Abstract
Sleep accounts for a third of one's lifetime, partial or complete deprivation of sleep could elicit sever disorders of body function. Previous studies have reported the higher prevalence of sleep disorders in glaucoma patients, but the definite mechanism for this phenomenon is unknown. On the other hand, it is well known by us that the intrinsically photosensitive retinal ganglion cells (ipRGCs) serve additional ocular functions, called non-image-forming (NIF) functions, in the regulation of circadian rhythm, melatonin secretion, sleep, mood and others. Specifically, ipRGCs can directly or indirectly innervate the central areas such as suprachiasmatic nucleus (SCN), downstream pineal gland (the origin of melatonin), sleep and wake-inducing centers and mood regulation areas, making NIF functions of ipRGCs relate to sleep. The more interesting thing is that previous research showed glaucoma not only affected visual functions such as the degeneration of classical retinal ganglion cells (RGCs), but also affected ipRGCs. Therefore, we hypothesize that higher prevalence of sleep disorders in glaucoma patients maybe result from the underlying glaucomatous injuries of ipRGCs leading to the abnormalities of diverse NIF functions corresponding to sleep.
Keywords: glaucoma, intrinsically photosensitive retinal ganglion cells, sleep disorders
INTRODUCTION
Glaucoma, the leading cause of irreversible blindness in the world, is characterized by a degenerative and progressive optic neuropathy that leads to structural and functional changes in the optic nerve and retinal ganglion cells (RGCs)[1]. Previous studies have reported the incidence of sleep disorders, which was characterized by excessive daytime sleepiness, delayed onset of sleep, shortened sleep duration, and increased spontaneous arousals[2]–[3], was higher in glaucoma patients than that in the control subjects[4]–[5]. It is well known that many factors contribute to sleep disorders in glaucoma patients, including concerns about the disease, ophthalmic pain, the burden of treatment, and the effects of comorbidities such as depression and anxiety. However, the pathogenic mechanism of these problems has not been fully characterized.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), are a distinct subpopulation of RGCs, functioning as a kind of novel photoreceptor which expresses melanopsin[6]. Studies recently show that ipRGCs can affect sleep through direct and indirect pathways. The direct pathway is to influence the onset and homeostasis of sleep by regulating the sleep and wake-inducing centers[7]. The indirect pathways, which contain the ipRGCs projection to suprachiasmatic nucleus (SCN) regulating melatonin secretion[8] and the projection to mood regulation areas[9], could impact many aspects of sleep. Some research suggested ipRGCs damage in glaucoma, along with diverse dysfunctions and a decrease in the number of ipRGCs[10]–[14]. These studies strongly indicate a connection between the ipRGCs and higher prevalence of sleep disorders in glaucoma patients.
INTRINSICALLY PHOTOSENSITIVE RETINAL GANGLION CELLS
The Characteristics of ipRGCs
More than a decade ago, it was reported that there were exclusively cone and rod light sensitive cells which transmit polysynaptic information via the optic nerve to the brain. Subsequently, scientists identified the third class of photoreceptor in rodent retina that was named melanopsin-containing RGCs or ipRGCs which exhibit intrinsic photosensitivity[6],[15]. More than 95% ipRGCs are localized in the ganglion cell layer, with 5% found in the inner nuclear layer in rodent retina[6]. These distinct light transduction cells, which can detectirradiance of light, are sensitive to the wavelength of around 480 nm[15]–[16]. The ocular light detecting system is therefore comprised of pathways containing the classic image-forming system involving rods and cones, and the non-visual phototransduction system involving the retinohypothalamic tract to the SCN, which is the central circadian pacemaker in the anterior hypothalamus. In addition to ipRGCs projection to the SCN, it also innervates other regions throughout the brain, such as the olivary pretectal nucleus[6], which is the relay system for the pupillary light reaction, the ventrolateral preoptic (VLPO) area and lateral hypothalamus (LH)[7], which are important for the regulation of sleep. Furthermore, the areas in relation to mood regulation, involving the medial amygdala and lateral habenula (LHb), as well as their downstream areas (i.e. the ventral tegmental area and raphe)[6],[9],[17]–[18], are also projected by ipRGCs. Recently, studies reported that the classic and non-classical visual system could influence each other as well[19], which deserves further study. However, the discoveries of more ipRGCs target areas and their related functions have suggested connections between ipRGCs and diseases.
The Relationship Between Sleep and ipRGCs
Although the intact physiological mechanism of sleep is unknown, it is thought that there are sleep and wake-inducing systems in the brain, which are mutually inhibiting in the maintenance of sleep homeostasis[20]–[21]. ipRGCs make direct projection to the VLPO and LH, the former of which expresses inhibitory neurotransmitters γ-aminobutyric acid (GABA) and galanin, and plays a key role in the promotion of sleep[7]. The LH expresses hypocretin, which has excitatory effects on almost every wake-promoting neuronal group of ascending reticular activating systems and enhances the wakeful state through activating wake-inducing systems[22]–[24]. Also, ipRGCs via SCN and downstream neurons indirectly project to the VLPO, LH, and the locus coeruleus (LC), which plays an important function in wake-inducing system[25]–[26]. ipRGCs perceive ambient light during daytime, and send projection to active the LH and the LC respectively through excitatory neurotransmitters glutamate and orexin[24],[27]–[28]. ipRGCs also via interneurons output the GABA-ergic signals to VLPO to inhibit sleep[28]. The overall effect of light during daytime is to maintain wakeful state. And during nighttime, ipRGCs without light input may disinhibit the VLPO and can sustain sleep state[23],[28]. These structural and functional connections between ipRGCs and sleep centers provide the basis for deciphering sleep disorders in glaucoma patients.
ipRGCs perceive the environmental zeitgebers and deliver signals via retinohypothalamic tract to the SCN, which oscillates with a periodicity that is slightly longer than a solar day[29]–[30]. The SCN integrates the ambient information perceived by ipRGCs and aligns with the environmental period of precisely 24h to adapt environment, then emits the corrected rhythmic signals to control the rhythm and concentrations of melantonin (MT)[8]. MT, a metabolite of tryptophan in the plasma, has periodic plasma concentrations with the peak concentration at approximately 2:00 a.m.[31]–[32]. Previous trials suggested short-wavelength light exposure of ipRGCs could elicit phase shift of MT's rhythm[33]. ipRGCs are exposed to blue light with different intensity or duration, which can inordinately inhibit MT secretion[34]–[35]. Considering that MT has close interaction with sleep[36] and could affect sleep through many aspects: altering neurotransmitters in the cerebrum involving norepinephrine, acetylcholine, and 5-hydroxytryptamine; regulating the rhythm of SCN by binding to MT receptors in the SCN; affecting slow-wave sleep corresponding to MT's effect on body temperature[8],[32],[37]–[38]. We could conclude that ipRGCs relaying at SCN can influence sleep by regulating the synthesis and secretion of MT.
In addition to these functions, ipRGCs also innervate to MA, LHb and their downstream areas, which are critical in regulating mood. Accumulating evidence in humans and animals has linked mood disorders to abnormalities of ipRGCs input, and exposure to light at night may alter mood by disrupting circadian rhythm[39]–[42]. Previous studies have reported that mice exposed to aberrant light directly influenced mood regulation, without disrupting circadian rhythms[43], suggesting that unnatural light exposure can directly affect mood. It is generally accepted that there exists bidirectional relationship between sleep disorders and depression, and an increased incidence of abnormal sleep is associated with mood disorders[44]. So we suggest that mood disorders resulting from abnormalities of ipRGCs can elicit sleep disorders in glaucoma patients.
The ipRGCs Lesions in Glaucoma
Previous studies have reported that glaucoma, an ocular disorder characterized by loss of RGCs, could affect the numbers and functions of ipRGCs[10]–[12],[14]. The initial studies using mutant DBA/2J mice reported that glaucomatous RGCs degeneration was not cell type specific, which indicated that ipRGCs might also be damaged in glaucoma[10]. Additional studies reported that melanopsin-containing RGCs were damaged in rats with chronic ocular hypertension[12], and decreased numbers of ipRGCs resulting from chronic ocular hypertension were observed[11],[14].
Besides these animal studies, there have been clinical studies focusing on glaucoma and ipRGCs. With the discovery of melanopsin and the characterization of the non-image-forming (NIF) functions system, studies showed that the post-illumination pupil response (PIPR) to blue light could be a specific measure for testing the intrinsic activity of ipRGCs[45]–[46]. Clinical researches showed that blue light PIPR significantly decreased in glaucomatous patients when compared with age-matched controls[13],[47]. And there was a correlated decrease in the PIPR with the increasing severity of glaucomatous neuropathy[47]. The discovery of a positive correlation between blue light PIPR and retinal nerve fiber layer (RNFL) thickness showed that decreased numbers of ipRGCs was potentially related to the reduced RNFL thickness[48]–[49]. Some works showed the abnormality of circadian rhythm or light-suppression of MT secretion, may also be caused by disrupted ipRGCs in glaucoma[50]–[51]. These results suggest that glaucoma could disrupt ipRGCs and leads to various dysfunctions of ipRGCs.
THE HYPOTHESIS
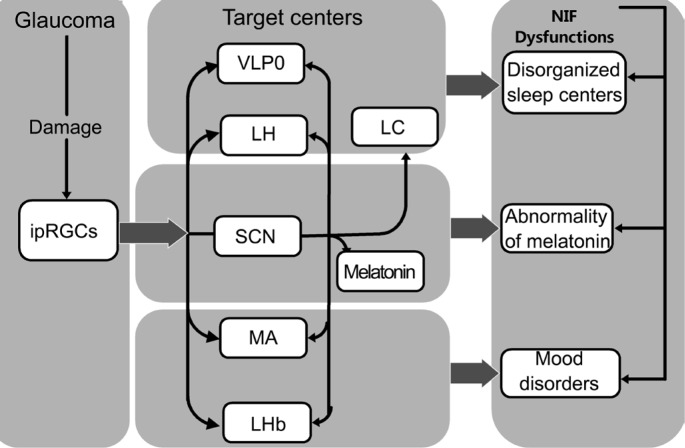
Previous studies have demonstrated the glaucomatous lesions of ipRGCs and the various relationships between ipRGCs and sleep. So we propose the following hypothesis about the mechanism of sleep disorders in glaucoma: the higher prevalence of sleep disorders in glaucoma patients may be caused by the underlying glaucomatous injuries of ipRGCs, leading to diverse NIF dysfunctions corresponding to sleep. Abnormal NIF functions related to sleep involve the disturbance of sleep centers, the abnormality of MT and mood disorders (Figure 1) [52]. Reproduced from reference[52].
Figure 1. A schematic view of the glaucomatous lesions in ipRGCs leading to various NIF dysfunctions corresponding to sleep.
DISCUSSION
Glaucoma, a progressive and to date incurable ocular disease, will affect 79.6 million people around the globe and 6 million in China by 2020[53], meanwhile the higher prevalence of sleep disorders in glaucoma worsens the life quality of glaucoma patients. Prior studies attributed the sleep disorders of glaucoma patients to the mental-psychological factors or ocular ache. Nevertheless, the discovery of ipRGCs and NIF functions submits us the implications that ipRGCs lesions in glaucoma leading to the disturbance of sleep centers, the abnormality of MT and mood disorders may be the possible causation accounting for sleep disorders in glaucoma patients. It should be mentioned that sleep disorders in the present article do not include (obstructive) sleep apnea syndrome, which is are search focus between sleep and glaucoma[54] and has it's specific pathomechanism.
Recent discoveries revealed that ipRGCs were not uniform population. Based on morphological and electrophysiological properties, the ipRGCs were identified as at least five subtypes, namely M1-M5[9],[55]. Each subtype has specific cell size, melanopsin protein level and central projections[56]–[58]. Specific central projections provide specific functions for each ipRGCs subtype. So the specific biological properties of each ipRGCs subtype should be taken into account in future experiments as well as in clinical studies. When exploring the correlation between disrupted NIF functions and abnormal structural parameters, the contribution of ipRGCs subtypes also should be allowed for.
In summary, there exists objective fact and data to support our hypothesis, which would be helpful for individual therapy of sleep disorders in glaucoma patients, thus increasing the life quality of glaucoma patients.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81200687); the National Major Scientific Equipment Program (No.2012YQ12008005); the Young Scholar for the Doctoral Program of Higher Education of China (No.20120181120014).
Conflicts of Interest: Guo ZZ, None; Jiang SM, None; Zeng LP, None; Tang L, None; Li N, None; Xu ZP, None; Wei X, None.
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Lanzani MF, de Zavalía N, Fontana H, Sarmiento MIK, Golombek D, Rosenstein RE. Alterations of locomotor activity rhythm and sleep parameters in patients with advanced glaucoma. Chronobiol Int. 2012;29(7):911–919. doi: 10.3109/07420528.2012.691146. [DOI] [PubMed] [Google Scholar]
- 3.Gracitelli CP, Duque-Chica GL, Moura AL, Roizenblatt M, Nagy BV, de Melo GR, Borba PD, Teixeira SH, Tufik S, Ventura DF, Paranhos A., Jr Relationship between daytime sleepiness and intrinsically photosensitive retinal ganglion cells in glaucomatous disease. J Ophthalmol. 2016;2016:5317371. doi: 10.1155/2016/5317371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Zhang Y, Ding J, Wang N. Changes in the circadian rhythm in patients with primary glaucoma. PLoS One. 2013;8(4):e62841. doi: 10.1371/journal.pone.0062841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agorastos A, Skevas C, Matthaei M, Otte C, Klemm M, Richard G, Huber CG. Depression, anxiety, and disturbed sleep in glaucoma. J Neuropsychiatry Clin Neurosci. 2013;25(3):205–213. doi: 10.1176/appi.neuropsych.12020030. [DOI] [PubMed] [Google Scholar]
- 6.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Shiromani P, Saper CB. Retinal input to the sleep-active ventrolateral preoptic nucleus in the rat. Neuroscience. 1999;93(1):209–214. doi: 10.1016/s0306-4522(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 8.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85(3):335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34(11):572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobs TC, Libby RT, Ben Y, John SWM, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171(2):313–325. doi: 10.1083/jcb.200506099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drouyer E, Dkhissi-Benyahya O, Chiquet C, WoldeMussie E, Ruiz G, Wheeler LA, Denis P, Cooper HM. Glaucoma alters the circadian timing system. PLoS One. 2008;3(12):e3931. doi: 10.1371/journal.pone.0003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HZ, Lu QJ, Wang NL, Liu H, Zhang L, Zhan GL. Loss of melanopsin-containing retinal ganglion cells in a rat glaucoma model. Chin Med J (Engl) 2008;121(11):1015–1019. [PubMed] [Google Scholar]
- 13.Feigl B, Mattes D, Thomas R, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(7):4362–4367. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- 14.de Zavalia N, Plano SA, Fernandez DC, Lanzani MF, Salido E, Belforte N, Sarmiento MI, Golombek DA, Rosenstein RE. Effect of experimental glaucoma on the non-image forming visual system. J Neurochem. 2011;117(5):904–914. doi: 10.1111/j.1471-4159.2011.07260.x. [DOI] [PubMed] [Google Scholar]
- 15.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 16.Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433(7027):745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 17.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23(18):7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hannibal J, Kankipati L, Strang CE, Peterson BB, Dacey D, Gamlin PD. Central projections of intrinsically photosensitive retinal ganglion cells in the macaque monkey. J Comp Neurol. 2014;522(10):2231–2248. doi: 10.1002/cne.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lall GS, Revell VL, Momiji H, Al EJ, Altimus CM, Guler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66(3):417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JR, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6(4):367–378. doi: 10.2174/157015908787386050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FSN, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 24.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29(1):70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4(7):732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 26.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130(1):165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23(33):10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabadi E. Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol. 2013;27(8):659–693. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- 29.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 30.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 31.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336(3):186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 32.Cagnacci A, Elliott JA, Yen SS. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75(2):447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- 33.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 34.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunz D, Mahlberg R, Muller C, Tilmann A, Bes F. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J Clin Endocrinol Metab. 2004;89(1):128–134. doi: 10.1210/jc.2002-021057. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19(1):91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 38.Krauchi K, Wirz-Justice A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology. 2001;25(5 Suppl):S92–S96. doi: 10.1016/S0893-133X(01)00315-3. [DOI] [PubMed] [Google Scholar]
- 39.Fonken LK, Finy MS, Walton JC, Weil ZM, Workman JL, Ross J, Nelson RJ. Influence of light at night on murine anxiety- and depressive-like responses. Behav Brain Res. 2009;205(2):349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Jean-Louis G, Kripke D, Cohen C, Zizi F, Wolintz A. Associations of ambient illumination with mood: contribution of ophthalmic dysfunctions. Physiol Behav. 2005;84(3):479–487. doi: 10.1016/j.physbeh.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Bedrosian TA, Nelson RJ. Influence of the modern light environment on mood. Mol Psychiatry. 2013;18(7):751–757. doi: 10.1038/mp.2013.70. [DOI] [PubMed] [Google Scholar]
- 42.Roecklein KA, Rohan KJ, Duncan WC, Rollag MD, Rosenthal NE, Lipsky RH, Provencio I. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114(1-3):279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riemann D, Berger M, Voderholzer U. Sleep and depression-results from psychobiological studies: an overview. Biol Psychol. 2001;57(1-3):67–103. doi: 10.1016/s0301-0511(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 45.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476(7358):92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52(5):2287–2292. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gracitelli CPB, Duque-Chica GL, Moura AL, Nagy BV, de Melo GR, Roizenblatt M, Borba PD, Teixeira SH, Ventura DF, Paranhos A., Jr A positive association between intrinsically photosensitive retinal ganglion cells and retinal nerve fiber layer thinning in glaucoma. Invest Ophthalmol Vis Sci. 2014;55(12):7997–8005. doi: 10.1167/iovs.14-15146. [DOI] [PubMed] [Google Scholar]
- 49.Gracitelli CP, Duque-Chica GL, Roizenblatt M, Moura AL, Nagy BV, Ragot de Melo G, Borba PD, Teixeira SH, Tufik S, Ventura DF, Paranhos A., Jr Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology. 2015;122(6):1139–1148. doi: 10.1016/j.ophtha.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Kashiwagi K, Tsumura T, Ishii H, Ijiri H, Tamura K, Tsukahara S. Circadian rhythm of autonomic nervous function in patients with normal-tension glaucoma compared with normal subjects using ambulatory electrocardiography. J Glaucoma. 2000;9(3):239–246. doi: 10.1097/00061198-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Rico C, de la Villa P, Arribas-Gomez I, Blanco R. Evaluation of functional integrity of the retinohypothalamic tract in advanced glaucoma using multifocal electroretinography and light-induced melatonin suppression. Exp Eye Res. 2010;91(5):578–583. doi: 10.1016/j.exer.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 52.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balbay EG, Balbay O, Annakkaya AN, Suner KO, Yuksel H, Tunc M, Arbak P. Obstructive sleep apnoea syndrome in patients with primary open-angle glaucoma. Hong Kong Med J. 2014;20(5):379–385. doi: 10.12809/hkmj134021. [DOI] [PubMed] [Google Scholar]
- 55.Sand A, Schmidt TM, Kofuji P. Diverse types of ganglion cell photoreceptors in the mammalian retina. Prog Retin Eye Res. 2012;31(4):287–302. doi: 10.1016/j.preteyeres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27(7):1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol. 2011;519(8):1492–1504. doi: 10.1002/cne.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estevez ME, Fogerson PM, Ilardi MC, Borghuis BG, Chan E, Weng S, Auferkorte ON, Demb JB, Berson DM. Form and function of the M4 cell, an intrinsically photosensitive retinal ganglion cell type contributing to geniculocortical vision. J Neurosci. 2012;32(39):13608–13620. doi: 10.1523/JNEUROSCI.1422-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]