Abstract
Background
Clinical data that support stereotactic body radiation therapy (SBRT) metastatic malignant melanoma (MM) are limited. Furthermore, functional imaging with 18F-fludeoxyglucose positron emission tomography (PET) may offer a more accurate post-SBRT assessment. Therefore, we assessed the clinical outcomes and metabolic response of metastatic MM after SBRT.
Methods and materials
Patients with MM who were treated with SBRT and had pre- and post-PET scans (>1) were included in this study. A total of 390 pre- and post-SBRT PET/computed tomography (CT) scans for 80 metastases were analyzed. The PET metabolic response was evaluated per the PET Response Criteria in Solid Tumors (PERCIST), version 1.0, criteria. Single-fraction equivalent dose (SFED) was calculated as per the standard. The Kaplan-Meier method was used for estimates of overall survival (OS) and progression-free survival. The cumulative incidence method was used to estimate metastasis control (MC). A Wilcoxon test was used to compare survival estimates. The prognostic factors for MC and OS were assessed using the Cox proportional hazards model, and the Likelihood Ratio was also used for comparisons between groups.
Results
A median of 6 PET scans (range, 2-6 scans) was evaluated for each metastasis. The median SFED was 42.8 Gy (range, 18-56.4 Gy) and the median biologically effective dose was 254.4 Gy2.5 (range, 100.8-540 Gy2.5). Twenty percent of patients received chemotherapy and 59% received immunotherapy: granulocyte-macrophage colony-stimulating factor (64%) and ipilimumab (34%). MC was 94% and 90% at 1 year and 3 years, respectively. The OS was 74% and 27% and 1 year and 3 years, respectively. Complete response was achieved in 90% at a median of 2.8 months (range, 0.4-25.2 months). SFED >24 Gy correlated with improved MC (93% vs 75%, P = .01). Acute and late grade 3+ toxicities were 4% and 11%, respectively, with no grade 5 toxicity.
Conclusions
Post-SBRT PET/CT for extracranial metastatic MM resulted in high rates of complete response at a median of 2.8 months, and durable MC was achieved with SFED >24 Gy. SBRT, in addition to surgery and ablation, should be discussed with patients with MM, especially those with oligometastases.
Summary.
This study assessed the outcomes for oligometastatic malignant melanoma (MM) treated with stereotactic body radiation therapy (SBRT). Patients with pre- and posttreatment 18F-fludeoxyglucose positron emission/computed tomography scans were included. Metastasis control was excellent after SBRT, and the majority of patients achieved a complete metabolic response. A single-fraction equivalent dose >24 Gy resulted in optimal metastasis control. Prospectively captured acute and late toxicity were minimal. SBRT should be discussed with patients with oligometastatic MM in addition to surgery and ablation options.
Introduction
Early in vitro data have suggested that malignant melanoma (MM) is a radioresistant malignancy, which is a characteristic that increases as it metastasizes.1, 2 Radiobiology attributes this radioresistance to a large “shoulder” on the cell survival curve.1, 3 Hypofractionated radiation therapy is thought to be advantageous when compared with conventional fractionation for tumors with large shoulders on the cell survival curve.4, 5, 6 In contrast, 2 prospective randomized trials failed to show a correlation between response rates and fraction size.7, 8 Furthermore, retrospective data have suggested that total dose correlates with tumor control.9, 10, 11 In the clinical setting, experiences with multiple stereotactic radiation surgery have shown high metastasis control (MC) for MM intracranial metastases.12, 13, 14 However, the data supporting the use of stereotactic body radiation therapy (SBRT) for extracranial MM is limited to a small series of 17 patients.15
In general, patients with metastatic MM have a dismal prognosis. Over the past 25 years, the concept of oligometastatic disease has become increasingly relevant when classifying patients with a limited burden of metastatic disease and a more favorable prognosis with aggressive metastases-directed therapy.16 Metastasectomy for patients with oligometastatic MM improved median survival from 6.9 months to 15.8 months when surgery was added to systemic therapy.17 Similarly, thermal ablation for patients with oligometastatic MM resulted in a 4-year overall survival (OS) of 44%.18
Tumor and surrounding normal tissue response after radiation therapy can often result in fibrosis and scarring, which can obscure treatment response. Thus, the utility of conventional imaging and Response Evaluation Criteria In Solid Tumors (RECIST) may be limited when assessing the efficacy of SBRT in MM.19 In addition to the 3-dimensional images provided by conventional imaging modalities, positron emission tomography/computed tomography (PET/CT) offers insight into the metabolic vitality of the underlying tumor. However, inflammation after treatment with radiation therapy can result in an initial increase in metabolic activity (pseudopregression). Therefore, the aim of this study was to quantify the changes in metabolic response of patients with MM metastases who were treated with SBRT. In addition, we sought to determine whether an initial response in the metabolic activity correlated with oncologic outcomes.
Methods
This study was reviewed and approved by the Mayo Clinic institutional review board. A prospective SBRT database was used to identify patients with MM who were treated between January 1999 and March 2015. To be included in the study, patients needed at least 1 pretreatment 18F-fludeoxyglucose PET/CT scan within 4 months of SBRT and at least 1 posttreatment PET/CT scan. The electronic medical records were reviewed for patient characteristics, including any prior treatment (local or systemic), before each course of SBRT. Patients were classified as having oligometastatic disease if they had 5 or fewer metastases noted on the pre-SBRT PET/CT scan. SBRT was defined as a single-fraction dose of at least 18 Gy, a cumulative 3-fraction dose of a least 24 Gy, or a cumulative 5-fraction dose of at least 40 Gy.
PET outcomes were evaluated retrospectively per the PET Response Criteria in Solid Tumors (PERCIST), version 1.0. A complete response (CR) was defined as a decrease in the maximum standard uptake value corrected for lean body mass (SUL) to 1.5 times the liver mean plus 2 standard deviations, and partial response (PR) was defined as a 30% decrease in SUL. Progressive disease (PD) was defined as a >30% increase in SUL, and stable disease (SD) was any metastasis not fitting these criteria.19 Each imaging study was reviewed independently to manually quantify the PERCIST parameters. MC included CR, PR, and SD. The single-fraction equivalent dose (SFED) was calculated as previously reported.20 SFED = D − (n − 1) × Dq, where D is the total dose, n is the number of fractions, and Dq was estimated as 1.8.20 The biologically effective dose (BED) was calculated using an alpha/beta ratio of 2.5.21 Toxicity was assessed prospectively using Common Terminology Criteria for Adverse Events, version 4.0.
Each metastasis treated with SBRT was analyzed independently. The Kaplan-Meier method was used to estimate OS and progression-free survival (PFS). The cumulative incidence method was used to estimate MC, and the Wilcoxon test was used to compare survival estimates. Prognostic factors for MC and/or OS were assessed using the Cox proportional hazards model. The Likelihood Ratio was used to compare between groups. A P-value < .05 was considered statically significant. Because the analyses were performed per treated metastasis, toxicities were scored if the treated metastasis could result in the toxicity exhibited. For example, if a patient had SBRT to 2 separate metastases in the lung and iliac crest, radiation pneumonitis would be attributed to the lung SBRT course and counted once. However, causality could not always be established when multiple sites were treated within close proximity of one another and any single treatment course could have resulted in a recorded toxicity. In these cases, all treated metastases were assigned a particular toxicity. All statistical analyses were reviewed by a statistician from the “xyz” Cancer Center.
Results
A total of 390 PET/CT scans were assessed for 80 metastases in 48 patients who were treated with SBRT. The median follow-up was 4.0 years for patients who were alive at the time of last follow-up. The characteristics of patients and metastases are shown in Table 1. The median time from initial PET/CT to SBRT treatment was 17 days (range, 3-109 days). A median of 6 scans were performed for each metastasis (range, 2-6 scans). Most (76%) had oligometastatic disease; similarly, the majority (68%) had all known sites of disease treated with SBRT. The most common sites treated were musculoskeletal (29%), lung (26%), and liver (23%). Few (18%) received chemotherapy within 1 month before starting SBRT. In contrast, many (61%) received immunotherapy within 1 month before initiating SBRT, most commonly granulocyte-macrophage colony-stimulating factor. Although a variety of dose and fractionation regimens were used, the most common schemes were 24 Gy in 1 fraction, 54 Gy in 3 fractions, 60 Gy in 3 fractions, 50 Gy in 5 fractions, and 60 Gy in 5 fractions. The median SFED was 42.8 Gy and the median BED was 254.4 Gy2.5.
Table 1.
Treatment characteristics
| Characteristics | n = 80 | Percentagea |
|---|---|---|
| PET/CT scans | ||
| Total | 390 | - |
| Median per lesion (range) | 6 (2-6) | - |
| Treated sites | ||
| Musculoskeletal | 23 | 29 |
| Lung | 21 | 26 |
| Liver | 18 | 23 |
| Abdomen | 16 | 20 |
| Extra-abdominal lymph nodes | 2 | 3 |
| Oligometastatic (≤5 metastases) | ||
| Yes | 61 | 76 |
| No | 19 | 24 |
| All lesions treated | ||
| Yes | 54 | 68 |
| No | 26 | 32 |
| Recent chemotherapyb | ||
| Yes | 14 | 18 |
| No | 66 | 83 |
| Agents | ||
| Paclitaxel | 6 | 27 |
| Bevacizumab | 5 | 23 |
| Carboplatin | 4 | 18 |
| Temozolomide | 7 | 32 |
| Recent immunotherapyb | ||
| Yes | 49 | 61 |
| No | 31 | 39 |
| Agents | ||
| GM-CSF | 30 | 64 |
| Ipilimumab | 16 | 34 |
| Pembrolizumab | 3 | 6 |
| Dose and fractionation | ||
| 18-22.8 Gy × 1 | 7 | 9 |
| 24-34 Gy × 1 | 13 | 16 |
| 8-12 Gy × 3 | 5 | 6 |
| 18-20 Gy × 3 | 20 | 25 |
| 12 Gy × 4 | 1 | 1 |
| 8-10 Gy × 5 | 24 | 30 |
| 12 Gy × 5 | 10 | 13 |
| SFED | ||
| Median (range) | 42.8 (18-56.4) | - |
| ≤24 Gy | 21 | 26 |
| >24 Gy | 59 | 74 |
| BED (Gy2.5) | ||
| Median (range) | 254.4 (100.8-540) | |
| ≤200 | 11 | 14 |
| >200 | 69 | 86 |
BED, biologically effective dose; CT, computed tomography; GM-CSF, granulocyte-macrophage colony-stimulating factor; PET, positron emission tomography; SFED, single-fraction equivalent dose.
Percentage shown when applicable.
Within 1 month of stereotactic body radiation therapy.
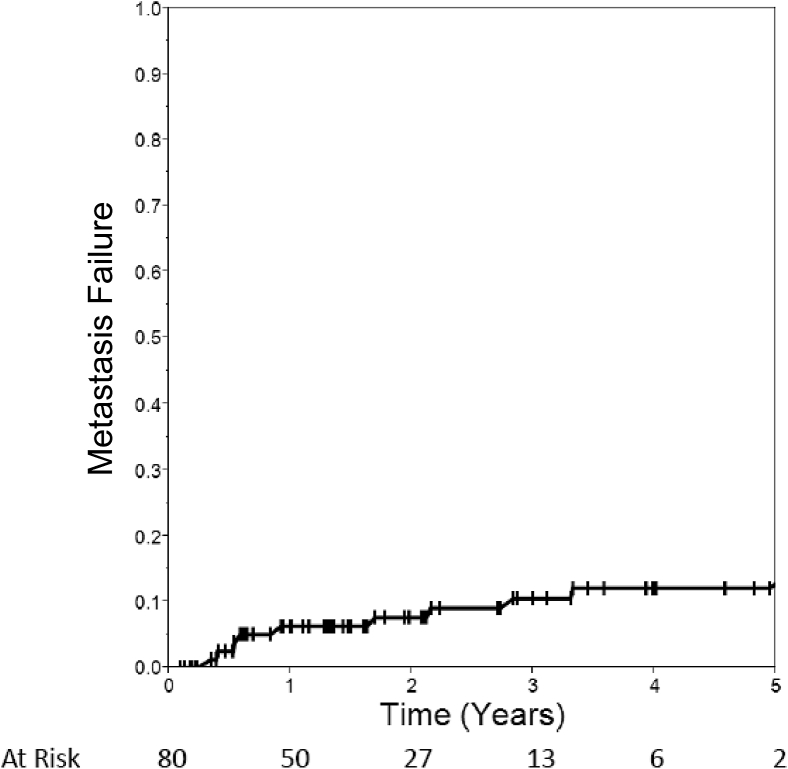
As shown in Table 2, the majority of metastases responded with a CR to treatment. Responses seen during the first PET/CT scan after treatment (median, 2.2 months) revealed metastasis control in 97% and a CR in 64%. Following the metastases until a patient's last available PET/CT scan (median, 16.3 months), the rate of CR increased to 79% but MC declined slightly to 89%. Using the cumulative incidence method, metastasis failure was 6% at 1 year and 10% at 3 years (Fig 1).
Table 2.
Positron emission tomography outcomes after SBRT
| F/U 1 | F/U 2 | F/U 3 | F/U 4 | F/U Final | |
|---|---|---|---|---|---|
| No. of metastases | 80 | 67 | 60 | 47 | 80 |
| Median months after SBRT (range) | 2.2 (0.4-6.1) | 4.7 (1.4-14.5) | 7.9 (3.2-25.9) | 11.5 (6.4-31.8) | 16.3 (1.1-71.7) |
| CR | 64% | 84% | 83% | 90% | 79% |
| PR | 14% | 14% | 5% | 4% | 5% |
| SD | 19% | 1% | 5% | 2% | 5% |
| PD | 3% | 1% | 7% | 4% | 11% |
| MC | 97% | 99% | 93% | 96% | 89% |
CR, complete response; F/U, follow-up; MC, metastasis control; PD, progressive disease; PR, partial response; SBRT, stereotactic body radiation therapy; SD, stable disease.
Figure 1.
Cumulative incidence of metastasis failure.
An initial increase in SUL was seen on the first PET/CT scan after SBRT in a total of 14 metastases (Table 2). Of these 14, only 3 had at least a 30% increase in SUL, resulting in a classification of PD. Subsequent follow-up revealed no association between an initial increase in SUL and an increased risk of metastasis failure (P = .71), PFS (P = .37), or OS (P = .67). PET/CT scans that showed an initial increase in SUL were more likely to be done at an earlier time point after SBRT, at a mean time of 1.8 months, compared with 2.3 months in those that did not show an initial increase in SUL (1-sided t test, P = .046). Therefore, the prognostic impact of an early scan that showed an increase in SUL was not clinically significant.
Improved MC was associated with an SFED of >24 Gy with 93% MC compared with 75% with a lower SFED (P = .01). At 2 years, MC was 100% with an SFED of >24 Gy and 88% with an SFED of ≤24 Gy (P = .01). There was no significant association between BED and MC (P = .14). MC was marginally better with fractionated regimens (92% vs 75%), but the result was not statistically significant (P = .06). Total doses ≥50 Gy (P = .10) and ≥11 Gy per fraction (P = .27) were not associated with MC. Furthermore, MC was not associated with the site treated, oligometastatic disease, or treatment of all known metastases.
In total, 72 metastases (90%) had a CR at some point during follow-up. No statistically significant correlations were seen between dose, fractionation, SFED, or BED with the development of a CR (P > .05 for all). The median time to CR was 2.8 months (range, 0.4-25.2 months). CR was not sustained in 10 metastases (14%) of which 7 developed PD, 2 regressed to SD, and 1 regressed to PR. In patients who had a CR and subsequently developed PD, the median duration of CR was 6.2 months (range, 1.9-70 months).
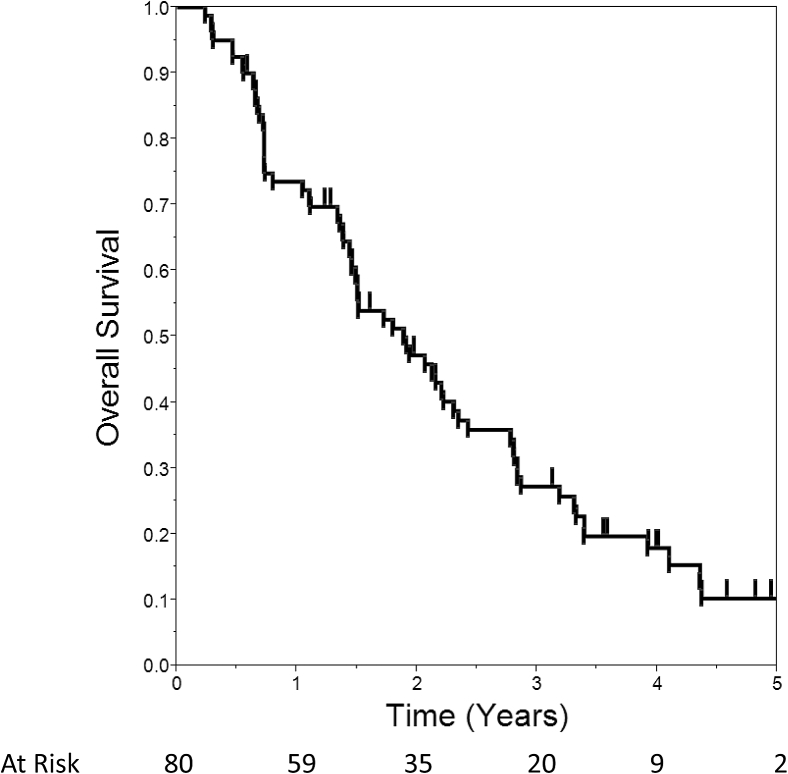
Median distant PFS was 3.6 months, which was higher (4.3 months) for patients with MC compared with those without MC (2.5 months; P = .02). Median OS was 1.9 years. OS was 74% at 1 year, 47% at 2 years, and 27% at 3 years (Fig 2). Survival was better for patients with oligometastatic disease (median 2.3 vs 0.7 years, P < .0001) and those with all known metastases treated (median 2.2 vs 1.1 years, P = .01). MC was not associated with OS (P = .99). However, median survival was 2.1 years after a CR compared with 0.7 years without a CR (P = .001). The administration of chemotherapy had no association with survival (P = .30). In contrast, median survival was longer in patients who received some form of immunotherapy within 1 month of SBRT (median 2.3 vs 1.4 years, P = .01).
Figure 2.
Kaplan-Meier plot of overall survival.
Acute toxicity is shown in Table 3. Notably, there was 1 case of grade 3 pain and 2 cases of grade 3 neuropathy, which were all thought to be definitely related to the tumor. Late toxicity is shown in Table 4. One patient had a perforated grade 4 gastric ulcer that resulted in grade 4 sepsis, which was probably related to the combination of liver SBRT and bevacizumab. Another patient had a grade 4 bowel obstruction, which was thought to be possibly related to liver SBRT. One patient had a grade 3 portal hypertension that was related to SBRT after 3 separate courses of liver SBRT treatment. Similarly, 1 patient had a grade 3 compression fracture at T12 after SBRT to the adrenal gland and a retroperitoneal lymph node. Grade 2 or greater pneumonitis occurred in 2 of 21 (10%) courses of lung SBRT and 1 of 18 (5%) of liver SBRT courses.
Table 3.
Acute toxicity
| None |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Pain | 66 | 83 | 4 | 5 | 9 | 11 | 1a | 1 | 0 | 0 | 0 | 0 |
| Nausea | 72 | 90 | 2 | 3 | 6 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 77 | 96 | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neuropathy | 78 | 98 | 0 | 0 | 0 | 0 | 2a | 3 | 0 | 0 | 0 | 0 |
| Esophagitis | 78 | 98 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 79 | 99 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cough | 79 | 99 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pericarditis | 79 | 99 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anorexia | 79 | 99 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Attribution: definitely related to tumor.
Table 4.
Late toxicity
| None |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Pain | 74 | 93 | 2 | 3 | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 75 | 94 | 2 | 3 | 2 | 3 | 1a | 1 | 0 | 0 | 0 | 0 |
| Fracture | 76 | 95 | 0 | 0 | 2 | 3 | 2b | 3 | 0 | 0 | 0 | 0 |
| Portal hypertension | 77 | 96 | 0 | 0 | 0 | 0 | 3c | 4 | 0 | 0 | 0 | 0 |
| Ulcer | 78 | 98 | 0 | 0 | 1 | 1 | 0 | 0 | 1d | 1 | 0 | 0 |
| Bowel obstruction | 79 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 1e | 1 | 0 | 0 |
| Sepsis | 79 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 1d | 1 | 0 | 0 |
Attribution: definitely related to radiation therapy.
Single patient with two courses possibly contributing to fracture.
Single patient with three liver stereotactic body radiation therapy treatments.
Attribution: probably related to radiation therapy.
Attribution: possibly related to radiation therapy.
Discussion
This study demonstrates excellent MC for extracranial MM treated with SBRT, especially with SFED >24 Gy. Observations of transient post-SBRT PET/CT increase in SUL (pseudoprogression) can be significantly reduced when the scan is performed after 2.3 months of SBRT. In addition, we report prospectively collected toxicity outcomes that reveal that SBRT is both safe and highly efficacious. Taken together, SBRT can be safely considered for patients with oligometastatic MM where optimizing MC may translate into survival benefit.
Scant evidence exists to inform treatment decisions for clinicians when evaluating patients for SBRT in the setting of metastatic MM. At present, only 1 study reported outcomes after treatment of 28 metastatic MM metastases with SBRT delivered in 3 or 5 fractions.15 Median follow-up for living patients was 28 months, and MC was 88% at 18 months. In the current study, median follow-up was 48 months and MC was 90% at 3 years. Similarly, MC ranges between 63% and 90% in small, retrospective studies of stereotactic radiation surgery for intracranial MM metastases.13, 14 Ultimately, our outcomes compare favorably with the limited existing literature.
While we found that SFED >24 Gy was associated with improved MC, the previously published SBRT MM report demonstrated improved MC when treating to an SFED of 45 Gy or higher.15 We found no difference in MC when using 45 Gy as a threshold. One explanation for the lower threshold reported here is the fact that 25% of our cohort was treated with single-fraction SBRT, but no patients were treated with single-fraction doses in the previous study. Because many patients with SFED ≤24 Gy were actually treated with a single fraction of radiation, this raises questions about the efficacy of single-fraction SBRT for metastatic MM. Our results showed a marginally statistically significant association with better MC and fractionated regimens (P = .06).
Previous comparisons between single- and multifraction SBRT regimens have not found a significant compromise in outcomes or increase in toxicity.22 In fact, there was better MC with high-dose single-fraction stereotactic radiation surgery for sarcomas metastasizing to the spine in a series by Folkert et al.23 Their hypofractionated treatments consisted of a relatively modest median of 28.5 Gy delivered in 3 to 6 fractions, but their median single-fraction dose was 24 Gy. At 12 months, MC was 91% with single-fraction treatment and 84% with hypofractionated regimens. Thus, it seems probable that single-fraction doses can be at least as efficacious as fractionated regimens, provided a sufficient dose is delivered.
While the metabolic information from PET/CT scans can be advantageous in addressing underlying tumor viability, inflammation after SBRT can raise suspicion for tumor progression. An initial increase in SUL was seen in 14 metastases in our study, although only 3 met PERICST criteria for PD. A follow-up of these metastases revealed a low rate of metastasis failure despite an initial increase in SUL. The optimal interval between SBRT and the first posttreatment PET/CT scan has not been established for metastatic MM. However, our data argue against imaging less than 2 months after SBRT because there was a significant association between an initial increase in SUL and early imaging (psuedoprogression).
This study has several strengths. We report oncologic outcomes for a large number of extracranial MM metastases after SBRT with long-term follow-up. In addition, we used modern metabolic imaging with PET/CT scans to quantify metastasis control. The prospectively captured toxicity outcomes are unique to our study. Our data are generalizable to many patients with extracranial metastatic MM because there was an even distribution of a variety of commonly treated sites.
Despite its strengths, this study also has several weaknesses. First, the timeline of PET/CT scans was not mandated or standardized, so the intervals between scans were variable. Although patients are commonly followed with PET/CT scans to verify responses to treatment for metastatic MM, no comparison was made between PET and standard axial CT outcomes using RECIST criteria. Given that PET/CT imaging is more expensive than CT, such a comparison would be useful to verify the cost-effectiveness of PET/CT. Furthermore, tumor size was not captured, and its influence on dose/fractionation and outcomes was not assessed. Because the vast majority of our patients were treated in the era preceding anti-programmed cell death protein 1 and programmed death-ligand 1 therapy, it is unknown whether these data are generalizable to the modern population receiving such agents. In addition, the impact of systemic agents initiated after SBRT was not studied. However, the prolonged responses seen in this study would be unlikely to occur solely from the systemic agents commonly used in the era studied.24, 25, 26 Moreover, the most meaningful method of quantifying efficacy of a local therapy remains classification of MC or lack thereof. Thus, our methods are considered standard and are similar to those used in many prior series.
In summary, SBRT is effective at controlling individual extracranial MM metastases with minimal toxicity. MC appears to be improved with SFED >24 Gy. Aggressive local therapies including SBRT, surgery, and thermal ablation should be discussed with patients with oligometastatic extracranial MM seen in the clinical setting.
Footnotes
Sources of support: None.
Conflicts of interest: None.
References
- 1.Barranco S.C., Romsdahl M.M., Humphrey R.M. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971;31:830–833. [PubMed] [Google Scholar]
- 2.Rofstad E.K. Radiation sensitivity in vitro of primary tumors and metastatic lesions of malignant melanoma. Cancer Res. 1992;52:4453–4457. [PubMed] [Google Scholar]
- 3.Dewey D.L. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1971;44:816–817. doi: 10.1259/0007-1285-44-526-816. [DOI] [PubMed] [Google Scholar]
- 4.Habermalz H.J., Fischer J.J. Radiation therapy of malignant melanoma: Experience with high individual treatment doses. Cancer. 1976;38:2258–2262. doi: 10.1002/1097-0142(197612)38:6<2258::aid-cncr2820380611>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Hornsey S. The radiosensitivity of melanoma cells in culture. Br J Radiol. 1972;45:158. doi: 10.1259/0007-1285-45-530-158. [DOI] [PubMed] [Google Scholar]
- 6.Hornsey S. The radiation response of human malignant melanoma cells in vitro and in vivo. Cancer Res. 1972;32:650–651. [PubMed] [Google Scholar]
- 7.Overgaard J., von der Maase H., Overgaard M. A randomized study comparing two high-dose per fraction radiation schedules in recurrent or metastatic malignant melanoma. Int J Radiat Oncol Biol Phys. 1985;11:1837–1839. doi: 10.1016/0360-3016(85)90042-2. [DOI] [PubMed] [Google Scholar]
- 8.Sause W.T., Cooper J.S., Rush S. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991;20:429–432. doi: 10.1016/0360-3016(91)90053-7. [DOI] [PubMed] [Google Scholar]
- 9.Rades D., Heisterkamp C., Huttenlocher S. Dose escalation of whole-brain radiotherapy for brain metastases from melanoma. Int J Radiat Oncol Biol Phys. 2010;77:537–541. doi: 10.1016/j.ijrobp.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Isokangas O.P., Muhonen T., Kajanti M., Pyrhonen S. Radiation therapy of intracranial malignant melanoma. Radiother Oncol. 1996;38:139–144. doi: 10.1016/0167-8140(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 11.Seegenschmiedt M.H., Keilholz L., Altendorf-Hofmann A. Palliative radiotherapy for recurrent and metastatic malignant melanoma: prognostic factors for tumor response and long-term outcome: A 20-year experience. Int J Radiat Oncol Biol Phys. 1999;44:607–618. doi: 10.1016/s0360-3016(99)00066-8. [DOI] [PubMed] [Google Scholar]
- 12.Manon R., O'Neill A., Knisely J. Phase II trial of radiosurgery for one to three newly diagnosed brain metastases from renal cell carcinoma, melanoma, and sarcoma: An Eastern Cooperative Oncology Group study (E 6397) J Clin Oncol. 2005;23:8870–8876. doi: 10.1200/JCO.2005.01.8747. [DOI] [PubMed] [Google Scholar]
- 13.Mori Y., Kondziolka D., Flickinger J.C., Kirkwood J.M., Agarwala S., Lunsford L.D. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42:581–589. doi: 10.1016/s0360-3016(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 14.Powell J.W., Chung C.T., Shah H.R. Gamma Knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J Neurosurg. 2008;109:122–128. doi: 10.3171/JNS/2008/109/12/S19. [DOI] [PubMed] [Google Scholar]
- 15.Stinauer M.A., Kavanagh B.D., Schefter T.E. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 17.Howard J.H., Thompson J.F., Mozzillo N. Metastasectomy for distant metastatic melanoma: analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I) Ann Surg Oncol. 2012;19:2547–2555. doi: 10.1245/s10434-012-2398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White M.L., Atwell T.D., Kurup A.N. Recurrence and survival outcomes after percutaneous thermal ablation of oligometastatic melanoma. Mayo Clin Proc. 2016;91:288–296. doi: 10.1016/j.mayocp.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C., Papiez L., Zhang S., Story M., Timmerman R.D. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 21.Overgaard J., Overgaard M., Hansen P.V., von der Maase H. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol. 1986;5:183–192. doi: 10.1016/s0167-8140(86)80048-2. [DOI] [PubMed] [Google Scholar]
- 22.Greco C., Pares O., Pimentel N. Spinal metastases: From conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother. 2015;20:454–463. doi: 10.1016/j.rpor.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folkert M.R., Bilsky M.H., Tom A.K. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88:1085–1091. doi: 10.1016/j.ijrobp.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki N., Kiyohara Y., Uhara H. Phase II study of ipilimumab monotherapy in Japanese patients with advanced melanoma. Cancer Chemother Pharmacol. 2015;76:997–1004. doi: 10.1007/s00280-015-2873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eroglu Z., Kong K.M., Jakowatz J.G., Samlowski W., Fruehauf J.P. Phase II clinical trial evaluating docetaxel, vinorelbine and GM-CSF in stage IV melanoma. Cancer Chemother Pharmacol. 2011;68:1081–1087. doi: 10.1007/s00280-011-1703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodi F.S., Lee S., McDermott D.F. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]