Dear Editor,
I am Dr. Daniel Russell Richardson from the West Virginia University Eye Institute in Morgantown, West Virginia, United States. I write to present a case of uveitis associated with nivolumab, which is a promising new immune checkpoint inhibitor (ICI) for metastatic melanoma and non-small cell lung carcinoma with expanding indications. As the use of nivolumab continues to increase, ophthalmologists must be aware of uveitis as an adverse event. Modulating the immune system to rid the body of cancer is the current trend in oncology. Nivolumab is one such agent that has shown promising results in metastatic melanoma and non-small cell lung carcinoma. Nivolumab acts on the programmed death 1 (PD-1) receptor and serves to tip the immune system into a more activated state. Immune related adverse events with regards to nivolumab include uveitis. However, to date there have been only a few published case reports in the literature regarding nivolumab and uveitis describing presentation, treatment and patient outcome.
A 74-year-old woman with scalp melanoma, stage IVb, BRAF+, presented with three weeks of blurry vision and headaches. Past medical history was significant for a fourteen year history of type 2 diabetes mellitus, coronary artery disease with prior pacemaker placement and subsequent removal, chronic kidney disease, chronic obstructive pulmonary disease, and essential hypertension. At presentation, she was taking amlodipine, aspirin, atorvastatin, carvedilol, fenofibrate, combivent, loratadine, guaifenesin, nitroglycerin, ranitidine, sitagliptin, trazodone and acetaminophen. Additionally, she was a current smoker, with a 28 pack-year history.
Regarding the therapy for metastatic melanoma, she initially underwent treatment with vemurafenib, a BRAF inhibitor, which was discontinued less than two months into treatment due to intolerable myalgia, arthralgia and renal failure. Subsequently, she received nivolumab without adverse events for five months at the time of presentation to the Eye Clinic.
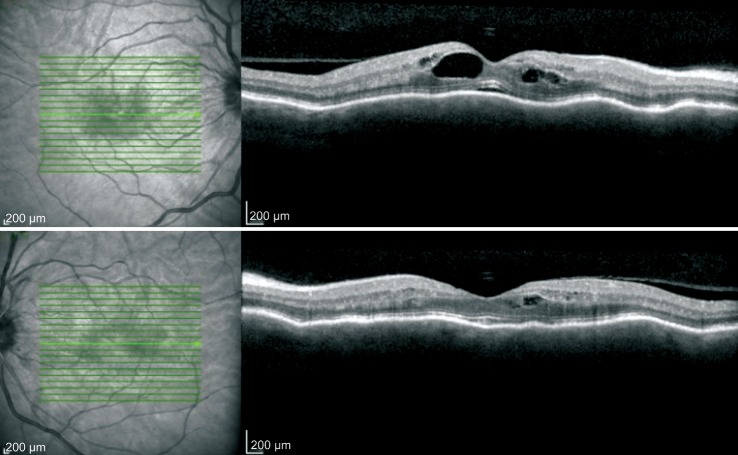
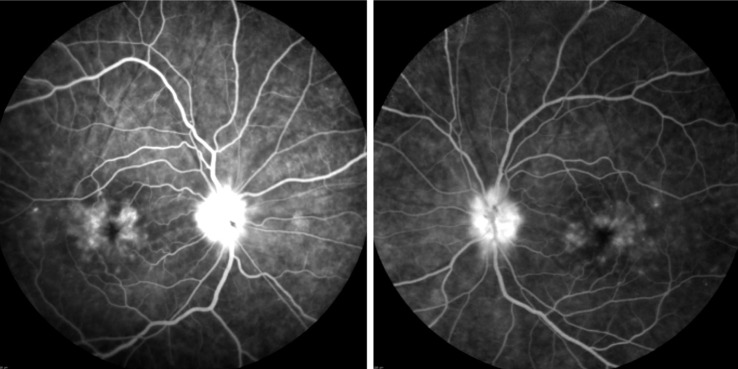
Her initial best corrected visual acuity (BCVA) was 20/100 in the right eye (OD) and 20/40 in the left eye (OS). Her exam was significant for conjunctival and scleral injection both eyes (OU), 2+ anterior chamber cell (using SUN Working Group nomenclature[1]), mild optic disc edema, cystoid macular edema (CME), and chorioretinal folds of OU. She was pseudophakic and intraocular pressure was 15 OD and 17 OS by applanation. Optical coherence tomography and fluorescein angiography (Heidelberg Spectralis) showed CME OU, with more edema OD (Figure 1). She had mild non-proliferative diabetic retinopathy with a few microaneurysms OU (Figure 2). Computed tomography (CT) scan of the brain and orbits was negative for mass or lesion. Magnetic resonance imaging (MRI) was unable to be performed due to presence of pacemaker leads. Laboratory evaluation of the patient's serum, including anti-neutrophil cytoplasmic antibody (ANCA) panel, serum syphilis testing, rheumatoid factor, Quantiferon Gold testing for tuberculosis, Lyme disease serology, angiotensin converting enzyme (ACE) level, anti-nuclear antibodies, was performed and was negative for alternative etiologies for the uveitis. CT scan of the chest did not reveal adenopathy. Nivolumab was discontinued and topical prednisolone acetate eyedrops every 2h OU and oral prednisolone (50 mg daily) were initiated, along with topical cyclosporine twice a day OU. Anterior chamber inflammation subsided and CME improved. Visual acuity returned to 20/25 OD and 20/20 OS by three weeks. The topical steroid course was tapered after two weeks to twice a day.
Figure 1. OCT imaging of OD (top image) and OS (bottom image) at initial presentation demonstrating chorioretinal folds, with cystoid macular edema OU.
Figure 2. Fluorescein angiography images of both eyes show cystoid macular edema, disc edema, choroidal folds and mild non-proliferative diabetic retinopathy.
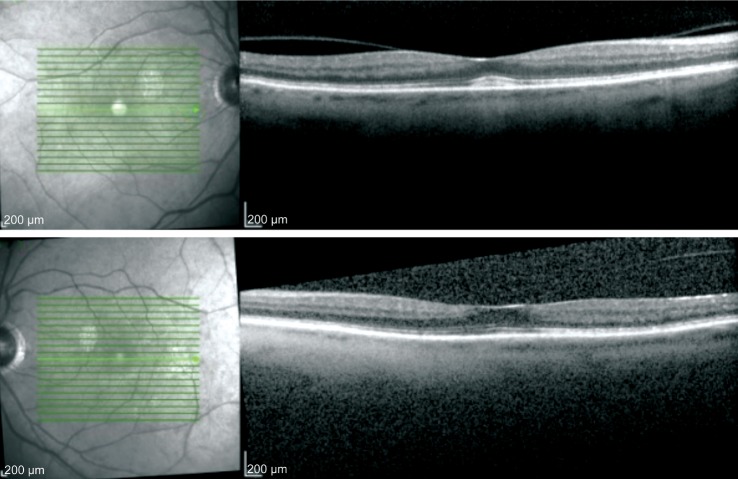
The patient was hospitalized with elevated blood sugars while taking oral prednisone and the prednisone was discontinued prior to the taper being completed. She was then lost to follow up for two months. Upon return to the eye clinic her vision was 20/60 OD and 20/40 OS with return of CME OU. She was given intravitreal triamcinolone acetonide OD and on subsequent visit in OS, following which the CME resolved and her vision returned to 20/40 OU (Figure 3). The prednisolone acetate dose was adjusted to qid during her return visit. Despite resolution of uveitis on subsequent visits and tapering off of topical steroid, her ocular surface remained dry with rapid tear breakup time and mild diffuse fluorescein staining.She was continued on preservative free artificial tears and topical cyclosporine, which we plan to continue indefinitely.Eight months after cessation of the nivolumab a second triamcinolone acetonide injection was given in the right eye for recurrent CME.
Figure 3. OCT images of OD (top image) and OS (bottom image) at 1mo follow up from intravitreal triamcinolone injection respectively demonstrating resolution of chorioretinal folds and cystoid macular edema.
The vitreomacular adhesion persists in both eyes.
ICIs are novel anti-cancer agents which act on specific receptors to enhance the immune system response in combating neoplastic cells. This class of medications includes ipilimumab, nivolumab, and pembrolizumab. ICIs inhibit the stimulatory signal to generate a T-cell response, thereby allowing T-cells to become activated and proliferate. Ipilimumab acts on cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), blocking the interaction of B7 and CTLA-4, and thus removing the inhibitory signal. Nivolumab and pembrolizumab act directly on the PD-1 receptor and block its interaction with its ligands PD-L1 and PD-L2, thus preventing co-inhibition and promoting T-cell proliferation and activation[2]. At this time, ICIs are thought to show benefit in the treatment of metastatic melanoma and squamous non-small cell lung carcinoma. Importantly, indications for currently approved ICIs are expanding as clinical trials are being performed and additional novel ICIs are in clinical trials. By modulating the immune system to induce an antitumor response, ICIs may also induce a shift toward autoimmune disease as an adverse effect of treatment. Lack of functionality of PD-1 has been associated with autoimmunity due to a shift in helper T cells towards a proinflammatory response[3]. In fact, Okazaki and Wang[3] have even suggested that agonizing PD-1 may be beneficial in treatment of autoimmune diseases such as type 1 diabetes mellitus, systemic lupus erythematosus, autoimmune myocarditis, while Gianchecchi et al[4] have further outlined support for PD-1 promotion to ameliorate such ailments as multiple sclerosis, inflammatory bowel disease, rheumatoid arthritis, ankylosing spondylitis, and Graves disease. Specifically of concern for ophthalmologists, PD-1+ T-cells have been shown to be heavily involved in anterior uveitis[5] and posterior uveitis[6]. As nivolumab acts directly on the PD-1 receptor, it is biologically plausible therefore that nivolumab is the etiology of our patient's uveitis.
We are aware of only three published case reports of a patient who experienced autoimmune uveitis associated with nivolumab treatment, as revealed by a PubMed.com search using the terms “uveitis” and “nivolumab”. de Velasco et al[7] report on the case of a patient undergoing nivolumab treatment for metastatic clear renal cell carcinoma with autoimmune uveitis, including CME, and Jaccoud's arthropathy. The patient was reported to have discontinued the nivolumab and received intravitreal steroids and remains cancer-free after 2y. No mention was made of visual acuity, and the only description of the uveitis is an included OCT figure. The other two cases were both published in September of 2016. Arai et al[8] report on a 55-year-old man with metastatic melanoma who experienced bilateral anterior uveitis and vitiligo, poliosis, and alopecia, treated with topical steroid and cycloplegic drops with successful continuation of nivolumab. Karlin et al[9] report a case of bilateral anterior uveitis in a 66-year-old woman undergoing therapy with nivolumab for metastatic non-small cell lung carcinoma. In this case, the nivolumab was able to be continued, and the anterior uveitis was treated successfully with topical prednisolone acetate and cyclopentolate.
Regarding pembrolizumab, Abu Samra et al[10] have described a case of an 82-year-old patient who presented with bilateral autoimmune uveitis and papillitis while undergoing pembrolizumab therapy for BRAF wild-type malignant melanoma. He was treated with topical difluprednate in addition to being on hydrocortisone 40 mg daily, and treatment with pembrolizumab was continued with last reported visual acuity being 20/20 in either eye.
Crews et al[11] have described a case of a 46-year-old man with metastatic melanoma who experienced bilateral serous retinal detachments as a complication of treatment with ipilimumab. However, this patient did not respond to intravenous dexamethasone given 10 mg four times daily for three days and had improvement of visual acuity and reduction of subretinal fluid only after cessation of ipilimumab therapy. Several cases have been described of a VKH-like syndrome previously[12]–[13]. Hahn and Pepple[14] described a bilateral neuroretinitis and anterior uveitis during ipilimumab treatment for metastatic melanoma. In this 44-year-old patient, ipilimumab was continued and the uveitis was treated with topical and oral steroids with resolution of ophthalmic complications.
In the case of our patient, nivolumab was discontinued after discussion between hematology/oncology and ophthalmology. The patient was started on T-VEC injections locally in scalp and neck and therapy with dabrafenib and trametinib, a BRAF/MEK inhibitor combination. As vemurafenib has been associated with uveitis[15], we may continue to inject intravitreal triamcinolone while she undergoes treatment.
Acknowledgments
Conflicts of Interest: Richardson DR, None; Ellis B, None; Mehmi I, None; Leys M, None.
REFERENCES
- 1.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbee MS, Ogyunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49(8):907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 3.Okazaki T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38(5):353–357. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 4.Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12(11):1091–1100. doi: 10.1016/j.autrev.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Meng Q, Yang P, Li B, Zhou H, Huang X, Zhu L, Ren Y, Kijlstra A. CD4+PD-1+ T cells acting as regulatory cells during the induction of anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 2006;47(10):4444–4452. doi: 10.1167/iovs.06-0201. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Pai V, Levinson R, Sharpe AH, Freeman GJ, Braun J, Gordon LK. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1), identified during experimental autoimmune uveitis. Occul Immunol Inflamm. 2009;17(1):47–55. doi: 10.1080/09273940802491884. [DOI] [PubMed] [Google Scholar]
- 7.de Velasco G, Bermas B, Choueiri TK. Autoimmune arthropathy and uveitis as complications of programmed cell death 1 inhibitor treatment. Arthritis Rheumatol. 2016;68(2):556–557. doi: 10.1002/art.39406. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Harada K, Usui Y, Irisawa R, Tsuboi R. Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol. 2016. [DOI] [PubMed]
- 9.Karlin J, Gentzler R, Golen J. Bilateral anterior uveitis associated with nivolumab therapy. Ocul Immunol Inflamm. 2016;6:1–3. doi: 10.1080/09273948.2016.1215473. [DOI] [PubMed] [Google Scholar]
- 10.Abu Samra K, Valdes-Navarro M, Lee S, Swan R, Foster CS, Anesi SD. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol. 2016;26(3):e46–e48. doi: 10.5301/ejo.5000724. [DOI] [PubMed] [Google Scholar]
- 11.Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD. Ipilimumab-associated retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):658–660. doi: 10.3928/23258160-20150610-10. [DOI] [PubMed] [Google Scholar]
- 12.Fierz FC, Meier F, Chaloupka K, Boni C. Intraocular inflammation associated with new therapies for cutaneous melanoma - case series and review. Klin Monbl Augenheilkd. 2016;233(4):540–544. doi: 10.1055/s-0042-102668. [DOI] [PubMed] [Google Scholar]
- 13.Mantopoulos D, Kendra KL, Letson AD, Cebulla CM. Bilateral choroidopathy and serous retinal detachments during ipilimumab treatment for cutaneous melanoma. JAMA Ophthalmol. 2015;133(8):965–967. doi: 10.1001/jamaophthalmol.2015.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn L, Pepple KL. Bilateral neuroretinitis and anterior uveitis following ipilimumab treatment for metastatic melanoma. J Ophthalmic Inflamm Infect. 2016;6(1):14. doi: 10.1186/s12348-016-0082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe CH, Mcarthur GA, Caro I, Kempen JH, Amaravadi RK. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am J Ophthalmol. 2014;158(4):831–837.e2. doi: 10.1016/j.ajo.2014.07.003. [DOI] [PubMed] [Google Scholar]