Abstract
AIM
To investigate the roles of PKC-α/ezrin signals in phagocytosis crisis of retinal pigment epithelium (RPE) cells in light damage model.
METHODS
Light induced mice RPE injury model was established by continuously irradiating cool white light at different exposure time (0, 4, 8h light intensity: 4.18×10−6 J/cm2). In vitro, human ARPE-19 cells treated with the doses and intensity (1.57×10−6 J/cm2) of laser irradiation. Histology analysis was evaluated by hematoxylin and eosin (HE) staining. In vivo RPE phagocytosis was quantified by measuring the accumulation of photoreceptor outer segments in the sub-retinal space. In vitro RPE phagocytosis was assessed by calculating the relative fluorescence intensity of FITC-labeled microspheres in ARPE-19 cells. To further investigate the molecular mechanism, the activation of PKC-α/ezrin signal was evaluated by Western blot in vivo and in vitro.
RESULTS
HE staining revealed that the thickness of outer nuclear layer decreased significantly after 4 and 8h light exposure. By immunostaining with rhodopsin, a significant greater accumulation of photoreceptor outer segment was noticed after light injury. In vitro, light injured RPE cells had less phagocytic activity in a dose dependent manner than that of the normal control (P<0.01). Western blot suggested the activation of PKC-α/ezrin signaling was down-regulated in a dose-dependent manner after light exposure.
CONCLUSION
Our data suggest that light induced phagocytic crisis of RPE cells may result from the down-regulation of PKC-α/ezrin signaling.
Keywords: age-related macular degeneration; retinal pigment epithelium; ezrin, light injury; phagocytosis
INTRODUCTION
Age-related macular degeneration (AMD), with an increasing prevalence, is one of the leading causes of irreversible visual impairment and blindness in the elderly worldwide[1]–[2]. Polarized retinal pigment epithelial cells act pivotal roles in retinoid cycle. Dysfunction of retinal pigment epithelium (RPE) phagocytic activity is one of the main mechanisms of dry AMD[3], and light induced RPE degeneration is a well-recognized AMD model. Overdoses of light exposure can cause a photochemical effect[4]–[5] which results in the activation of oxidative stress[6] and decrease of RPE phagocytosis[7]. Ezrin, a member of ezrin/radixin/moesin (ERM) protein family, is an important polarity protein mainly located in the apical side of RPE cells[8]–[9]. Recent studies suggested that it acts a pivotal role in RPE phagocytosis[10]–[11], adhesion, migration as well as membrane transportation[12]. Protein kinase C alpha (PKC-α) is the upstream regulator of ezrin[13]–[14]. PKC-α/ezrin has been reported as the key signal pathway regulating the phagocytosis of many types of cells[14], including RPE cells, but its roles in light induced dysfunction of RPE phagocytosis is still largely unknown. The objective of this study is to investigate the potential roles of PKC-α/ezrin signaling in light induced dysfunction of RPE phagocytosis. We hypothesize that the mixed wavelength white light can cause the dysfunction of RPE phagocytic activity and this effect is regulated by PKC-α/ezrin signal.
MATERIALS AND METHODS
Study Design
In vivo and in vitro light-induced RPE injury models were established by continuous laser irradiation with a cool white LED emitter (model LG-150W, Beijing Paidiwei Instrument Co., Ltd, Beijing, China). In vivo RPE phagocytosis was evaluated by measuring the accumulation of rhodopsin positive photoreceptor outer segment (POS) in mice retina. In vitro RPE phagocytosis was quantified by the intensity of engulfed fluorescein isothiocyanate microbeads in human RPE cells. Western blotting was utilized to identify the activation of ezrin/p-ezrin/PKC-α pathway before and after the light injury. Hematoxylin and eosin (HE) staining was used for the histology.
Animals
The study was approved by the Animal Care and Use Committee of Jinzhou Medical University (SCXK-Jing 2012-0001) in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Eight-week-old C57BL/6J mice purchased from Beijing Vital River Laboratory Animal Technology (Beijing, China) were maintained in a 12h light/dark cycle and freely accessed to food and water in a SPF laboratory Animal Center.
Cell Culture
Human ARPE-19 cells were purchased from American Tissue Culture Collection (ATCC; Rockville, MD, USA) and maintained in Dulbecco's modified eagle's medium (DMEM; Hyclone, gelifesciences, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies, NY, USA) in a 37°C incubator. The medium was changed every three days. Cells were digested with 0.25% trypsin/0.02% ethylenediaminetetraacetic acid solution and passaged at 100% confluency.
Light Injury Model
In vivo model
Mice pupils were dilated by 0.5% tropicamide (Shanghai Shentian Pharmaceutical Co., Shanghai, China) 30min before light exposure. Then the mice were exposed to cool white light from 8 a.m. to 4 p.m., 12 a.m. to 4 p.m., respectively. The control mice were sham irradiated.
In vitro model
After the cells became 80% confluent, they were trypsinized and seeded into six-well-plate at a density of 3×105 cells per well. After 24h, they were exposed to the white light at the intensity and doses of 1.57×10−6 J/cm2 from 8 a.m. to 4 p.m., 12 a.m.to 4 p.m., respectively. The cell without light exposure served as the control. The detailed information about the laser instrument was described as below (Table 1).
Table 1. Device information.
| Manufacturer | Beijing Paidiwei Instrument Co., Ltd, Beijing, China |
| Year of product | 2015 |
| No. of emitter | 1 |
| Emitter type | Semiconductor diode laser |
| Beam delivery system | Fiber optic |
| Wavelength | White light with mixed wavelength |
| Irradiation model | Continuous |
| Max output power | 150 W |
| Light intensity | 0.8 W/cm2; cell: 0.3 W/cm2 |
Phagocytosis Assay
In vivo phagocytosis
After treatment, the mice were sacrificed and the eyes were enucleated and fixed by 4% paraformaldehyde (PFA) for 24h. After dehydration with 30% sugar solution, the eyes were frozen and sectioned. Retinal sections were blocked by 1% goat serum for 1h and incubated with rat-anti-rabbit rhodopsin antibody (1:200, ab 3424, Abcam, USA) overnight at 4 degrees. After washing with phosphate buffered saline (PBS) for three times, and the sections were labeled by primary rhodopsin antibody and secondary antibody conjugated with Alexa Fluor. The cell nuclears were counterstained with 4′-6-diamidino-2-phenylindole (DAPI). Pictures were taken and POS thickness was measured.
In vitro phagocytosis
To evaluate the RPE phagocytosis in vitro, the cells were incubated with FITC-labelled microbeads (0.1 µm diameter, Invitrogen, Karlsruhe, Germany) at a microbead/cell ratio of 1:16 000 in a 37°C incubator for 1h, then washed with PBS three times. After fixation with 4% PFA for 10min, 20 pictures were taken from each sample using a confocal microscope with the same settings (FV10C-W3, Olympus, Tokyo, Japan). The relative amount of fluorescence intensity was semi-quantified using ImageJ.
Western blot
After harvest, the retinas and ARPE-19 cells were lysed on ice. The total protein was extracted and titered by bicinchoninic acid assay kit (#P0012, Beyotime Biotechnology, Nanjing, China). Samples were denatured at 98°C for 5min and 20 µL of protein was loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, separated by electrophoresis and transferred onto poly vinylidene fluoride membrane. After blocking with 1% bovine serum albumin (BSA; Sigma, Deisenhofen, Germany)/TBST (1 mL Tween 20/1 L Tris-buffer saline), the members were incubated with primary antibodies at 4°C overnight. After three times washing with TBST, the members were incubated with goat-anti-rabbit or goat-anti-mouse IgG (H+L), horseradish peroxidase conjugate secondary antibodies (#SA00001-2, #SA00001-1; Proteintech Group Inc., IL, USA) at room temperature for 1h. The bands were visualized by an enhanced chemiluminescence substrate (Bio-Rad, Hercules, CA, USA). The protein expression was semi-quantified by ImageJ. The primary antibodies used in this study were: mouse anti-ezrin, rabbit anti-ezrin (phospho Thr 567)/Radixin (phospho Thr 564)/Moesin (phospho Thr 558), rabbit anti-PKC-α and mouse anti-beta actin. All the antibodies were purchased from Abcam. The working dilution is 1:1000.
Histology
The eyes were fixed with 4% PFA and dehydrated by sucrose gradients, then embedded in paraffin. Five micron sections were obtained and stained with HE. Pictures were taken under conventional light microscope.
Statistical Analysis
The data was presented as mean±standard error. One-way analysis of variance (ANOVA) was performed to calculate the statistical difference. A minimum of six samples were obtained from each group. Alpha=0.05.
RESULTS
The Loss of Photoreceptors After Light Injury
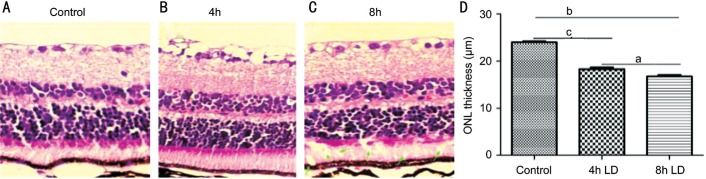
To evaluate the effect of light damage on retinal photoreceptors, the sections were stained with HE. The average thickness of outer nuclear layer in light damaged groups were 92.08±0.6067 µm (Figure 1B), and 81.17±0.8250 µm (Figure 1C) in 4-hour group and 8-hour group, respectively, significantly thicker than that of the control (122.42±0.2183 µm), both P<0.01. Additionally, the POS layer was not well organized in the 8-hour-light damaged groups and some giant vacuoles can be found (Figure 1C, green arrows).
Figure 1. Reduction of outer nuclear layer thickness after light exposure.
A: Retinas were fixed by 4% PFA, sectioned and stained with HE. Normal retina was well-organized; B, C: Compare to the normal control, the thickness of outer nuclear layers in light damaged groups were significant thinner (P<0.01); C: Additionally, some giant vacuoles can be seen in 8-hour-light exposure group (green arrows); D: The average thickness of outer nuclear layer (ONL) showed a dose dependent decrease manner. aP<0.05, bP<0.01, cP<0.001.
Decrease of Retinal Pigment Epithelium Phagocytosis After Light Exposure
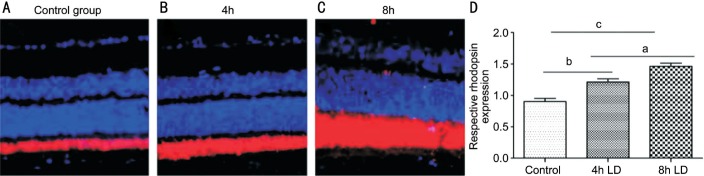
We then evaluate the effects of light induced dysfunction of RPE phagocytosis. In vivo, the thickness of rhodopsin labeled POS in normal control was 10.25±0.008 µm (Figure 2A), significantly thinner than light damaged groups in a dose-dependent manner 10.25±0.008 vs 17.53±0.125 µm in 4-hour group, and 10.25±0.008 vs 28.00±0.303 µm in 8-hour group, both P<0.01 (Figure 2B, 2C).
Figure 2. Decrease RPE phagocytosis after light injury in vivo.
A: Dysfunction of RPE phagocytosis in vivo was evaluated by measuring the thickness of rhodopsin labelled POS. In normal retina, the average thickness of POS; B-D: Light injury induced a dose dependent accumulation of POS in mice retina. aP<0.05, bP<0.01, cP<0.001.
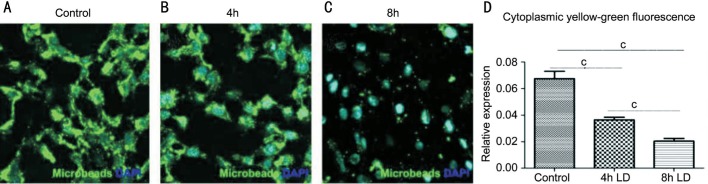
Higher dose of light irradiation induced greater accumulation of POS (P<0.05). In vitro, RPE phagocytosis was quantified by measuring the average fluorescence intensity of FITC-labeled microbeads engulfed by RPE cells. The normal control group without light treatment showed the highest fluorescence intensity (mean fluorescence intensity: 0.067±0.0147; Figure 3A) compared to the light injured groups in Figure 3B and 3C. The decrease of RPE phagocytosis showed a dose dependent manner 0.0365±0.005 vs 0.021±0.006, P<0.05 (Figure 3D).
Figure 3. Decrease of RPE phagocytosis after light injury in vitro.
RPE phagocytosis was quantified by the mean fluorescence intensity of FITC-labeled microbeads engulfed by RPE cells. The normal control group showed the highest fluorescence intensity (A) compared to the light injured groups (B, C). The decrease of RPE phagocytosis showed a dose dependent manner (D). cP<0.001.
Down-regulation of Ezrin/Protein Kinase C Alpha Signalings After Light Exposure
To investigate the potential roles of ezrin/PKC signaling in the dysfunction of RPE phagocytosis in vivo and in vitro, the expression of ezrin, p-ezrin and PKC-α were semi-quantified by Western blotting. Compared to the normal control, the expression of ezrin, p-ezrin and PKC-α decreased in a dose dependent manner in vitro (P<0.05 in all three proteins; Figure 4). Consistently, in vivo experiment showed a similar result (P<0.05 in all three proteins; Figure 5).
Figure 4. Decrease the expression of ezrin, p-ezrin and PKC-α after light injury in vitro.
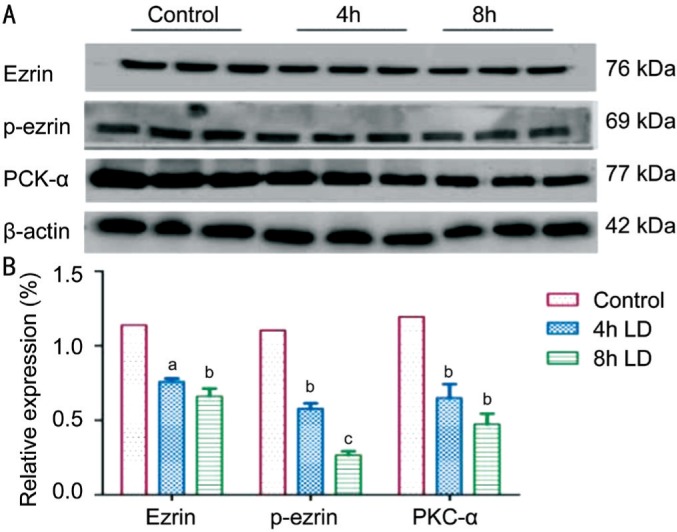
A: The expression of ezrin, p-ezrin and PKC-α were semi-quantified by Western blotting; B: Compared to the normal control, the expression of ezrin, p-ezrin and PKC-α all decreased in a dose dependent manner in vitro. aP<0.05, bP<0.01, cP<0.001.
Figure 5. Decrease the expression of ezrin, p-ezrin and PKC-α after light damage in vivo.
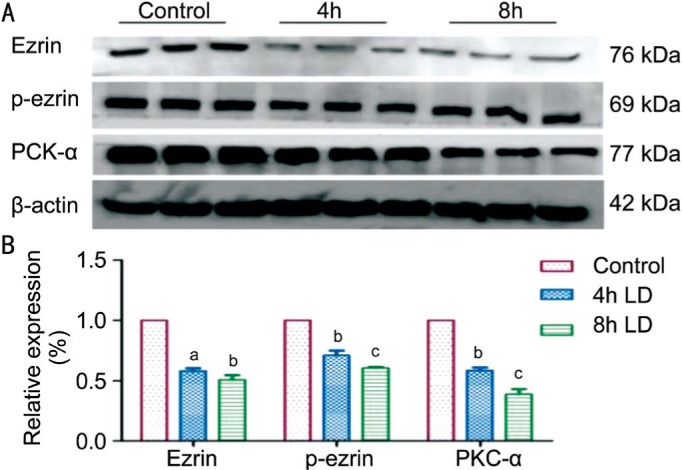
A: The expression of ezrin, p-ezrin and PKC-α were semi-quantified by Western blotting; B: Consistent with the in vitro study, the expression of ezrin, p-ezrin and PKC-α all decreased statistically in a dose dependent manner after light exposure. aP<0.05, bP<0.01, cP<0.001.
DISCUSSION
Light induced dysfunction of RPE phagocytosis plays a pivotal role in the pathogenesis of dry AMD. Our results suggested mixed wavelength white light can cause the loss of retinal photoreceptor, disorganization and accumulation of POS. Decrease of phagocytic activity might result from the inhibition of ezrin/PKC signaling. Accumulation of POS between photoreceptor and RPE layer may cause by the dysfunction of RPE phagocytosis. Our research showed that the dysfunction of phagocytosis in mice and ARPE-19 cells was in a time-dependent manner, especially the increasing of rhodopsin under the light damage. It is well known that ezrin plays a major role of phagocytosisin RPE[15], primary human malignant melanomas and etc[16]–[17]. We further showed that the gradual down-regulated expression of ezrin accompanied by the decreased ability for phagocytosis of POS by light damage induced RPE. C-terminal threonine T567 of ezrin is the phosphorylated target by PKC-α[13]–[14],[18]–[19], which is responsible for maintenance cell phagocytosis, polarity and etc. We went further to detect the expression of phosphorylated ezrin and PKC-α in light damage induced RPE. The results showed a down regulation of phosphorylated ezrin and PKC-α in a time-dependent manner both in vivo and in vitro. In the experiment described by Ueta et al[20], the physiological range of light stimulus was from 5 to 15 lx. In this study, RPE cells might be damaged by toxic light exposure-8000 lx in C57BL/6J mice. This may lead to the pyknotic nuclei of photoreceptor, diffuse swelling and disruption of the inner segment. The thickness of outer nuclear layer adjacent to disorganization POS was significantly decreased. The pathological changes of photoreceptor and RPE lead to dysfunction of opsin synthesis result in RPE overloaded with undigested POS. Indeed, with toxic levels of white light, RPE is no longer to maintain photoreceptor homeostasis[21]. In addition, histological analysis indicated that exposure time may enhance susceptibility to light damage in mouse and exceed retinal neuronal cells to death with their loss function. Therefore, phagocytic clearance is required to remove death retinal cells and metabolic waste. With the major function of phagocytosis and autophagy of RPE[22], daily clearance of shedding POS and metabolic waste is important to maintain disk renewal and preservate the visual cycle. It has been reported by Ferguson and Green[22] that a non-canonical autophagy named LC3-associated phagocytosis (LAP) was related to the mechanism of AMD. Actin filaments and microtubule-dependent motor proteins, major components of cytoskeleton elements, have been reported as a critical part in the internalization of phagosomes after POS ingested by RPE cells[23]. Ezrinis essential for the maintenance in morphogenesis of apical microvilli and basal infoldings in RPE[8]–[9]. It has been reported that decreased ezrin in ezrin−/− mice and in ezrin antisense oligonucleotides added primary cultures of rat RPE reduced the length and number of apical microvilli and the elaborate basal infoldings typical of these cells[8]–[9]. As conserved C-terminal ERM association domain residue in human ezrin, Thr567 is phosphorylated coincides with activation[24]. It is reported that ezrin involves in phagocytosis[25]–[26] and C-terminal threonine Thr567 is the phosphorylated target by PKC-α[13]–[14]. In this study, we assess light damaged RPE phagocytosis was reduced, which was consisted with the down-regulated expression of PKC/ezrin in light damaged RPE cells.
In conclusion, we performed the effect of light damage on RPE phagocytosis. Firstly, excessive light exposure damaged the morphology of retina; secondly, we have uncovered a phenomenon in which excessive light exposure reduces RPE phagocytosis; lastly, down-regulation of PKC-α/ezrin signal is related to light induced phagocytosis crisis of RPE cells.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81641057); the Natural Science Foundation of Liaoning Province (No.201602292; No.201602298).
Conflicts of Interest: Zhang YQ, None; Fan YG, None; Dang YL, None; Liu YL, None; Liu H, None; Li LH, None.
REFERENCES
- 1.Klein R, Knudtson MD, Lee KE, Gangnon RE, Klein BE. Age-period-cohort effect on the incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2008;115(9):1460–1467. doi: 10.1016/j.ophtha.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Merry GF, Munk MR, Dotson RS, Walker MG, Devenyi RG. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 4.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010;29(2):113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24(2):275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Pinazo-Duran MD, Gallego-Pinazo R, Garcia-Medina JJ, Zanón-Moreno V, Nucci C, Dolz-Marco R, Martínez-Castillo S, Galbis-Estrada C, Marco-Ramírez C, López-Gálvez MI, Galarreta DJ, Díaz-Llópis M. Oxidative stress and its downstream signaling in aging eyes. Clin Interv Aging. 2014;9:637–652. doi: 10.2147/CIA.S52662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- 8.Bonilha VL, Finnemann SC, Rodriguez-Boulan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147(7):1533–1548. doi: 10.1083/jcb.147.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilha VL, Rayborn ME, Saotome I, McClatchey AI, Hollyfield JG. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82(4):720–729. doi: 10.1016/j.exer.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997;110(Pt 24):3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J, Forte JG. High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol. 2007;293(3):C874–884. doi: 10.1152/ajpcell.00111.2007. [DOI] [PubMed] [Google Scholar]
- 12.Celik H, Sajwan KP, Selvanathan SP, Marsh BJ, Pai AV, Kont YS, Han J, Minas TZ, Rahim S, Erkizan HV, Toretsky JA, Üren A. Ezrin binds to DEAD-Box RNA Helicase DDX3 and regulates its function and protein level. Mol Cell Biol. 2015;35(18):3145–3162. doi: 10.1128/MCB.00332-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Wu Z, Shi X, Li W, Liu C, Wang D, Ye X, Liu L, Na J, Cheng H, Chen L. Atypical PKC, regulated by Rho GTPases and Mek/Erk, phosphorylates Ezrin during eight-cell embryocompaction. Dev Biol. 2013;375(1):13–22. doi: 10.1016/j.ydbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, Parker PJ. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. EMBO J. 2001;20(11):2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murad N, Kokkinaki M, Gunawardena N, Gunawan MS, Hathout Y, Janczura KJ, Theos AC, Golestaneh N. miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration. FEBS J. 2014;281(23):5251–5264. doi: 10.1111/febs.13066. [DOI] [PubMed] [Google Scholar]
- 16.Lugini L, Lozupone F, Matarrese P, Funaro C, Luciani F, Malorni W, Rivoltini L, Castelli C, Tinari A, Piris A, Parmiani G, Fais S. Potent phagocytic activity discriminates metastatic and primary human malignant melanomas: a key role of ezrin. Lab Invest. 2003;83(11):1555–1567. doi: 10.1097/01.lab.0000098425.03006.42. [DOI] [PubMed] [Google Scholar]
- 17.Celik H, Bulut G, Han J, Graham GT, Minas TZ, Conn EJ, Hong SH, Pauly GT, Hayran M, Li X, Özdemirli M, Ayhan A, Rudek MA, Toretsky JA, Üren A. Ezrin inhibition up-regulates stress response gene expression. J Biol Chem. 2016;291(25):13257–13270. doi: 10.1074/jbc.M116.718189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong SH, Osborne T, Ren L, Briggs J, Mazcko C, Burkett SS, Khanna C. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Vet Comp Oncol. 2011;9(3):207–218. doi: 10.1111/j.1476-5829.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJ. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J Cell Sci. 2008;121(Pt 5):644–654. doi: 10.1242/jcs.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueta T, Inoue T, Yuda K, Furukawa T, Yanagi Y, Tamaki Y. Intense physiological light upregulates vascular endothelial growth factor and enhances choroidal neovascularization via peroxisome proliferator-activated receptor gamma coactivator-1alpha in mice. Arterioscler Thromb Vasc Biol. 2012;32(6):1366–1371. doi: 10.1161/ATVBAHA.112.248021. [DOI] [PubMed] [Google Scholar]
- 21.D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson TA, Green DR. Autophagy and phagocytosis converge for better vision. Autophagy. 2014;10(1):165–167. doi: 10.4161/auto.26735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnside B, Bost-Usinger L. The retinal pigment epithelium: function and disease. New York: Oxford University Press; 1998. The retinal pigment epithelial cytoskeleton; pp. 41–46. [Google Scholar]
- 24.Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxyl-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270(52):31377–31385. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- 25.Marion S, Hoffmann E, Holzer D, Le Clainche C, Martin M, Sachse M, Ganeva I, Mangeat P, Griffiths G. Ezrin promotes actin assembly at the phagosome membrane and regulates phago-lysosomal fusion. Traffic. 2011;12(4):421–437. doi: 10.1111/j.1600-0854.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 26.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Nati Acad Sci U S A. 2006;103(34):12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]