Abstract
AIM
To investigate the role of oxidative stress in regulating the functional expression of P-glycoprotein (P-gp) in mitochondria of D407 cells.
METHODS
D407 cells were exposed to different ranges of concentrations of H2O2. The mitochondrial location of P-gp in the cells subjected to oxidative stress was detected by confocal analysis. Expression of P-gp in isolated mitochondria was assessed by Western blot. The pump activity of P-gp was evaluated by performing the efflux study on isolated mitochondria with Rhodamine 123 (Rho-123) alone and in the presence of P-gp inhibitor (Tariquidar) using flow cytometry analysis. The cells were pretreated with 10 mmol/L N-acetylcysteine (NAC) for 30min before exposing to H2O2, and analyzed the mitochondrial extracts by Western blot and flow cytometry.
RESULTS
P-gp was co-localized in the mitochondria by confocal laser scanning microscopy, and it was also detected in the mitochondria of D407 cells using Western blot. Exposure to increasing concentrations of H2O2 led to gradually increased expression and location of P-gp in the mitochondria of cells. Rho-123 efflux assay showed higher uptake of Rho-123 on isolated mitochondria in the presence of Tariquidar both in normal and oxidative stress state. H2O2 up-regulated P-gp in D407 cells, which could be reversed by NAC treatment.
CONCLUSION
H2O2 could up-regulate the functional expression of P-gp in mitochondria of D407 cells, while antioxidants might suppress oxidative-stress-induced over-expression of functional P-gp. It is indicative that limiting the mitochondrial P-gp transport in retinal pigment epithelium cells would be to improve the effect of mitochondria-targeted antioxidant therapy in age-related macular degeneration-like retinopathy.
Keywords: P-glycoprotein, retinal pigment epithelium, oxidative stress, mitochondria
INTRODUCTION
Oxidative stress in the mitochondria of retinal pigment epithelium (RPE) is thought to play a causative role in age-related macular degeneration (AMD)[1]–[5]. RPE is a part of the outer blood-retinal barrier (BRB). A primary function of RPE is to maintain the metabolic homeostasis of the retina by removing the waste products and providing nutrients for the neural retina[6]–[7]. Dysfunction and degeneration of RPE by increased oxidative stress contributes to the irreversible loss of photoreceptors and central vision in AMD[8]–[9]. One reason that RPE is sensitive to oxidative stress induced injury and dysfunction is the very high density of mitochondria in RPE. Mitochondrion is a rich source of reactive oxygen species (ROS) and vulnerable to oxidative stress, so RPE with abundant mitochondria is destine to be especially susceptible target of oxidative stress[4]. It has been recognized that mitochondrial dysfunction due to oxidative stress aggregates in the AMD eyes and the anti-oxidative capacity of RPE from AMD patients decreases with age[10]–[11]. There is a need for mitochondria-targeted therapy in AMD-like retinopathy.
Mitochondrial pharmaceutics, particularly those involving mitochondria-targeted antioxidants, execute therapeutic potential by maintaining the energy production and ameliorating apoptosis[12]–[17]. However, some mitochondrial medicines may have fewer efficacies than expected due to their limited access to the mitochondria, although they are accumulated several hundred-fold into energized mitochondria. One potential mechanism that the efficacy of these mitochondrial medicines is limited may be the challenging of the drug efflux pump[18]–[19]. If these mitochondrial medicines are happened to be substrates of some efflux pumps, such as P-glycoprotein (P-gp), mitochondria-targeted therapies may be less efficient.
P-gp is the longest identified efflux pump, which is encoded by the multidrug resistance (MDR) gene. Overexpression of P-gp and resistance to apoptosis are correlated well with MDR, a very common observed molecular mechanism in chemotherapeutic therapy. P-gp can pump anticancer drugs and other compounds out of the cells, reducing the intracellular concentration and chemotherapeutic efficacy of these agents[20]–[21]. P-gp has been also recognized to be involved in other cell functions such as cellular metabolism and cell survival[22]–[24]. It is generally considered that P-gp expresses on cell surface, but increasingly growing evidences show the functional localization of P-gp in mitochondria[25]–[27]. In response to a broad range of endogenous and xenobiotic stimuli, P-gp is proposed to either pump these substrates out of mitochondria or pump them into mitochondria for sequestering, both reducing the effect of substrates in mitochondria[25],[28]. Some novel mitochondria-targeting carrier has been designed to transport drugs to mitochondria as well as limit the effects of P-gp efflux pump[29]. We propose that P-gp is present in the mitochondria of RPE cells and may influence mitochondrial medicines by changing transport across mitochondrial membranes. We therefore built hydrogen peroxide (H2O2)-induced oxidative stress in vitro model in D407 cells to mimic RPE dysfunction in AMD-like retinopathy, and investigated the functional expression of P-gp in mitochondria of D407 cells under oxidative stress conditions. This study will indicate a possible role of P-gp activity regulation in the mitochondria targeted therapeutic intervention in AMD.
MATERIALS AND METHODS
Cell Culture and Treatment
The human RPE D407 cells were from the Experimental Animal Center of Sun Yat-Sen University, Guangzhou, China. Cells were routinely kept in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 0.1 mg/mL streptomycin) at 37°C in a 95% air and 5% CO2. All cell culture reagents were from Gibco BRL (Grand Island, NY, USA). Treatment of D407 cells in this study included the incubation with fresh medium containing different concentrations of H2O2 (Sigma-Aldrich, Shanghai, China) or antioxidant N-acetylcysteine (NAC, Sigma-Aldrich, Shanghai, China) or P-gp pump inhibitor, Tariquidar (Selleckchem, China).
Measurement of Reactive Oxygen Species
D407 cells at a density of 1×106 cells/mL were incubated in serum-free medium containing 10 µmol/L dichloro-dihydro-fluorescein diacetate (Beyotime Biotechnology, Jiangsu Province, China) for 20min at 37°C after exposing to varying concentrations of H2O2. Following washing, cells were collected by trypsinization. Intracellular ROS was analyzed from the conversion of nonfluorescent dichloro-dihydro-fluorescein diacetate to its fluorescent derivatives. The fluorescence intensity was measured at 488 nm excitation and 525 nm emission wavelengths by flow cytometry.
Immunofluorescence Assay
Cells at a density of 2000 cells/chamber were seeded onto polylysine-coated glass chamber slides. Dilute 1 mmol/L MitoTracker Red CMXRos probe (Life technology, USA), widely used to identify mitochondria, to the final working concentration 100 nmol/L in DMEM. Cells were reacted with MitoTracker probe for 20min, and then fixed by ice-cold 4% Paraformaldehyde for 15min and permeabilized for 10min in 1% Triton X-100. They were immunolabeled with mouse monoclonal Mdr-1 antibody (D-11, 1:200 dilution, Santa Cruz Biotechnology, USA) at room temperature for 2h and were washed and incubated for 30min with anti-mouse IgG Alexa flour 488 (1:5000, Life technology, USA), followed by the addition of 4′, 6-diamidino-2-phenylindole (ROCH, USA) to stain nucleus. The staining pattern was visualized using a confocal microscope (Carl Zeiss Jena GmbH, Jena, Germany).
Preparation of Isolated Mitochondria from D407 Cells
The preparation of isolated mitochondria was performed using the mitochondria isolation kit for mammalian cells (Rockford, IL USA). Cells at a density of 2×106 cells/mL following H2O2 incubation were collected by centrifugation (850 g for 10min at 4°C) in a 2.0 mL micorocentrifuge tube. Carefully remove and discard the supernatant, and re-suspend cells in 10 packed cell volumes of buffer (1 mmol/L TrisHCl, pH 7.4, 0.13 mol/L NaCl, 5 mmol/L KCl, 7.5 mmol/L MgCl2). Pellet cells and decant supernatant, repeat this washing step 2 times. Resuspend cells in 6 packed cell volumes of homogenization buffer. Transfer cells to a glass homogenizer and incubate for 10min on ice. Using a tight pestle, homogenize the cells. Check under the microscope for cell breakage, the optimum is around 60% which may require 35 strokes. Pour homogenate into a conical centrifuge tube containing 1 packed cell volume of 2 mol/L sucrose solution and mixed gently. Pellet unbroken cells, nuclei, and large debris at 700 g for 10min at 4°C and transfer the supernatant to another tube. This treatment is repeated twice, transferring the supernatant to a new tube each time, discarding the pellet. Pellet the mitochondria by centrifuging at 12 000 g for 15min at 4°C. Resuspend the mitochondrial pellet in 3 packed cell volumes of mitochondrial suspension buffer (10 mmol/L TrisHCl, pH 6.7, 0.15 mmol/L MgCl2, 0.25 mol/L sucrose, 1 mmol/L PMSF, 1 mmol/L DTT), and centrifuge at 12 000 g for 5min. Discard the supernatant, and maintain the mitochondrial pellet on ice before downstream processing.
Western Blot
The proteins of isolated mitochondria were extracted by resuspending the cells in RIPA buffer with protease inhibitor, and incubated on ice for 30min. The samples were cleared by centrifuging at 12 000 g for 5min at 4°C and supernatants were collected. The protein concentration was determined using a protein assay kit (Sangon Biotech, Shanghai, China). Equal amounts of protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electropheresis and transferred electrically onto polyvinylidene fluoride membranes (ROCH, USA) and blocked with 5% non-fat dry milk in Tris-buffered saline with 0.05% Tween-20. The membranes were probed with mouse monoclonal antibodies to P-gp (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, USA) or voltage-dependent anion channel 1 (VDAC1, 1:100 dilution, Santa Cruz Biotechnology) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1: 3000, Santa Cruz Biotechnology) overnight at 4°C. The anti-mouse secondary antibodies (1:5000 dilution; Santa Cruz Biotechnology) were incubated for 1.5h at room temperature. The immunoreactive bands were detected with enhanced chemiluminescence kit (Pulilai, Beijing, China). The gray value of the target band was determined by using the LabWorks 4.6 gel imaging and analysis software (UVP BioImaging Systems). The expression of VDCA1 was used to normalize levels of the mitochondrial P-gp. To exclude the possibility of contamination of mitochondria by cytoplasm, we also detected the expression of GAPDH which is a loading control and cytoplasm marker.
Measurement of Membrane Potential in Mitochondria
The mitochondrial membrane potential (ΔΨm) was estimated in D407 cells using the dye JC-1 (5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazole carbocyanine iodide) by flow cytometry. Cells with 80% confluence were incubated for 20min at 37°C after the corresponding treatment in medium containing 10 µmol/L JC-1 (Beyotime Biotechnology, Jiangsu Province, China), a lipophilic fluorescent cation that exists as green fluorescent monomers at a low ΔΨm or as red fluorescent aggregates at a greater ΔΨm. The cells were then rinsed twice in PBS and immediately analyzed by flow cytometry. Data were collected at a 529 nm emission for green fluorescence and at 590 nm for red fluorescence. The green/red fluorescence intensity ratio is expressed as decreased ΔΨm, which represents the mitochondrial depolarization ratio.
Rhodamine 123 Accumulation Assay
P-gp efflux activity was determined by flow cytometry with intracellular accumulation of rhodamine 123 (Rho-123, Sigma-Aldrich, Shanghai, China), which has been used as a typical probe to assess P-gp function in various cells and tissues. D407 cells were pretreated with 10 mmol/L NAC for 30min or 1 µmol/L tariquidar for 1h before exposing of different concentrations of H2O2 for 24h. The optimal dose of tariquidar employed in this study was determined by the sub-toxic in D407 cells. Tariquidar was added to the medium for 1h and cells were then maintained in conditioning media at least for another 12h following medium was removed and then various detections were performed. To eliminate the influence of ΔΨm and keep Rho-123 out of mitochondria, cells were treated with the ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP, 10 µg/mL, Sigma) for 30min to induce the loss of ΔΨm completely. After depolarization of the ΔΨm using the CCCP, cells were collected by trypsinization, washed in PBS, and then incubated for 1h at 37°C in the dark with 500 nmol/L Rho-123. Cells were then washed and fed with Rho-123-free culture medium. The cultured cells were pelleted at 200 g for 5min, washed twice in PBS, and then analyzed immediately by flow cytometry analysis using a BD Accuri C6 flow cytometer. In each experiment, at least 20 000 events were analyzed. All experiments used six wells per condition and were repeated on two to three separate occasions. The mean fluorescence intensity in arbitrary units was used for data presentation.
Statistical Analysis
Data were presented as the mean±SE and analyzed using the SPSS package for Windows (version 17.0). The differences between data sets were analyzed by a one-way ANOVA and P value less than 0.05 was considered to be statistically significant.
RESULTS
H2O2-induced Oxidative Stress in D407 Cells
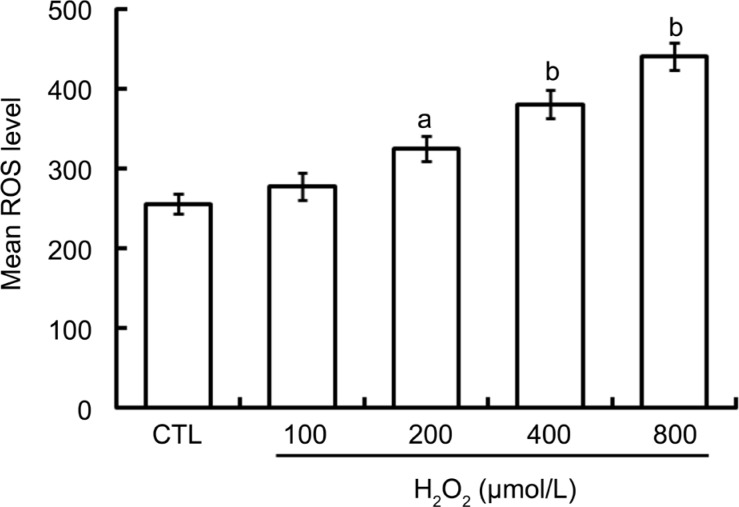
The oxidative stress state in D407 cells following exposure to different concentrations of H2O2 was determined by detecting the intracellular ROS level, indicated by DCF fluorescence using flow cytometry. As shown in Figure 1, the intracellular ROS level was increased in a concentration-dependent manner after exposing D407 cells to varying concentrations of H2O2 for 24h.
Figure 1. H2O2 increases ROS production in D407 cells in a dose-dependent manner.
D407 cells subjected to varying concentrations of H2O2 for 24h were loaded with DCFH-DA and the intracellular ROS level was measured by flow cytometry. Results shown are the mean±SE of three separate experiments performed in triplicate. Treatment with H2O2 for 24h at a concentration range of 0 to 800 µmol/L caused a linear rise in ROS level and reached a maximum at 800 µmol/L in D407 cells. aP<0.05, bP<0.01 versus CTL for one-way ANOVA. CTL: Untreated cells.
Mitochondrial Location and Expression of P-glycoprotein in D407 Cells Subjected to Oxidative Stress
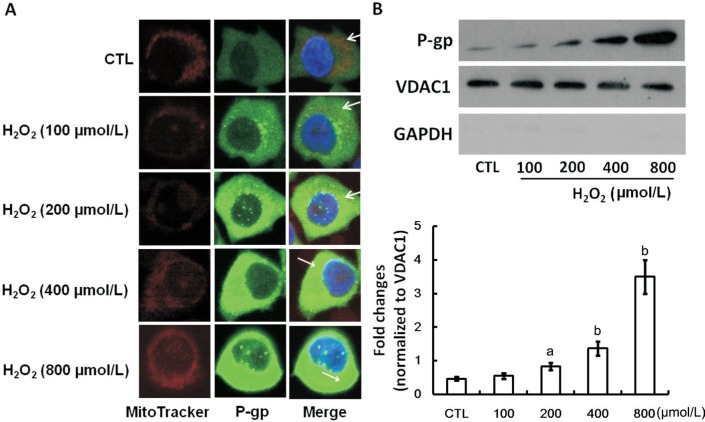
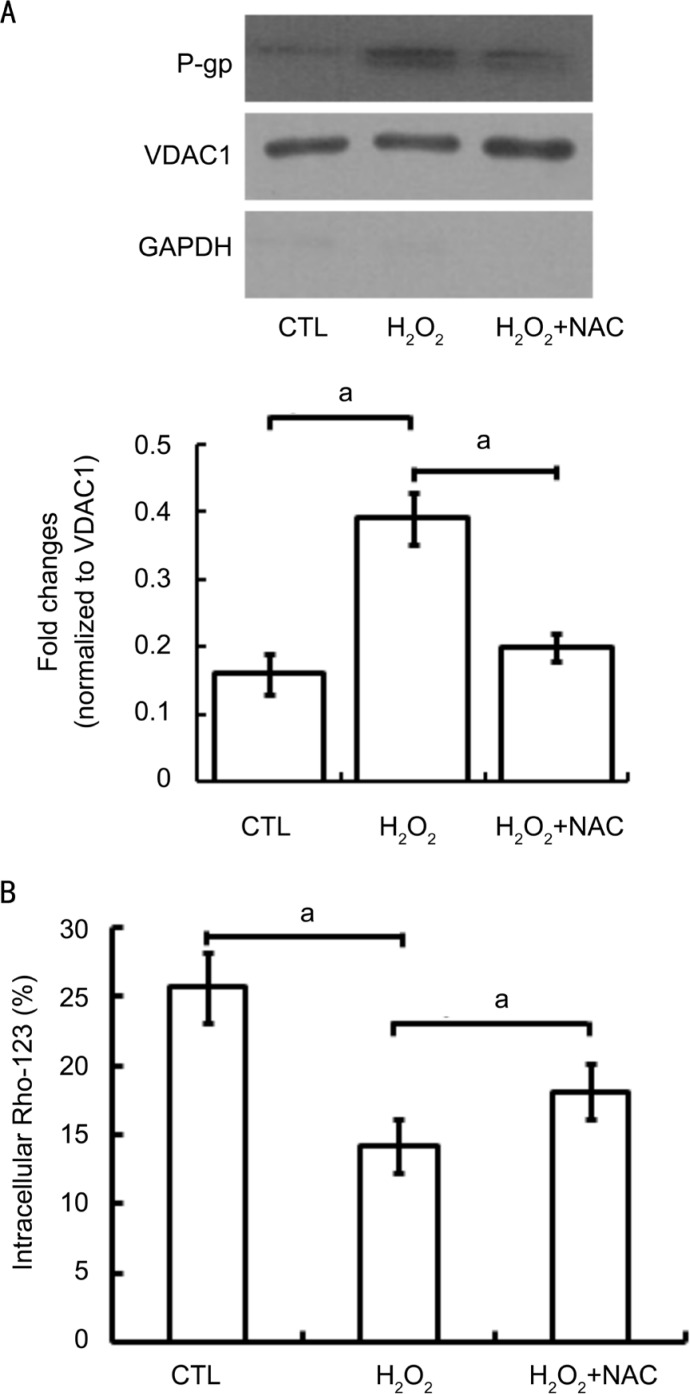
Figure 2A is the confocal microscopic picture presenting the mitochondrial location of P-gp in D407 cells subjected to oxidative stress. We found a visible but very faint overlap of P-gp and mitochondria fluorescence in untreated cells, indicating the colocalization of P-gp and mitochondria in D407 cells. A gradually increasing overlap of P-gp and mitochondria fluorescence was found in cells exposure to increased concentration of H2O2, indicating that P-gp mitochondrial expression increases with the increase of H2O2 exposure concentration. We next examined the P-gp expression in the mitochondria of D407 cells under oxidative stress state. The mitochondrial proteins of D407 cells were extracted after exposing to 0-800 µmol/L H2O2 and P-gp expression was analyzed by Western blot. Figure 2B shows that P-gp expression was observed in the mitochondrial extracts of untreated D407 cells and gradually increased expressions were observed in the increasing exposure concentrations of H2O2. No expression of GAPDH was observed in all samples.
Figure 2. Mitochondrial expression and location of P-gp in untreated and H2O2-incubated D407 cells.
A: Representative confocal micrographs of D407 monolayers showed that D407 cells have exact overlapping P-gp staining (green) and mitochondria staining (red), indicating colocalization of P-gp and mitochondria (as shown in white arrowhead); B: A representative Western blot picture showed the alterations of mitochondrial P-gp in translational level in D407 cells after exposing to varying concentration of H2O2 for 24h. VDAC1 was used as the internal loading control of mitochondrial proteins and GAPDH was used as a cytoplasm marker. The relative protein expression was expressed as the gray value ratio of target protein to respective VDCA1. The average expression of each protein was calculated from three independent experiments. aP<0.05, bP<0.01 versus CTL for one-way ANOVA. CTL: Untreated cells.
Effect of Oxidative Stress on the Activity of P-glycoprotein in Mitochondria of D407 Cells
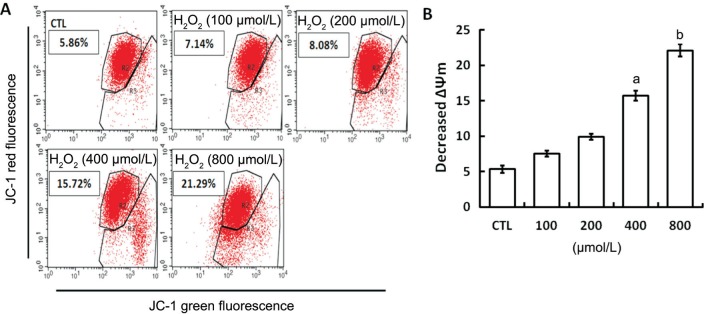
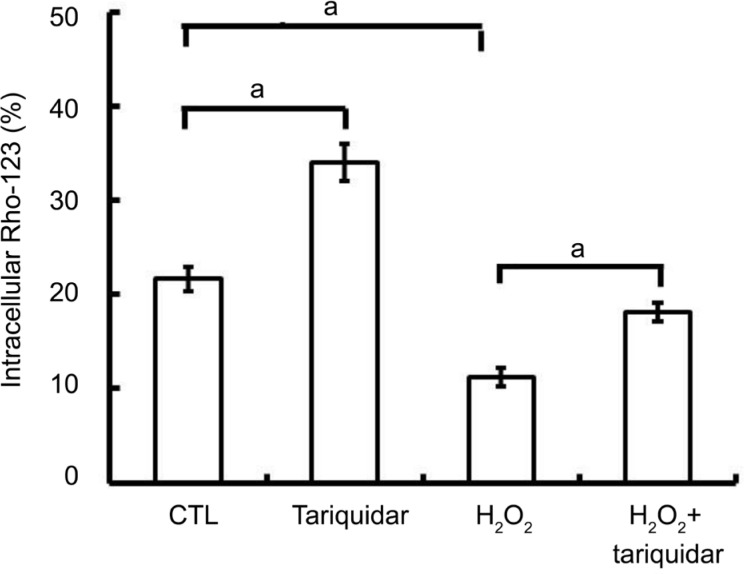
To excel the influence of mitochondrial integrity destruction induced by oxidative stress on P-gp activity detection, we first measured the alterations of ΔΨm using flow cytometry (Figure 3). When cells were exposed to 100 or 200 µmol/L H2O2, the ΔΨm had only a slight decrease (both P>0.05 when comparing to untreated cells) which indicates that the mitochondrial integrity has not been significantly damaged by exposure to H2O2 less than 200 µmol/L for 24h. When cells were exposed to 400 or 800 µmol/L H2O2 for 24h, the levels of ΔΨm were significantly decreased (both P<0.05 when comparing to untreated cells). To further exclude cytotoxic effects, the CCK-8 assay was performed. We found that the cell viability was 82.16%±5.03% when incubated with 200 µmol/L H2O2 for 24h. Pursuant to ISO 10993-5, percentages of cell viability above 80% are considered as non-cytotoxicity. We therefore chose cells exposure to 200 µmol/L H2O2 to investigate the effects of oxidative stress on P-gp activity in mitochondria of D407 cells. Figure 4 showed that in untreated cells, the intracellular Rho-123 fluorescence intensity was 22.31%±1.15% and it was increased to 33.98%±1.98% after P-gp activity was inhibited by 1 µmol/L tariquidar pretreatment for 1h (P<0.05). When cells were treated with 200 µmol/L H2O2 for 24h or combined pretreatment with 1 µmol/L tariquidar for 1h, the intracellular Rho-123 fluorescence intensity was 11.20%±1.01% and 18.10%±0.98%, respectively (P<0.05). These results indicate that the functional expression of P-gp be detected in the normal mitochondria of D407 cells and its functional expression was increased under oxidative stress state.
Figure 3. Changes of ΔΨm induced by H2O2 incubation in D407 cells.
A: The changes of ΔΨm induced by H2O2 incubation in D407 cells were detected by flow cytometry. JC-1 was a ΔΨm indicator which could be used to demonstrate the changes in ΔΨm. The dye fluoresces red when it aggregates in cells with high ΔΨm, whereas it fluoresces green in cells with low ΔΨm. The green/red fluorescence intensity ratio is expressed as decreased ΔΨm, which represents the mitochondrial depolarization ratio; B: Quantitative densitometric analysis of the decreased ΔΨm by exposure to increasing concentrations of H2O2 was performed in three independent experiments. aP<0.05, bP<0.01 versus CTL for one-way ANOVA. CTL: Untreated cells.
Figure 4. Effect of oxidative stress on the activity of P-gp in mitochondria of D407 cells.
D407 cells were treated with 200 µmol/L H2O2 alone or in combination with 1 µmol/L of tariquidar (1h prior to H2O2 treatment) for up to 24h. Untreated cells were set as the control (CTL). The activity of P-gp was determined by measuring the intracellular Rho-123 fluorescence intensity using flow cytometry. Data are the mean±SE of three separate experiments. aP<0.05.
Involvement of Antioxidants in the Regulation of P-glycoprotein Functional Expression in Mitochondria of D407 Cells
To further determine whether antioxidants could change the functional expression of P-gp in mitochondria of D407 cells, we pretreated cells with 10 mmol/L NAC (a ROS scavenger) for 30min before exposing D407 cells to H2O2, and analyzed the mitochondrial extracts by Western blot and flow cytometry. The pretreatment of NAC decreased the intracellular ROS level when comparing to cells exposure to H2O2 (data not shown). In Figure 5A, the Western blot result showed that P-gp expression was increased after 200 µmol/L H2O2 exposure while its expression was decreased after NAC pretreatment. Rho-123 accumulation assay further showed that H2O2 treatment induced a significant decrease in the fluorescence intensity of intracellular Rho-123, indicative of an increased P-gp function. As expected, the Rho-123 retention was increased with NAC pretreatment before exposure of 200 µmol/L H2O2 when compared with H2O2 alone, but did not fully return to the level of the control, untreated cultures (Figure 5B). These results indicate that the antioxidant may be involved in the regulation of P-gp functional expression in D407 cells.
Figure 5. Effects of antioxidants on the functional expression of P-gp in mitochondria of D407 cells.
D407 cells were treated with 200 µmol/L H2O2 alone or in combination with 10 mmol/L NAC (30min prior to H2O2 treatment) for up to 24h. Untreated cells were set as the control (CTL). The mitochondrial expression of P-gp was detected by Western blot and the activity of P-gp was determined by measuring intracellular Rho-123 accumulations by flow cytometry. A: A representative Western blot image shows the mitochondrial expression of P-gp. VDAC1 was used as the internal loading control of mitochondrial proteins and GAPDH was used as a cytoplasm marker; B: Quantitative densitometric analysis of the mean fluorescence intensity of intracellular Rho-123 following different treatment was performed in three independent experiments. aP<0.05 versus CTL for one-way ANOVA.
DISCUSSION
Mitochondrion is becoming an attractive target for drug-delivery in many oxidative stress-induced diseases[30]. It remains elusive that whether the mitochondrial drug-delivery executes less therapeutic potential than expected due to the disturbance of drug efflux pump. We therefore conducted this study to investigate the functional expression of P-gp in mitochondria of human D407 RPE cells under normal and oxidative stress conditions and its influence to the drug-delivery across mitochondrial membranes. Here we demonstrated the mitochondrial presence of P-gp in the untreated D407 cells and increased mitochondrial expression under oxidative stress conditions. The efflux assay detected by quantifying the fluorescence intensity of intracellular Rho-123 shows that H2O2-exposed cells have increased transport of Rho-123 across mitochondrial membranes than untreated cells. In addition, administration of the antioxidant NAC led to the down-regulation of P-gp functional expression in mitochondria of H2O2-exposed D407 cells.
Age is the strongest risk factor for AMD[6] and the oxidative stress is believed to be a primary contributor to the pathogen-esis of AMD[3]. Oxidative stress sensitizes RPE cells to injury and mediates the RPE cells dysfunction, so growing increasing studies on the AMD pathogenesis have been focused on defects in RPE function induced by oxidative stress[31]–[32]. The sources of oxidative stress in RPE cells are diverse. High oxygen tension exposure from the choriocapillaris, long-term sunlight exposure and excess accumulation of lipofuscin are all significant source of oxidative stress to the RPE, which can lead to destruction of the RPE mitochondrial network and induce further pathological cascades of toxicity, inflammation and neurodegeneration process[33]–[34]. Although some protective mechanisms work in RPE during the early phases of AMD, mitochondria are vulnerable to oxidative stress[4]. We chose the point of mitochondrial network damage during oxidative stress in RPE cells because of the evidences that mitochondrial damage in AMD eyes parallels disease severity[35] and eyes obtained postmortem from AMD patients have more obvious free radical damage to mitochondria of RPE cells than controls[36].
Expression of P-gp efflux transporter has been detected in different RPE models including primary RPE cells and secondary ARPE-19 and D407 RPE cell lines. It was indicated a different efflux transporter expression profile in the different RPE model. Constable et al[37] showed that P-gp was expressed in D407 cells but was not in ARPE-19 cells. Chen et al[38] found a significantly different efflux transporter expression profile in ARPE-19 cells when comparing other RPE models and suggested to use ARPE-19 with caution in the efflux transporter research. However, Mannermaa et al[39] thought that ARPE-19 and primary RPE cells had similar efflux protein profile. We think this discrepancy from different laboratory may be caused by the different process of cell culture and cell culture materials such as P-gp substrate and inhibitor. We previously identified expression of P-gp efflux transporter in our laboratory on human D407 RPE cell line[40]–[41]. To see from these previously published literatures, we chose D407 cells to investigate the efflux pump in mitochondria of outer BRB in the present study. We demonstrated the mitochondrial localization and expression of P-gp in D407 cells by cellular staining assays and Western blot. In obtained confocal microscopic pictures, the exact overlapping P-gp (green) and mitochondria staining (red) indicates colocalization of P-gp and mitochondria in D407 cells. Purified mitochondria were isolated from D407 cells and the visible band of about 170 kDa following reacting with anti-P-gp antibody further indicated the presence of P-gp on the mitochondria of D407 cells. The functional expression of P-gp in mitochondria has been increasingly recognized[25],[27]. However, some researchers queried the location of P-gp in the mitochondrial membranes and they attributed the presence of P-gp in the mitochondrial fraction to the plasma membrane contamination[42]. To expel the possibility of false positive induced by contaminated plasma membrane fractions, we chose both VDAC1 and GAPDH as the internal loading controls. VDAC1 was used as the internal loading control of mitochondrial proteins and GAPDH was used as a cytoplasm marker. Our findings showed the negative GAPDH expression in isolated mitochondria, which indicated that the isolated mitochondria had not been contaminated by plasma membrane fractions.
We found that the intracellular ROS levels gradually increased in response to increased H2O2 concentration. Accordingly, P-gp expression in mitochondria also increased with the increased oxidative stress. P-gp expresses in a wide range of excretory and barrier tissues and it is proposed that P-gp serves a protective function in these tissues by limiting the absorption and distribution of harmful xenobiotics[43]. Induction of P-gp activity is becoming a new therapeutic focus to limit the toxicity caused by its substrates[44]. Alterations in P-gp functional expression following H2O2 exposure have been found in several tissues and cell lines and the potential role of P-gp under oxidative stress in these in vitro models of H2O2 exposure is also indicated. In most of the reports, P-gp expression was up-regulated after exposure of H2O2[45]–[46], while only few reported the down-regulated expression[47]. It was proposed to be relative between the amount of ROS and P-gp expression following H2O2 exposure in these published literatures. Because RPE is functionally very similar to the blood-brain barrier (BBB), we focused on some investigations of P-gp in BBB and found that consistent with some of the reports, the finding that up-regulated P-gp expression parallels with the increased ROS generation in our study was also found in brain endothelial cells[46],[48].
In some published literatures, it is proposed that P-gp executes the protective effects from oxidative injury might be realized by activating the antioxidant defenses or up regulating some protective anti-oxidative signal pathways[23]–[25]. It needs further investigation whether P-gp has a specific role in regulating the caspase dependent apoptotic pathway or not in RPE cells. In our study, the fact that NAC attenuated P-gp expression induced by H2O2 indicates that the altered P-gp expression might affect the transport of P-gp substrates in RPE cells under conditions of oxidative stress. Another indication of our findings was that the oxidative stress in RPE may induce P-gp functional expression to an extent, but the excessive oxidative damage might increase difficulties in protection against toxicity caused by its substrates or alteration pharmacokinetics of therapeutic drugs targeted to the retinopathies.
In summary, we investigated the location and expression of P-gp in mitochondria of D407 cells under oxidative stress conditions. One implication of our study is to propose a potential role of P-gp in the mitochondria targeted therapeutic intervention by changing drug transport across mitochondrial membranes. Another implication is to propose the necessity of concurrent application of antioxidant in limiting the mitochondrial P-gp transport and improving the effect of mitochondria-targeted therapy in AMD-like retinopathy.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81400424); Guangdong Medical Science Foundation (No.A2015151).
Conflicts of Interest: Zhang YH, None; Li J, None; Yang WZ, None; Xian ZH, None; Feng QT, None; Ruan XC, None.
REFERENCES
- 1.Cai L, Liao HF, Zhang XJ, Shao Y, Xu M, Yi JL. Acetylcholinesterase function in apoptotic retina pigment epithelial cells induced by H2O2. Int J Ophthalmol. 2013;6(6):772–777. doi: 10.3980/j.issn.2222-3959.2013.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao H, Seo SJ, Biswal MR, Li H, Conners M, Nandyala A, Jones K, Le YZ, Lewin AS. Mitochondrial oxidative stress in the retinal pigment epithelium leads to localized retinal degeneration. Invest Ophthalmol Vis Sci. 2014;55(7):4613–4627. doi: 10.1167/iovs.14-14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plafker SM, O'Mealey GB, Szweda LI. Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol. 2012;298:135–177. doi: 10.1016/B978-0-12-394309-5.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cano M, Wang L, Wan J, Barnett BP, Ebrahimi K, Qian J, Handa JT. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic Biol Med. 2014;69:1–14. doi: 10.1016/j.freeradbiomed.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Zhuang P, Xiao T, Chiou GC. Effect of cytokeratin 17 on retinal pigment epithelium degeneration and choroidal neovascularization. Int J Ophthalmol. 2016;9(3):363–368. doi: 10.18240/ijo.2016.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopdar A, Chakravarthy U, Verma D. Age related macular degeneration. BMJ. 2003;326(7387):485–488. doi: 10.1136/bmj.326.7387.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simó R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier-implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Liu RT, Cao S, Cui JZ, Wang A, To E, Matsubara JA. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators Inflamm. 2015;2015:690243. doi: 10.1155/2015/690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hytti M, Piippo N, Salminen A, Honkakoski P, Kaarniranta K, Kauppinen A. Quercetin alleviates 4-hydroxynonenal-induced cytotoxicity and inflammation in ARPE-19 cells. Exp Eye Res. 2015;132:208–215. doi: 10.1016/j.exer.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Nowak JZ. AMD-the retinal disease with an unprecised etiopathogenesis: in search of effective therapeutics. Acta Pol Pharm. 2014;71(6):900–916. [PubMed] [Google Scholar]
- 11.Joseph K, Kulik L, Coughlin B, Kunchithapautham K, Bandyopadhyay M, Thiel S, Thielens NM, Holers VM, Rohrer B. Oxidative stress sensitizes retinal pigmented epithelial (RPE) cells to complement-mediated injury in a natural antibody-, lectin pathway-, and phospholipid epitope-dependent manner. J Biol Chem. 2013;288(18):12753–12765. doi: 10.1074/jbc.M112.421891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saprunova VB, Lelekova MA, Kolosova NG, Bakeeva LE. SkQ1 slows development of age-dependent destructive processes in retina and vascular layer of eyes of wistar and OXYS rats. Biochemistry (Mosc) 2012;77(6):648–658. doi: 10.1134/S0006297912060120. [DOI] [PubMed] [Google Scholar]
- 13.Markovets AM, Fursova AZ, Kolosova NG. Therapeutic action of the mitochondria-targeted antioxidant SkQ1 on retinopathy in OXYS rats linked with improvement of VEGF and PEDF gene expression. PLoS One. 2011;6(7):e21682. doi: 10.1371/journal.pone.0021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, Liu J. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Invest Ophthalmol Vis Sci. 2007;48(1):339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova N, Victor VM. Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal. 2015;22(8):686–729. doi: 10.1089/ars.2014.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobales N, Nuñez RE, Jang S, Parodi-Rullan R, Ayala-Peña S, Sacher JR, Skoda EM, Wipf P, Frontera W, Javadov S. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J Mol Cell Cardiol. 2014;77:136–146. doi: 10.1016/j.yjmcc.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dranka BP, Gifford A, McAllister D, Zielonka J, Joseph J, O'Hara CL, Stucky CL, Kanthasamy AG, Kalyanaraman B. A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 [LRRK2(R1441G)] transgenic mouse model of Parkinson's disease. Neurosci Lett. 2014;583:159–164. doi: 10.1016/j.neulet.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porteous CM, Menon DK, Aigbirhio FI, Smith RA, Murphy MP. P-glycoprotein (Mdr1a/1b) and breast cancer resistance protein (Bcrp) decrease the uptake of hydrophobic alkyl triphenylphosphonium cations by the brain. Biochim Biophys Acta. 2013;1830(6):3458–3465. doi: 10.1016/j.bbagen.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fetisova EK, Avetisyan AV, Izyumov DS, Korotetskaya MV, Chernyak BV, Skulachev VP. Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress. FEBS Lett. 2010;584(3):562–566. doi: 10.1016/j.febslet.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Anand BS, Dey S, Mitra AK. Current prodrug strategies via membrane transporters/receptors. Expert Opin Biol Ther. 2002;2(6):607–620. doi: 10.1517/14712598.2.6.607. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 22.Guenova ML, Balatzenko GN, Nikolova VR, Spassov BV, Konstantinov SM. An anti-apoptotic pattern correlates with multidrug resistance in acute myeloid leukemia patients: a comparative study of active caspase-3, cleaved PARPs, Bcl-2, Survivin and MDR1 gene. Hematology. 2010;15(3):135–143. doi: 10.1179/102453309X12583347113690. [DOI] [PubMed] [Google Scholar]
- 23.Tainton KM, Smyth MJ, Jackson JT, Tanner JE, Cerruti L, Jane SM, Darcy PK, Johnstone RW. Mutational analysis of P-glycoprotein: suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004;11(9):1028–1037. doi: 10.1038/sj.cdd.4401440. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani I, Cappellini A, Tazzari PL, Papa V, Cocco L, Martelli AM. Caspase-dependent cleavage of 170-kDa P-glycoprotein during apoptosis of human T-lymphoblastoid CEM cells. J Cell Physiol. 2006;207(3):836–844. doi: 10.1002/jcp.20628. [DOI] [PubMed] [Google Scholar]
- 25.Solazzo M, Fantappiè O, Lasagna N, Sassoli C, Nosi D, Mazzanti R. P-gp localization in mitochondria and its functional characterization in multiple drug-resistant cell lines. Exp Cell Res. 2006;312(20):4070–4078. doi: 10.1016/j.yexcr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Barot M, Gokulgandhi MR, Pal D, Mitra AK. Mitochondrial localization of P-glycoprotein and peptide transporters in corneal epithelial cells-novel strategies for intracellular drug targeting. Exp Eye Res. 2013;106:47–54. doi: 10.1016/j.exer.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munteanu E, Verdier M, Grandjean-Forestier F, Stenger C, Jayat-Vignoles C, Huet S, Robert J, Ratinaud MH. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem Pharmacol. 2006;71(8):1162–1174. doi: 10.1016/j.bcp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Chu Y, Yang Y, Wang Z. Mitochondrial localization of P-glycoprotein in the human breast cancer cell line MCF-7/ADM and its functional characterization. Oncol Rep. 2012;27(5):1535–1540. doi: 10.3892/or.2012.1671. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Lee TH, Tung CH, Lee DY. Design and synthesis of a mitochondria-targeting carrier for small molecule drugs. Org Biomol Chem. 2014;12(48):9793–9796. doi: 10.1039/c4ob01981d. [DOI] [PubMed] [Google Scholar]
- 30.Anders MW, Robotham JL, Sheu SS. Mitochondria: new drug targets for oxidative stress-induced diseases. Expert Opin Drug Metab Toxicol. 2006;2(1):71–79. doi: 10.1517/17425255.2.1.71. [DOI] [PubMed] [Google Scholar]
- 31.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol Histopathol. 2007;22(12):1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 32.Miter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, Bowes Rickman C, Boulton M. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10(11):1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229(5):729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284(25):16939–16947. doi: 10.1074/jbc.M808166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin H, Xu H, Liang FQ, Liang H, Gupta P, Havey AN, Boulton ME, Godley BF. Mitochondrial DNA damage and repair in RPE associated with aging and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3521–3529. doi: 10.1167/iovs.10-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(11):5470–5479. doi: 10.1167/iovs.10-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006;83(1):24–30. doi: 10.1016/j.exer.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Chen P, Chen H, Zang X, Chen M, Jiang H, Han S, Wu X. Expression of efflux transporters in human ocular tissues. Drug Metab Dispos. 2013;41(11):1934–1948. doi: 10.1124/dmd.113.052704. [DOI] [PubMed] [Google Scholar]
- 39.Mannermaa E, Vellonen KS, Ryhanen T, Kokkonen K, Ranta VP, Kaarniranta K, Urtti A. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26(7):1785–1791. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Lu M, Sun X, Li C, Kuang X, Ruan X. Expression and activity of p-glycoprotein elevated by dexamethasone in cultured retinal pigment epithelium involve glucocorticoid receptor and pregnane X receptor. Invest Ophthalmol Vis Sci. 2012;53(7):3508–3515. doi: 10.1167/iovs.11-9337. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Li C, Sun X, Kuang X, Ruan X. High glucose decreases expression and activity of p-glycoprotein in cultured human retinal pigment epithelium possibly through iNOS induction. PLoS One. 2012;7(2):e31631. doi: 10.1371/journal.pone.0031631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paterson JK, Gottesman MM. P-Glycoprotein is not present in mitochondrial membranes. Exp Cell Res. 2007;313(14):3100–3105. doi: 10.1016/j.yexcr.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nawa A, Fujita Hamabe W, Tokuyama S. Inducible nitric oxide synthase-mediated decrease of intestinal P-glycoprotein expression under streptozotocin-induced diabetic conditions. Life Sci. 2010;86(11-12):402–409. doi: 10.1016/j.lfs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Silva R, Vilas-Boas V, Carmo H, Dinis-Oliveira RJ, Carvalho F, de Lourdes Bastos M, Remião F. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1–123. doi: 10.1016/j.pharmthera.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Laberge RM, Ambadipudi R, Georges E. P-glycoprotein (ABCB1) modulates collateral sensitivity of a multidrug resistant cell line to verapamil. Arch Biochem Biophys. 2009;491(1-2):53–60. doi: 10.1016/j.abb.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Robertson SJ, Kania KD, Hladky SB, Barrand MA. P-glycoprotein expression in immortalised rat brain endothelial cells: comparisons following exogenously applied hydrogen peroxide and after hypoxia-reoxygenation. J Neurochem. 2009;111(1):132–141. doi: 10.1111/j.1471-4159.2009.06306.x. [DOI] [PubMed] [Google Scholar]
- 47.Terada Y, Ogura J, Tsujimoto T, Kuwayama K, Koizumi T, Sasaki S, Maruyama H, Kobayashi M, Yamaguchi H, Iseki K. Intestinal P-glycoprotein expression is multimodally regulated by intestinal ischemia-reperfusion. J Pharm Pharm Sci. 2014;17(2):266–276. doi: 10.18433/j3jg7d. [DOI] [PubMed] [Google Scholar]
- 48.Nwaozuzu OM, Sellers LA, Barrand MA. Signalling pathways influencing basal and H(2)O(2)-induced P-glycoprotein expression in endothelial cells derived from the blood-brain barrier. J Neurochem. 2003;87(4):1043–1051. doi: 10.1046/j.1471-4159.2003.02061.x. [DOI] [PubMed] [Google Scholar]