P = .50 and P = .43, respectively) or OS (P = .37 and P = .44, respectively), as assessed by univariate Cox regression. Five patients could not be evaluated for pathologic response: 1 patient died of sepsis related to chemoradiation and another died of unrelated end-stage renal disease in the follow-up period. The remaining 3 patients who could not be assessed for pCR had cCR but did not receive vulvar biopsy or surgery within 1 year. One of these patients developed vulvar recurrence after 1 year, 1 developed distant metastatic disease, and the final patient was free from recurrence at 14 months of follow-up.
Overall survival
At the time of last follow-up, 5 patients had died of disease and 3 patients had died of intercurrent disease. The actuarial OS rates of the entire cohort were 82%, 72%, and 68% at 1, 2, and 3 years, respectively. The 3-year actuarial OS rate was 67% for those receiving postoperative irradiation, 49% for those receiving irradiation alone, and 100% for those receiving preoperative irradiation, as shown in Fig 2. On univariate analysis, age >70 years was associated with worse OS (HR, 5.15; 95% CI, 1.62-16.36; P < .01). Development of distant metastatic disease was associated with worse OS (HR, 15.42; 95% CI, 3.04-78.27; P = .01), but locoregional recurrence was not significantly correlated with OS (P = .26).
Distant metastasis
At last follow-up, 3 (8%) patients developed distant metastasis, with 2 occurring in patients treated with definitive radiation and 1 in a patient treated with adjuvant radiation. Median time to distant recurrence was 6 months (range, 3.2-7.2 months). One patient had metastatic disease to the lung, 1 to the liver, and 1 had widespread disease, including the liver, lung, and bone. No clinical variables were associated with distant metastatic disease on univariate analysis.
Toxicity
Treatment-related toxicities are shown in Table 3. Grade 2 or greater acute dermatologic toxicity was seen in 36 (92%) patients. No patients developed acute or late grade 3-4 GI or GU toxicities. All acute and late GI toxicity and lymphangitis occurred in patients receiving IMRT to both the vulva and regional lymph nodes. Of the 14 patients who received concurrent chemotherapy, 5 (36%) developed acute grade 2 leukopenia and 3 (21%) developed acute grade 2 anemia. There were no acute grade 3-4 hematologic toxicities. In regard to late skin toxicity, 2 (5%) patients developed lymphangitis, and no patients had vulvar necrosis or nonhealing ulceration. One death occurred while a patient was receiving definitive chemoradiation therapy for a cT3N2M0, 9-cm vulvar squamous cell carcinoma with rectal and anal involvement. Weekly concurrent cisplatin and daily IMRT was stopped at 4800 cGy (planned, 6080 cGy) after the patient developed a rectovaginal fistula. She received a diverting colostomy 3 days after her last IMRT fraction. Unfortunately, she developed sepsis and died postoperatively.
Table 3.
Acute and late toxicity
| Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Hematological | ||||||||||||
| WBC | 7 | 50 | 2 | 14 | 4 | 28 | 0 | 0 | 0 | 0 | 1 | 7 |
| Platelets | 12 | 86 | 2 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutrophils | 13 | 93 | 0 | 0 | 1 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemoglobin | 9 | 64 | 2 | 14 | 3 | 21 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acute skin | 3 | 8 | 9 | 23 | 27 | 69 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acute GI | 24 | 62 | 2 | 5 | 13 | 33 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acute GU | 33 | 85 | 2 | 5 | 4 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Late GI | 37 | 95 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Late GU | 37 | 95 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Fourteen patients treated with concurrent chemotherapy could be evaluated for hematological toxicity. Other toxicity could be evaluated in 39 patients. Percentages are of the total number of patients in the row of the table.
GI, gastrointestinal; GU, genitourinary; WBC, white blood cell.
Discussion
In our study, surgical resection of the primary vulvar tumor was associated with improved LRC. Postoperative radiation may also improve LRC by treating residual or microscopic disease in patients with high-risk features as defined by Heaps et al,14 or with close or positive margins.17 Dusenbery et al reported that full-dose radiation to the vulva was necessary in patients with locally advanced disease, even after radical vulvectomy.18 Additionally, Viswanathan et al reported that adjuvant RT doses >5600 cGy were associated with improved LRC, especially in the setting of close or positive margins.19 Furthermore, a review of the National Cancer Database suggests that patients with node-positive vulvar cancer after surgery may benefit from chemotherapy in addition to adjuvant radiation20 and that the optimal postoperative radiation dose for margin positive vulvar cancer is likely in the range of 5400 to 6000 cGy.21 Data in the literature are limited on the use of IMRT in the adjuvant setting, and previous studies of adjuvant 3D-CRT reported LRC rates of 67% to 82%.17, 18, 19 In our study, we observed a 3-year LRC of 89% in patients treated with adjuvant IMRT, suggesting that the increased conformality of adjuvant IMRT does not compromise LRC in the adjuvant setting.
Definitive or neoadjuvant chemoradiation are also reasonable treatment strategies in patients who do not qualify for primary surgery. The goal of radiation in this setting is to induce a complete tumor response or to decrease the extent of tumor to allow for resection. These strategies are supported by a Cochran review that found no difference in survival between primary chemoradiation and surgery.22 GOG 205, a phase 2 trial of neoadjuvant 3D conformal chemoradiation with weekly cisplatin, had a cCR rate of 64% and a pCR rate of 50%.23 IMRT in the neoadjuvant setting has been extensively investigated by Beriwal et al, who treated patients with twice-daily IMRT to a median dose of 4640 cGy concurrent with cisplatin/5-fluorouracil.3, 9, 10 They observed a pCR rate of 48.5% and 3-year recurrence free survival of 65.9%. These promising results have led to an ongoing phase 2 trial, GOG 279, which is investigating definitive IMRT to 6400 cGy with concurrent weekly gemcitabine and cisplatin. At our institution, in patients receiving definitive radiation, we delivered a median total dose from IMRT plus brachytherapy of 7000 cGy to the vulvar tumor. In our series, these patients achieved an unadjusted cCR rate of 61% and an unadjusted pCR rate of 44%, which are similar to the response rates of prior studies of radiation with or without IMRT. Despite these favorable initial response rates, LRC in patients treated with definitive radiation was unsatisfactory at 42% at 3 years. Surgery either before or after radiation is associated with improved local control in our study, and should be considered for patients with vulvar cancer. For patients who cannot undergo surgery, additional therapy such as concurrent chemotherapy, dose escalation, and consolidative brachytherapy may be considered. Additional research is needed to determine patient selection and evaluate the efficacy of these treatment strategies.
In our series, some patients who later developed vulvar recurrence after definitive radiation were treated with an initial IMRT dose of 5040 cGy and planned for consolidation by brachytherapy or surgery, but they later received no further local therapy for various reasons, such as noncompliance or early tumor progression. Patients completing a course of definitive IMRT to a dose of 6000 cGy followed by brachytherapy did not develop vulvar recurrence; therefore, in patients who are treated with definitive or potentially preoperative radiation, an external beam dose to the vulvar tumor GTV of less than 6000 cGy should be discouraged. Additional research investigating brachytherapy consolidation in vulvar cancer is warranted. We also observed that patients with a prolonged IMRT treatment time of >50 days also had a high recurrence rate. Furthermore, quality control checks should be considered for patients treated with definitive IMRT to avoid the possibility of unintentionally underdosing the vulvar tumor. These checks could include in vivo dose measurement using a thermoluminescent dosimeter, verification of the bolus positioning at the time of the first fraction, cone beam CT imaging, and monitoring for the necessity of treatment replanning because of tumor shrinkage, vulvar edema, or other changes in anatomy.
Appropriate radiation dose coverage of the inguinal lymph nodes has long been a topic of interest in vulvar cancer. GOG 88 demonstrated that a high rate of recurrence was attributed to underdosing of inguinal lymph nodes.24, 25 More recently, Glaser et al26 have expressed concern regarding appropriate nodal coverage in modern radiation protocols, especially after an interim analysis of the Observation in Patients With Early-Stage Vulvar Cancer Undergoing Sentinel Lymph Node Dissection (GROINSS-V) II trial found an inguinal recurrence rate of 12.2%, a higher rate than expected.27 It is important to note that GROINSS-V II used 3D-CRT planning and a 5-mm CTV margin around the femoral vessels; additionally, FDG-PET was not required as part of staging or treatment planning. This differs from Kim et al's analysis that 2-cm margins around the femoral vessels are required for coverage of potentially involved lymph nodes.28 Additionally, in the ongoing GOG 279 trial, the inguinal region is defined as a compartment similar to the method described in the RTOG contouring guidelines for anal cancer.29 Recently published vulvar cancer consensus guidelines11 and National Comprehensive Cancer Network guidelines12 also advocate contouring of the inguinal nodes as a compartment.
We defined the inguinal nodal CTV as a 7-mm expansion of the femoral vessel contour; planning target volume was an additional 5-mm expansion. These definitions have historically been used at our institution but were smaller than those advocated by recent guidelines11, 12; however, as described previously, these expansions were associated with a 3-year recurrence rate of 7% in the inguinal nodes. The actuarial rate of inguinal recurrence at 3 years was 6% (crude rate of 5%, 1 of 21) in patients treated with postoperative radiation and 12% (crude rate of 8%, 1 of 13) in patients treated with definitive radiation. However, the single nodal recurrence in the patient treated for definitive radiation was within a previously involved node, and therefore larger CTV expansions would not have influenced the outcome in this patient. A potential reason for our recurrence rate may be the use of FDG-PET/CT in the guidance of IMRT treatment volumes. A subset of patients in the series by Beriwal et al had treatment planning with FDG-PET/CT; the authors also reported a low rate of inguinal recurrence of 0%.9 However, because our study has a small number of patients with limited follow-up, additional data are needed on whether treatment volumes can be reduced with FDG-PET/CT imaging. We acknowledge that the recently published consensus guidelines, which recommend including the entire inguinal compartment in the CTV, are the most conservative approach to ensure coverage of the inguinal nodal region.11 The dose to the sites of inguinal recurrences in our study were 5040 cGy in a patient with a resected 3-cm lymph node with extracapsular extension and 6000 cGy in a patient with a previously unresected 4-cm lymph node. Dose escalation by IMRT above these values for future patients with extracapsular extension or unresected lymph nodes may be considered. We present suggested doses to the vulvar primary tumor, elective lymph nodes, and involved lymph nodes (Table 4) based on our experience with IMRT, as presented here, and taking into account data from other published studies.
Table 4.
Suggested doses to the vulva, nodal elective volume, and nodal boost
| Vulva GTV dose | Elective nodes | Nodal boost | |
|---|---|---|---|
| Postoperative radiation | |||
| Negative margins and no ECE | 5040-5120 cGy | 5040-5120a cGy | None |
| Vulva positive margin | 6000 cGy | 5040-5120a cGy | None |
| Node with ECE | 5040-5120 cGy | 5040-5120a cGy | 6000 cGy |
| Definitive radiation | |||
| No brachytherapy | 6000-7000b cGy | 5040-5120a cGy | 6600c cGy |
| With brachytherapy | 6000b cGy (IMRT) + 1200d cGy (BT) | 5040-5120a cGy | 6600c cGy |
BT, brachytherapy; ECE, extracapsular extension; GTV, gross tumor volume; IMRT, intensity modulated radiation therapy.
Dose per fraction of the elective volumes are adjusted to maintain 200 cGy per fraction in the integrated boost volume.
Consider increased dose to vulva tumor GTV of 6600 or 7000 cGy for large tumors.
Consider higher dose of 7000 cGy for large lymph nodes.
Consider brachytherapy dose of 1600 cGy or higher if residual disease is present after IMRT.
A goal of IMRT treatment is to reduce dose to organs at risk and therefore decrease treatment-related toxicity. The series by Beriwal et al reported no grade ≥3 chronic GI or GU toxicity.9 We also observed no late grade 3 or greater GI or GU toxicities. Additionally, in patients receiving concurrent chemotherapy, we observed no grade 3-4 hematological toxicity. Rates of grade 2 leukopenia and anemia were 36% and 21%, respectively, which are comparable to the hematological toxicity rates in a trial investigating IMRT for cervical cancer.6 It is important to note, however, that 1 death from sepsis was observed shortly after chemoradiation; therefore, caution should be used when considering concurrent chemoradiation in elderly or frail patients.
A limitation of this study includes the relatively short median follow-up time of 34 months, which precludes us from evaluating long-term outcomes. Additionally, the decision between treatment with definitive radiation, adjuvant radiation, and neoadjuvant radiation was not based on a randomization but on an individualized plan using tumor and patient characteristics. As a result, a comparison of the relative efficacy of these strategies may be confounded by selection bias. We allowed negative biopsies to be included in our calculation of pCR, which may be problematic because of sampling error. Our observed toxicities post-IMRT treatment may be underreported when evaluated retrospectively. Finally, as discussed previously, our data cannot establish whether a 7-mm expansion on the inguinal vessels is sufficient for the CTV because of the limited follow-up and the small number of patients.
Conclusion
Our data add to the published literature on IMRT by supporting its use in the postoperative setting. Definitive treatment of vulvar cancer remains challenging, and the LRC rates for patients treated without surgery were unsatisfactory. We suggest several potential refinements to our definitive treatment technique based on our patterns of recurrence. IMRT generally resulted in a low incidence of severe side effects. Treatment of vulvar cancer with IMRT is feasible and may be considered in future patients. Additional studies are needed to evaluate the efficacy and benefit of this treatment technique.
Footnotes
Conflicts of interest: None.
References
- 1.Perez C.A., Grigsby P.W., Chao C. Irradiation in carcinoma of the vulva: Factors affecting outcome. Int J Radiat Oncol Biol Phys. 1998;42:335–344. doi: 10.1016/s0360-3016(98)00238-7. [DOI] [PubMed] [Google Scholar]
- 2.Perez C.A., Grigsby P.W., Galakatos A. Radiation therapy in management of carcinoma of the vulva with emphasis on conservation therapy. Cancer. 1993;71:3707–3716. doi: 10.1002/1097-0142(19930601)71:11<3707::aid-cncr2820711139>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Beriwal S., Heron D.E., Kim H. Intensity-modulated radiotherapy for the treatment of vulvar carcinoma: A comparative dosimetric study with early clinical outcome. Int J Radiat Oncol Biol Phys. 2006;64:1395–1400. doi: 10.1016/j.ijrobp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bloemers M.C.W.M., Portelance L., Ruo R., Parker W., Souhami L. A dosimetric evaluation of dose escalation for the radical treatment of locally advanced vulvar cancer by intensity-modulated radiation therapy. Med Dosim. 2012;37:310–313. doi: 10.1016/j.meddos.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Kachnic L.A., Winter K., Myerson R.J. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klopp A.H., Moughan J., Portelance L. Hematologic toxicity in RTOG 0418: A phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90. doi: 10.1016/j.ijrobp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra S., Engineer R., Mahantshetty U.M. Phase III RCT of Postoperative Adjuvant Conventional Radiation (3DCRT) Versus IGIMRT for Reducing Late Bowel Toxicity in Cervical Cancer (PARCER) ( NCT01279135/CTRI2012/120349): Results of interim analyses. Int J Radiat Oncol Biol Phy. 2015;93:S4. [Google Scholar]
- 8.Klopp A., Yeung A., Deshmukh S. A phase III randomized trial comparing patient-reported toxicity and quality of life (QOL) during pelvic intensity modulated radiation therapy as compared to conventional radiation therapy. Int J Radiat Oncol Biol Phys. 2016;96:S3. [Google Scholar]
- 9.Beriwal S., Shukla G., Shinde A. Preoperative intensity modulated radiation therapy and chemotherapy for locally advanced vulvar carcinoma: Analysis of pattern of relapse. Int J Radiat Oncol Biol Phys. 2013;85:1269–1274. doi: 10.1016/j.ijrobp.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Beriwal S., Coon D., Heron D.E. Preoperative intensity-modulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol. 2008;109:291–295. doi: 10.1016/j.ygyno.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Gaffney D.K., King B., Viswanathan A.N. Consensus recommendations for radiotherapy contouring and treatment of vulvar carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:1191–1200. doi: 10.1016/j.ijrobp.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Clinical practice guidelines in oncology: Vulvar cancer. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp Available at: Accessed March 1, 2017.
- 13.Radiation therapy, gemcitabine hydrochloride, and cisplatin in treating patients with locally advanced squamous cell carcinoma of the vulva. https://clinicaltrials.gov/ct2/show/NCT01595061 Available at: Accessed March 1, 2017.
- 14.Heaps J.M., Fu Y.S., Montz F.J., Hacker N.F., Berek J.S. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38:309–314. doi: 10.1016/0090-8258(90)90064-r. [DOI] [PubMed] [Google Scholar]
- 15.Dyk P.T., Richardson S., Badiyan S.N. Outpatient-based high-dose-rate interstitial brachytherapy for gynecologic malignancies. Brachytherapy. 2015;14:231–237. doi: 10.1016/j.brachy.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 17.Faul C.M., Mirmow D., Huang Q., Gerszten K., Day R., Jones M.W. Adjuvant radiation for vulvar carcinoma: Improved local control. Int J Radiat Oncol Biol Phys. 1997;38:381–389. doi: 10.1016/s0360-3016(97)82500-x. [DOI] [PubMed] [Google Scholar]
- 18.Dusenbery K.E., Carlson J.W., LaPorte R.M. Radical vulvectomy with postoperative irradiation for vulvar cancer: Therapeutic implications of a central block. Int J Radiat Oncol Biol Phys. 1994;29:989–998. doi: 10.1016/0360-3016(94)90393-x. [DOI] [PubMed] [Google Scholar]
- 19.Viswanathan A.N., Pinto A.P., Schultz D., Berkowitz R., Crum C.P. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol Oncol. 2013;130:545–549. doi: 10.1016/j.ygyno.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 20.Gill B.S., Bernard M.E., Lin J.F. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A National Cancer Data Base (NCDB) analysis. Gynecol Oncol. 2015;137:365–372. doi: 10.1016/j.ygyno.2015.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Chapman B.V., Gill B.S., Viswanathan A.N., Balasubramani G.K., Sukumvanich P., Beriwal S. Adjuvant radiation therapy for margin-positive vulvar squamous cell carcinoma: Defining the ideal dose-response using the National Cancer Data Base. Int J Radiat Oncol Biol Phys. 2017;97:107–117. doi: 10.1016/j.ijrobp.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Shylasree T.S., Bryant A., Howells R.E. Chemoradiation for advanced primary vulval cancer. Cochrane Database Syst Rev. 2011;4:CD003752. doi: 10.1002/14651858.CD003752.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore D.H., Ali S., Koh W.-J. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;124:529–533. doi: 10.1016/j.ygyno.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Koh W.J., Chiu M., Stelzer K.J. Femoral vessel depth and the implications for groin node radiation. Int J Radiat Oncol Biol Phys. 1993;27:969–974. doi: 10.1016/0360-3016(93)90476-c. [DOI] [PubMed] [Google Scholar]
- 25.Stehman F.B., Bundy B.N., Thomas G. Groin dissection versus groin radiation in carcinoma of the vulva: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1992;24:389–396. doi: 10.1016/0360-3016(92)90699-i. [DOI] [PubMed] [Google Scholar]
- 26.Glaser S., Olawaiye A., Huang M., Beriwal S. Inguinal nodal region radiotherapy for vulvar cancer: Are we missing the target again? Gynecol Oncol. 2014;135:583–585. doi: 10.1016/j.ygyno.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Groningen International study on sentinel nodes in vulvar cancer (GROINSS-V) II: An observational study. May 2011. http://www.dgog.nl/images/stories/studies/groinssvii/groinssviistudyprotocolversion07052011.pdf Available at: Accessed March 1, 2017. [DOI] [PubMed]
- 28.Kim C.H., Olson A.C., Kim H., Beriwal S. Contouring inguinal and femoral nodes; how much margin is needed around the vessels? Pract Radiat Oncol. 2012;2:274–278. doi: 10.1016/j.prro.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Myerson R.J., Garofalo M.C., El Naqa I. Elective clinical target volumes for conformal therapy in anorectal cancer: A Radiation Therapy Oncology Group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824–830. doi: 10.1016/j.ijrobp.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]