Abstract
IMPORTANCE
Tinnitus affects more than 40 million people in the Unites States, and cognitive difficulties are among the most commonly associated symptoms.
OBJECTIVE
To test the feasibility and preliminarily the effectiveness of using a putative neuroplasticity-enhancing drug, D-cycloserine, to facilitate a computer-assisted CT program for improving tinnitus bother and related cognitive difficulties.
DESIGN, SETTING, AND PARTICIPANTS
Double-blind, randomized clinical trial at an outpatient academic medical center of 34 participants aged 35 to 65 years with subjective, unilateral or bilateral, nonpulsatile tinnitus of at least 6 months’ duration.
INTERVENTIONS
Five weeks of twice-weekly computer-based CT with either 250 mg D-cycloserine or placebo orally prior to computer CT sessions.
MAIN OUTCOMES AND MEASURES
Difference in the change in Tinnitus Functional Index (TFI) score between the 2 groups.
RESULTS
After excluding 1 participant lost to follow-up, 1 who withdrew, 1 who did not complete 90% of sessions, and 1 outlier, 30 participants were included in the analysis. The D-cycloserine plus CT group showed a significant improvement in median TFI score (−5.8 [95% CI, −9.4 to −1.1]) and self-reported cognitive deficits (−4.5 [95% CI, −11.5 to −1.0]), but the placebo group did not (−1.0 [95% CI, −11.7 to 4.9] and −2.0 [95% CI, −5.1 to 2.0], respectively). After controlling for age and duration of tinnitus, there was no significant difference in TFI score change between the 2 groups (P = .41). After confounders were controlled for, the D-cycloserine group demonstrated a significantly greater improvement in self-reported cognitive deficits as compared with the placebo group (P = .03). No serious adverse events were reported.
CONCLUSIONS AND RELEVANCE
Use of a computer-based CT program with a putative neuroplasticity-sensitizing drug, D-cycloserine, was feasible and well tolerated. With the limited sample size, the adjuvant use of D-cycloserine was no more effective than placebo at improving tinnitus bother. The finding that D-cycloserine use was more effective than placebo at improving self-reported cognitive difficulties could be important given the high rate of concern for cognitive deficits in patients with tinnitus. D-cycloserine and other putative neuroplasticity-facilitating agents could be investigated in the future as a strategy to enhance neuroplasticity-based tinnitus treatments.
Trial Registration
clinicaltrials.gov Identifier: NCT01550796
Tinnitus, the perception of a “ringing or hissing” sound in the absence of an acoustic stimulus, affects more than 40 million people in the United States.1,2 For a substantial subset of this population, tinnitus can be highly distressing and result in a variety of physical, functional, cognitive, and emotional impairments. Although many treatments will mitigate the tinnitus for some patients, unfortunately, no cures for tinnitus are available. The exact etiology of chronic tinnitus is unknown; however, current evidence suggests that most cases of tinnitus, regardless of their origin, have correlates in the central nervous system.3–6 In support of this idea, studies have found that the perception of tinnitus exists even after transsection of the auditory nerve between the cochlea and the brain.7 Recent neuroimaging studies have demonstrated that patients with tinnitus demonstrate abnormalities not only in the central auditory pathways8 but also in nonauditory areas of the brain involved in the allocation of attention, perception, and emotional processes.3–6,9,10
Cognitive difficulties are among the most commonly reported symptoms of tinnitus.11,12 Using the Cognitive Failures Questionnaire (CFQ),13 patients with tinnitus have endorsed a greater number of cognitive impairments than healthy controls and those with acquired hearing loss.11 Indeed, in a recent survey, 70% of patients with tinnitus reported difficulty concentrating, representing the single largest self-reported psychological consequence of tinnitus.14 The self-reported cognitive deficits of patients with tinnitus have been corroborated by their performance on routinely performed neurocognitive assessments.15 For instance, people with tinnitus have been found to have deficits in working memory,16 cognitive efficiency,11 attention control,17 and processing speeds on neurocognitive testing.16
Neuroplasticity refers to the brain’s ability to change and adapt through reorganization of its structure and synaptic connections.18 With growing evidence that neuroplasticity exists throughout life,19–21 computerized training programs aimed at recovering cognitive functioning have increased in popularity and have been studied in numerous disorders. For example, computer-based cognitive training (CT) programs have been used in an effort to slow typical age-related cognitive decline22–25 and ameliorate the sequelae of attention deficit disorder,26 mild cognitive impairment,27 depression,28 and schizophrenia.29–31 The Brain Fitness Program, developed by Posit Science, is a CT program in which participants listen to recorded sounds, including simple acoustic stimuli and continuous speech, for approximately 1 hour per day, 5 days per week. Program use extends for 8 weeks (ie, approximately 40 hours of training). Exercise parameters calibrate to individual performance at the onset of training and adapt in difficulty with performance progression, giving constant progress feedback. Each exercise focuses on 1 of the following cognitive processes: (1) auditory processing speed, (2) discriminating sounds, (3) sound precision, (4) sound sequencing, (5) working memory, and (6) narrative memory designed to improve cognitive functioning and reorganize aberrant neural networks such as those affected by tinnitus.21,22,29 In a 6-week open-label pilot study of 13 participants at Washington University with severe bothersome tinnitus, 10 of 13 (77%) reported improvement in attention and/or memory, 6 of 13 (46%) reported changes for the better in their tinnitus, and 11 of 13 (85%) would recommend the Brain Fitness Program to a friend (J. F. Piccirillo, MD, J. Nicklaus, RN, BSN, CRNC, and D. Kallogjeri, MD, MPH, unpublished data, 2011). If the hypothesis that tinnitus results in aberrant neural networks is accurate, then a novel way to facilitate treatment for tinnitus is through the enhancement of training programs designed to target and reorganize aberrant neural networks. D-Cycloserine is a medication that has shown mixed but encouraging results32–41 at augmenting learning therapies through increasing neuroplasticity.42–44 Evidence suggests that D-cycloserine at single oral doses of 50 to 250 mg acts as a partial agonist at the N-methyl-D-aspartate (NMDA) receptor in the brain, enhancing long-term potentiation and thus strengthening new synaptic connections that may occur during a treatment.45 D-Cycloserine has been studied in numerous trials as an adjuvant therapy in the treatment of panic disorder,32 social anxiety disorder,33,46 obsessive-compulsive disorder,34,35,47 posttraumatic stress disorder,36 traumatic brain injury,37,48 schizophrenia,43,49–51 Alzheimer disease,38,39 substance.abuse disorders,52 and autistic spectrum disorders.40 However, the ability of D-cycloserine to enhance learning has been most robust and consistent in trials related to anxiety disorders.53 In particular, D-cycloserine has been found in both human and animal studies to enhance the learning of information that disconfirms fears, such as in exposure therapy for anxiety.32,33,35,41,46,53 Given its ability to augment these therapies, studies have found that D-cycloserine is able to accelerate symptom reduction, lessen the time burden of learning therapies, and allow therapies to be administered at previously subtherapeutic levels.53
This study aimed to investigate a novel approach to targeting the aberrant neural network changes and cognitive deficits found in tinnitus by using the Brain Fitness Program, a computer-based program, and a medication shown to increase neuroplasticity. Our overall research hypothesis was that a neuroplasticity-sensitizing medication (D-cycloserine), compared with placebo, would lead to greater improvements in the severity of tinnitus bother and cognitive functioning when given with the Brain Fitness Program.
Methods
Study Design
This study was a double-blind randomized clinical trial. The study was approved by the institutional review boards of Washington University in St Louis and Stanford University on full-board review and was registered through ClinicalTrials.gov (NCT01550796). All participants provided written informed consent. Participants were enrolled for a period of approximately 5 weeks; the date of first participant contact was December 12, 2011, and the date of last participant completion was May 16, 2012. All participants received treatment with an abbreviated computer-based CT program and were randomized to 1 of 2 groups: a group that received adjuvant treatment with D-cycloserine or placebo.
Eligible patients were between the ages of 35 and 65 years with subjective, unilateral or bilateral, nonpulsatile tinnitus of at least 6 months’ duration who scored at least 30 on the Tinnitus Handicap Inventory (THI).54 Patients were excluded if they had any acute or chronic neurological condition, or any psychiatric comorbidity that may have complicated the interpretation of study results. Female participants of childbearing potential were required to have a negative result on a urine pregnancy test and to use a study-approved form of contraception throughout the study. The project’s biostatistician (D.K.) was responsible for generating the randomization procedure. Block randomization with blocks of 2 was used to provide a balanced design.
Measurements
After enrollment, all patients completed a demographic collection form and the Patient Health Questionnaire (PHQ-9)55 designed to screen for depression. Clinically depressed patients (score, >15 on the PHQ-9) were removed from the study. Validated forms from the Oregon Hearing Research Center (http://www.tinnitusarchive.org/forms) were administered to assess a participant’s tinnitus at the first study visit and final study visit. Tinnitus assessments and questionnaires included THI, Tinnitus Functional Index (TFI),56 and a Tinnitus Description and History form. For the purposes of this study, the TFI was used to assess changes in tinnitus bother through the study. The TFI assessment was chosen on the basis of its high convergent validity and greater responsiveness when compared with the THI.56 In addition, the TFI, unlike most other tinnitus assessments, probes respondents on their experiences with tinnitus “over the last week,” making it conducive to detect changes in tinnitus bother over the relatively short time frame of this study.
Cognitive functioning was assessed by self-report using the CFQ13 and standard validated neurocognitive testing using the Stroop Color and Word Test (SCWT)57,58 and the Paced Auditory Serial Addition Test (PASAT).59 The CFQ is a 25-item questionnaire that assesses cognitive slips, difficulties in attention, and forgetfulness using a 5-point Likert scale. Sample questions include “Do you find you forget people’s names?” and “Do you start doing one thing at home and get distracted into doing something else (unintentionally)?” Neurocognitive tests were chosen that specifically measure the cognitive domain of attention and that were used in prior tinnitus studies.
Intervention
At the first visit, participants were randomly assigned to either the treatment group receiving the Brain Fitness Program and 250 mg D-cycloserine or to the control group receiving the Brain Fitness Program and an identical-appearing placebo. Participants were instructed to work on the Brain Fitness Program 1 hour per day, 2 days per week, for 5 consecutive weeks, while taking their assigned pills 1 hour prior to working on the training program.The dose of 250 mg of D-cycloserine given 1 hour prior to the Brain Fitness Program session was chosen on the basis of other studies involving D-cycloserine in the treatment of anxiety disorders.32,53,60 This dose is substantially less than the maximal recommended daily dosage of 1000 mg and has been found to have minimal adverse effects in other studies.33,41,46,53 Participants completed the first study treatment, involving the first dose of D-cycloserine or placebo in addition to the first session of the Brain Fitness Program, in the Clinical Outcomes Office at Washington University in St Louis. The first study treatment was done at Washington University to ensure initial adherence and comfort with the Brain Fitness Program and to observe for potential adverse effects. Subsequently, all participants took the study medication or placebo at home and worked on the Brain Fitness Program on their personal computer. The degree of adherence to the intervention was assessed in several ways, including a participant-completed study journal, open-ended questioning at the final study visit, a pill count performed at the conclusion of the study, and information provided from the Brain Fitness Program software. Participants were considered to have completed the treatment if 90% (9 of10) of the recommended sessions of the Brain Fitness Program were completed and the study medication or placebo was taken prior to working on the program.
Throughout the study, all participants and research team members were blinded to the participants’ treatment group assignments. After the first treatment session at Washington University, both the participant and the researcher who dispensed the medication guessed which intervention, D-cycloserine or placebo, was administered in order to assess the integrity of the double-blinding.
Sample Size
To our knowledge, this is the first published study to examine the impact of D-cycloserine on CT in patients with tinnitus. Therefore, no effect size is known, which would have been necessary for any sample size estimate. However, a power computation and sample size justification for this study was made on the basis of previous tinnitus treatment studies conducted in the Outcomes Office. With the assumptions of the t test and on the basis of the observations from previous treatment studies, a sample size of 15 participants per group was calculated to provide 80%power to detect a change in the THI of 17 points or greater between those who receive treatment with the Brain Fitness Program and D-cycloserine and those who receive the Brain Fitness Program and placebo (SD of the difference, 16) at the .05 α level.
Statistical Analyses
Standard descriptive statistics were used to describe the distribution of the study population, their tinnitus characteristics, and results on all neurocognitive assessments in both treatment groups. Because the assumptions of parametric testing were not met, the nonparametric equivalent for the independent samples t test, Mann-Whitney U, was used to compare the distribution of continuous level characteristics and baseline test scores between participants randomized to each group. Prior to further analysis, sensitivity analyses were performed to assess for extreme outliers. The Wilcoxon signed rank test was used to compare preintervention and postintervention test results in each treatment group. The change in scores (Δscore) on TFI and neurocognitive assessments was calculated for each participant, and the median and 95% confidence interval of the Δscore was explored. To further explore the change in tinnitus bother and cognitive scores after controlling for potential confounders, a mixed-design analysis of covariance (ANCOVA) model was used to assess the within-subjects vs between-subjects interaction. All statistical tests used were 2 sided and evaluated at the α level of .05. Data were collected and managed throughout the study using the REDCap61 electronic database developed by Vanderbilt University. Statistical analyses were performed using SPSS Statistics, version 20.0.0 (IBM Corporation).
Results
Description of the Population
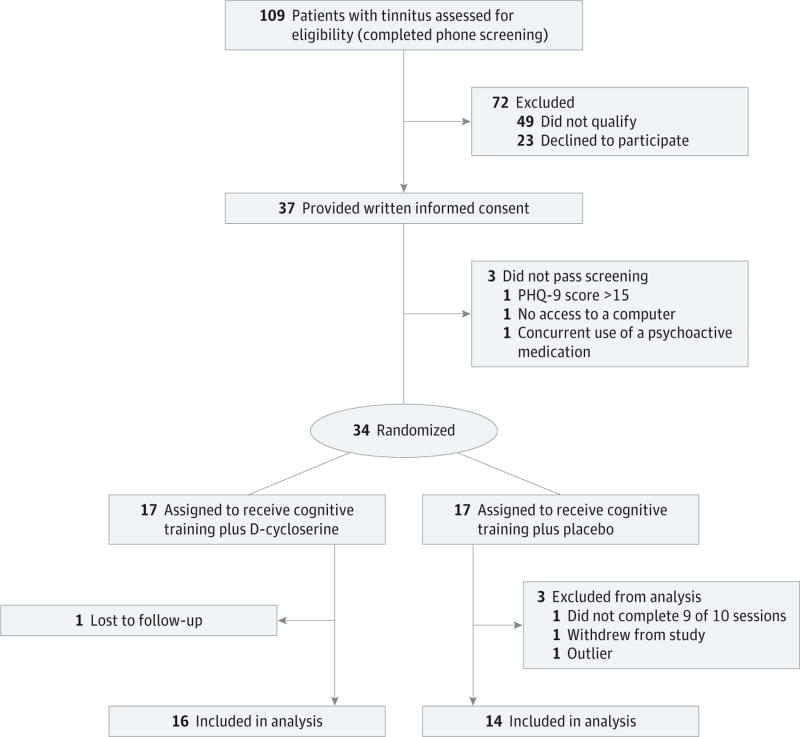
Of the 109 patients who expressed interest in the study, 37 passed the telephone screening and provided written informed consent. As shown in the Figure, 17 participants were assigned to the D-cycloserine group and 17 were assigned to the placebo group. In total, 31 of 34 participants completed the program and 30 participants were included in the analyses. The response of 1 participant on several measures was in the opposite direction of the others and, in this small sample size pilot study, was overly influential and was excluded from further analysis. Demographic characteristics, baseline tinnitus characteristics, and scores on neurocognitive tests of the analyzed participants are provided in Table 1. The median (range) age of the D-cycloserine group was 59 (49–63) years and for the placebo group was 55 (37–63) years. There were no significant differences between the intervention groups in terms of demographic characteristics, baseline tinnitus scores, or baseline neurocognitive assessment scores.
Figure.
Flow Diagram of Participation
PHQ-9 indicates Patient Health Questionnaire.
Table 1.
Description of Study Population at Baseline
| Characteristic | Placebo and Cognitive Training (n = 14) |
D-Cycloserine and Cognitive Training (n = 16) |
P Value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, median (range), y | 55 (37–63) | 59 (49–63) | .06 |
| Female sex, No. (%) | 6 (43) | 8 (50) | .70 |
| Race, No. (%) | |||
| White | 11 (79) | 16 (100) | .09 |
| Other | 3 (21) | 0 | |
| Education, median (range), y | 17 (14–18) | 16 (12–20) | .39 |
| Tinnitus characteristics at baseline | |||
| Duration of tinnitus, median (range), y | 10.0 (5–57) | 4.8 (1–37) | .047 |
| Subjective loudness, median (range) | 6 (4–10) | 7 (3–9) | .85 |
| Effort to ignore, No. (%) | |||
| Can never ignore | 3 (21) | 3 (19) | .48 |
| Considerable effort | 1 (7) | 2 (12) | |
| Some effort | 10 (71) | 8 (50) | |
| Slight effort | 0 | 3 (19) | |
| TFI score, median (range) | 44 (14–65) | 47 (10–76) | .66 |
| Bother level, No. (%) | |||
| Extremely bothered | 0 | 0 | .46 |
| Bothered a lot | 4 (29) | 6 (38) | |
| Bothered more than a little but not a lot | 8 (57) | 10 (63) | |
| Bothered a little, but not much | 2 (14) | 0 | |
| Not bothered | 0 | 0 | |
| PHQ-9 score, median (range) | 4 (0–10) | 5 (1–11) | .74 |
| Cognitive assessments, median (range) | |||
| CFQ score | 43 (19–60) | 41 (18–51) | .85 |
| Stroop Color-Word T-score | 58 (43–64) | 54 (40–71) | .28 |
| PASAT, No. correct | 156 (68–194) | 128 (65–229) | .48 |
Abbreviations: CFQ, Cognitive Failures Questionnaire; PASAT, Paced Auditory Serial Addition Test; PHQ-9, Patient Health Questionnaire-9; TFI, Tinnitus Functional Index.
Change in Tinnitus Bother and Cognitive Assessments
Baseline and postintervention scores on the TFI and neurocognitive assessments are provided by treatment group in Table 2. The median change in TFI score after intervention as compared with baseline for the D-cycloserine group was −5.8 (95% CI, −9.4 to −1.1). Although this difference of 5.8 points achieved statistical significance, it did not achieve the value of 13, which is the minimally clinically significant difference.56 The median change in TFI score for the placebo group was −1.0 (95% CI, −11.7 to 4.9). There were 3 participants in each of the intervention groups who experienced a clinically meaningful change in tinnitus (defined as a decline in TFI of ≥13 points). The median change in CFQ score for the D-cycloserine group was −4.5 (95% CI, −11.5 to −1.0), whereas the placebo group had a median change of −2.0 (95% CI, −5.1 to 2.0). Finally, the median change in PASAT and SCWT scores, respectively, was 20 (95% CI, 12.4 to 37.0) and 4.0 (95% CI, −1.6 to 8.0) for the D-cycloserine group and 23.0 (95% CI, 3.4 to 31.9) and 2 (95% CI, −0.3 to 6.0) for the placebo group.
Table 2.
Summary of Change in Tinnitus Bother and Cognitive Functioning by Treatment Group
| Assessments | Median (95% CI) | P Value | Difference, Final-Baseline, Median (95% CI) |
|
|---|---|---|---|---|
| Baseline Visit | Final Visit | |||
| Placebo and cognitive training (n = 14) | ||||
| TFI score | 44 (14 to 65) | 41 (13 to 61) | .35 | −1.0 (−11.7 to 4.9) |
| CFQ score | 43 (19 to 60) | 43 (13 to 52) | .16 | −2.0 (−5.1 to 2.0) |
| SCWT T-score | 58 (43 to 64) | 60 (45 to 70) | .09 | 2.0 (−0.3 to 6.0) |
| PASAT, No. correct | 156 (68 to 194) | 174 (103 to 225) | .005 | 23.0 (3.4 to 31.9) |
| D-cycloserine and cognitive training (n = 16) | ||||
| TFI score | 47 (10 to 76) | 38 (8 to 70) | .04 | −5.8 (−9.4 to −1.1) |
| CFQ score | 41 (18 to 51) | 34 (18 to 47) | .005 | −4.5 (−11.5 to −1.0) |
| SCWT T-score | 54 (40 to 71) | 60 (30 to 73) | .12 | 4.0 (−1.6 to 8.0) |
| PASAT, No. correct | 128 (65 to 229) | 157 (83 to 233) | .001 | 20.0 (12.4 to 37.0) |
Abbreviations: CFQ, Cognitive Failures Questionnaire; PASAT, Paced Auditory Serial Addition Test; SCWT, Stroop Color Word Test; TFI, Tinnitus Functional Index.
A general linear model mixed-design ANCOVA was used to explore differences in the change of all outcomes of interest after the intervention vs baseline. In this model, changes in test scores represented the within-subjects variable and treatment group represented the between-subjects variable. After controlling for age and duration of tinnitus, no significant difference existed for changes in neurocognitive score or TFI scores (F1,26 = 0.695, P = .41 for the intervention*time interaction) between the D-cycloserine and placebo groups. However, after confounders were controlled for, the improvement in CFQ score was significantly larger in the D-cycloserine group compared with the placebo group (F1,26 = 5.27, P = .03 for the intervention*time interaction).
Adherence and Tolerability
In this study, 31 of 34 participants completed the study (Figure). In the D-cycloserine group, 1 participant was considered to be lost to follow-up after the study team was no longer able to get in contact with him via mailings and telephone calls. In the placebo group, 1 participant asked to withdraw from the study after 2 treatment sessions as a result of difficulties with “a busy schedule.” In addition, on final assessment of adherence, 1 participant in the placebo group was found not to have completed 90% of treatments. In this study, there were no serious adverse events reported. In the D-cycloserine group, 2 participants reported experiencing a sensation of “tingling skin” after 1 treatment session. In the placebo group, 1 participant reported 2 exacerbations of his Ménière’s disease, and 1 participant reported an increased frequency of headaches.
Discussion
We aimed to evaluate the feasibility and preliminarily the effectiveness of D-cycloserine, a neuroplasticity-sensitizing drug, in conjunction with a computer-based CT program on improving tinnitus bother and related cognitive difficulties. The main finding was that those participants who received D-cycloserine with CT, compared with those who received placebo with CT, demonstrated a significantly greater improvement in self-reported tinnitus bother and cognitive deficits from baseline. In this study, D-cycloserine and computer-based CT were well tolerated with sufficient adherence and limited adverse effects. The results of this study suggest that the use of D-cycloserine with an abbreviated computer-based CT program may be an effective approach for those participants with bothersome tinnitus.
The finding that the added use of D-cycloserine with CT may be more effective than placebo and CT at improving self-reported cognitive difficulties is noteworthy. As mentioned in the Introduction, 70% of patients with tinnitus report concentration difficulties, representing the single largest self-reported psychological comorbidity of tinnitus.14 Furthermore, in a model constructed to predict quality of life in patients with severe tinnitus, no audiological or psychological variable contributed more to a decreased quality of life than self-reported impairments in concentration.62 As noted in the Introduction, studies have found that D-cycloserine use is able to accelerate treatment programs, lessen the time burden of therapies, and demonstrate therapeutic improvements at earlier time points in therapy.53 As such, in this study, the decision was made, similar to other early studies investigating D-cycloserine,32,33,35,41,46,53 to assess the effectiveness of the CT program after an abbreviated number of treatment sessions. In this study, 10 sessions with CT, rather than the previously studied 40 sessions,21,22 were administered to participants. The decision to curtail the number of treatment sessions was made to decrease the likelihood of desensitization to D-cycloserine after repeated use63 and to avoid a “ceiling effect,” wherein with enough treatment sessions no augmenting effect of D-cycloserine would be observed.53 However, the encouraging possibility exists that with full administration of the studied computer-based CT program, even more substantial improvements in self-reported cognitive deficits would be observed.
The results of this study provoke the question, Why should the adjuvant use of D-cycloserine, an NMDA agonist, in this treatment demonstrate a statistically significant improvement in self-reported cognitive difficulties yet not affect objectively assessed cognitive performance or more strongly reduce tinnitus bother since NMDA receptors are also present in the inner hair cell and/or auditory nerve synapse? One potential explanation for our results is that an effect was observed only in the most strongly affected outcome, and the sample size was inadequate to detect changes in tinnitus bother or objective neurocognitive scores. The possibility also exists that the computer-based CT program might more strongly affect transient, day-to-day cognitive issues, assessed by the CFQ, compared with effortful performance on standardized cognitive tasks or tinnitus bother. Therefore, if the CT program had not been given on an abbreviated basis or if it had been performed in a larger sample, the effects of the treatment program on standardized cognitive testing and tinnitus bother may have been observed. A second potential explanation for our findings is related to the fact that adjuvant D-cycloserine use, as discussed in the Introduction, most clearly augments learning related to fear extinction.53 Thus, in this study, D-cycloserine may have helped enhance reduction in fears (or, more broadly, concerns) about cognitive performance, rather than learning reflected in scores on neurocognitive testing. In other words, D-cycloserine might enhance only the subjective experience of cognitive problems, which themselves might be as much due to fears about cognitive problems, as compared with objective cognitive performance itself.
If the hypothesis that D-cycloserine use enhances learning of information that disconfirms fears is correct, D-cycloserine might be more likely to reduce tinnitus bother if the intervention clearly involves exposure to tinnitus-related fears, which was not the case in this study. Therapies specifically targeting catastrophizing misinterpretations and fears of tinnitus have been proposed recently in the literature. In a recent study, Cima et al64 noted that tinnitus-related fears and catastrophizing about tinnitus were associated with increased attention toward tinnitus and decreased quality of life. In a recent meta-analysis of randomized clinical trials examining cognitive behavioral therapy (CBT) for tinnitus, Andersson and Lyttkens65 concluded that CBT was an effective treatment at reducing annoyance and distress associated with tinnitus and was worthy of additional research. However, CBT treatment techniques for tinnitus are not uniform, with techniques ranging from cognitive therapy to relaxation strategies.66,67 Similar to chronic pain syndrome,68 with which tinnitus is speculated to have mechanistic similarities,69–71 CBT techniques could be developed specifically aimed at reducing tinnitus-related fears. Unfortunately, change-oriented strategies for addressing chronic conditions that are inherently difficult or impossible to change may contribute to increased distress and interference with life.72,73 A relatively new alternative to CBT and other change-oriented strategies involves mindfulness. Mindfulness meditation emphasizes focused, nonjudgmental awareness of present moment experiences without efforts to alter or avoid them.74,75
There are several limitations to this study that should be considered when the results are interpreted. Because this is the first time that a study examining CT and D-cycloserine use has been carried out in the tinnitus population, no effect size was known, which is necessary for sample size estimate. Therefore, this study may be underpowered to detect differences in changes in tinnitus bother or cognitive deficits. Second, the external validity of this study may be limited given the unique characteristics of the study population. In this study, the majority of patients were bothered by tinnitus and only those with no neurologic or psychiatric comorbidities were included. However, prior studies have estimated that nearly 50% of patients with severe tinnitus had a concurrent diagnosis of major depression.76 Consequently, participants in this study may not reflect the usual characteristics of a patient with severe tinnitus. Furthermore, this study was aimed at assessing the feasibility and effectiveness of D-cycloserine compared with placebo as an adjuvant therapy with computer-based CT. Therefore, in this study every participant received some form of treatment. Thus, we cannot comment on the effectiveness of the computer-based CT program, which was not compared with a sham treatment.
Conclusions
This novel approach to treating tinnitus using a computer-based CT program with a neuroplasticity-sensitizing drug, D-cycloserine, was feasible and well tolerated. In the limited sample size of this study, the adjuvant use of D-cycloserine was not associated with improvement in tinnitus bother. However, the adjuvant use of D-cycloserine with CT was more effective than placebo at improving the self-reported cognitive difficulties associated with tinnitus. This finding may be important given the particularly high rate of concerns about cognitive difficulties associated with tinnitus, and this treatment could be particularly useful in a subset of patients with tinnitus. Future research is needed to replicate these findings, preferably in a larger sample that might allow detection of additional effects. Finally, this study shows that D-cycloserine and other putative neuroplasticity-facilitating agents could be investigated in the future as a strategy to enhance various neuroplasticity-based tinnitus treatments.
Acknowledgments
Funding/Support: This research was supported by a grant from the Doris Duke Clinical Research Foundation, as well as support from the Stanford University Medical Scholars Fellowship. Posit Science provided access to the Brain Fitness Program for all participants.
Footnotes
Author Contributions: Drs Krings and Piccirillo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Krings, Wineland, Rodebaugh, Piccirillo.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Krings, Kallogjeri, Nicklaus, Piccirillo.
Critical revision of the manuscript for important intellectual content: Krings, Wineland, Rodebaugh, Nicklaus, Lenze, Piccirillo.
Statistical analysis: Krings, Kallogjeri, Rodebaugh, Piccirillo.
Obtained funding: Krings, Piccirillo
Administrative, technical, or material support: Krings, Kallogjeri, Nicklaus.
Study supervision: Krings, Wineland, Nicklaus, Piccirillo.
Conflict of Interest Disclosures: Dr Lenze receives support from Roche and Lundbeck to conduct research unrelated to that described in this article. No other disclosures are reported.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005;48(5):1204–1235. doi: 10.1044/1092-4388(2005/084). [DOI] [PubMed] [Google Scholar]
- 2.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS. Neuroanatomy of tinnitus. Scand Audiol Suppl. 1999;51:47–52. [PubMed] [Google Scholar]
- 4.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347(12):904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 5.Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. J Am Acad Audiol. 2000;11(3):125–137. [PubMed] [Google Scholar]
- 6.Lenarz T, Schreiner C, Snyder RL, Ernst A. Neural mechanisms of tinnitus. Eur Arch Otorhinolaryngol. 1993;249(8):441–446. doi: 10.1007/BF00168851. [DOI] [PubMed] [Google Scholar]
- 7.Bauer CA. Mechanisms of tinnitus generation. Curr Opin Otolaryngol Head Neck Surg. 2004;12(5):413–417. doi: 10.1097/01.moo.0000134443.29853.09. [DOI] [PubMed] [Google Scholar]
- 8.Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49(8):669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- 9.Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hear Res. 2009;253(1–2):15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Schecklmann M, Landgrebe M, Poeppl TB, et al. Neural correlates of tinnitus duration and distress: a positron emission tomography study. Hum Brain Mapp. 2013;34(1):233–240. doi: 10.1002/hbm.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallam RS, McKenna L, Shurlock L. Tinnitus impairs cognitive efficiency. Int J Audiol. 2004;43(4):218–226. doi: 10.1080/14992020400050030. [DOI] [PubMed] [Google Scholar]
- 12.Andersson G, McKenna L. The role of cognition in tinnitus. Acta Otolaryngol Suppl. 2006;(556):39–43. doi: 10.1080/03655230600895226. [DOI] [PubMed] [Google Scholar]
- 13.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersson G, Lyttkens L, Larsen HC. Distinguishing levels of tinnitus distress. Clin Otolaryngol Allied Sci. 1999;24(5):404–410. doi: 10.1046/j.1365-2273.1999.00278.x. [DOI] [PubMed] [Google Scholar]
- 15.Pierce KJ, Kallogjeri D, Piccirillo JF, Garcia KS, Nicklaus JE, Burton H. Effects of severe bothersome tinnitus on cognitive function measured with standardized tests. J Clin Exp Neuropsychol. 2012;34(2):126–134. doi: 10.1080/13803395.2011.623120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossiter S, Stevens C, Walker G. Tinnitus and its effect on working memory and attention. J Speech Lang Hear Res. 2006;49(1):150–160. doi: 10.1044/1092-4388(2006/012). [DOI] [PubMed] [Google Scholar]
- 17.Stevens C, Walker G, Boyer M, Gallagher M. Severe tinnitus and its effect on selective and divided attention. Int J Audiol. 2007;46(5):208–216. doi: 10.1080/14992020601102329. [DOI] [PubMed] [Google Scholar]
- 18.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(pt 6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: further evidence for cognitive plasticity in attentional control in late adulthood. Exp Aging Res. 2008;34(3):188–219. doi: 10.1080/03610730802070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28(2):272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- 22.Mahncke HW, Connor BB, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103(33):12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolinsky FD, Mahncke H, Vander Weg MW, et al. Speed of processing training protects self-rated health in older adults: enduring effects observed in the multi-site ACTIVE randomized controlled trial. Int Psychogeriatr. 2010;22(3):470–478. doi: 10.1017/S1041610209991281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GE, Housen P, Yaffe K, et al. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis SL, Tennstedt SL, Marsiske M, et al. ACTIVE Study Group. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingberg T, Fernell E, Olesen PJ, et al. Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Barnes DE, Yaffe K, Belfor N, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morimoto SS, Wexler BE, Alexopoulos GS. Neuroplasticity-based computerized cognitive remediation for geriatric depression. Int J Geriatr Psychiatry. 2012;27(12):1239–1247. doi: 10.1002/gps.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell M, Bryson G, Greig T, Corcoran C, Wexler BE. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Arch Gen Psychiatry. 2001;58(8):763–768. doi: 10.1001/archpsyc.58.8.763. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson D, Tenhula W, Morris S, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry. 2010;167(2):170–180. doi: 10.1176/appi.ajp.2009.09020264. [DOI] [PubMed] [Google Scholar]
- 32.Otto MW, Tolin DF, Simon NM, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67(4):365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 33.Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63(6):544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm S, Buhlmann U, Tolin DF, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165(3):335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 35.Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62(8):835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Heresco-Levy U, Kremer I, Javitt DC, et al. Pilot-controlled trial of D-cycloserine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol. 2002;5(4):301–307. doi: 10.1017/S1461145702003061. [DOI] [PubMed] [Google Scholar]
- 37.Adeleye A, Shohami E, Nachman D, et al. D-cycloserine improves functional outcome after traumatic brain injury with wide therapeutic window. Eur J Pharmacol. 2010;629(1–3):25–30. doi: 10.1016/j.ejphar.2009.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer’s disease with short-term D-cycloserine treatment. Am J Psychiatry. 1999;156(3):467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI. D-Cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996;46(2):420–424. doi: 10.1212/wnl.46.2.420. [DOI] [PubMed] [Google Scholar]
- 40.Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. Am J Psychiatry. 2004;161(11):2115–2117. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 41.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 42.Krystal JH, Tolin DF, Sanacora G, et al. Neuroplasticity as a target for the pharmacotherapy of anxiety disorders, mood disorders, and schizophrenia. Drug Discov Today. 2009;14(13–14):690–697. doi: 10.1016/j.drudis.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goff DC. D-Cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012;38(5):936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouaud E, Billard JM. D-Cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br J Pharmacol. 2003;140(6):1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaka R, Biegon A, Grigoriadis N, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21(9):2033–2041. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- 46.Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63(3):298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 47.Storch EA, Murphy TK, Goodman WK, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68(11):1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temple MD, Hamm RJ. Chronic, post-injury administration of D-cycloserine, an NMDA partial agonist, enhances cognitive performance following experimental brain injury. Brain Res. 1996;741(1–2):246–251. doi: 10.1016/s0006-8993(96)00940-7. [DOI] [PubMed] [Google Scholar]
- 49.Gottlieb JD, Cather C, Shanahan M, Creedon T, Macklin EA, Goff DC. D-Cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1–3):69–74. doi: 10.1016/j.schres.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goff DC, Herz L, Posever T, et al. A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl) 2005;179(1):144–150. doi: 10.1007/s00213-004-2032-2. [DOI] [PubMed] [Google Scholar]
- 51.Heresco-Levy U, Javitt DC, Ermilov M, Silipo G, Shimoni J. Double-blind, placebo-controlled, crossover trial of D-cycloserine adjuvant therapy for treatment-resistant schizophrenia. Int J Neuropsychopharmacol. 1998;1(2):131–135. doi: 10.1017/S1461145798001242. [DOI] [PubMed] [Google Scholar]
- 52.Kamboj SK, Massey-Chase R, Rodney L, et al. Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory study of the effects of D-cycloserine in heavy drinkers. Psychopharma-cology (Berl) 2011;217(1):25–37. doi: 10.1007/s00213-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 53.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143–148. doi: 10.1001/archotol.1996.01890140029007. [DOI] [PubMed] [Google Scholar]
- 55.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meikle MB, Henry JA, Griest SE, et al. The Tinnitus Functional Index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33(2):153–176. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- 57.Golden C. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Skoelting; 1978. [Google Scholar]
- 58.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 59.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 60.Behar E, McHugh RK, Peckham A, Otto MW. D-Cycloserine for the augmentation of an attentional training intervention for trait anxiety. J Anxiety Disord. 2010;24(4):440–445. doi: 10.1016/j.janxdis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erlandsson SI, Hallberg LR. Prediction of quality of life in patients with tinnitus. Br J Audiol. 2000;34(1):11–20. doi: 10.3109/03005364000000114. [DOI] [PubMed] [Google Scholar]
- 63.Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83(3):224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Cima RF, Maes IH, Joore MA, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet. 2012;379(9830):1951–1959. doi: 10.1016/S0140-6736(12)60469-3. [DOI] [PubMed] [Google Scholar]
- 65.Andersson G, Lyttkens L. A meta-analytic review of psychological treatments for tinnitus. Br J Audiol. 1999;33(4):201–210. doi: 10.3109/03005369909090101. [DOI] [PubMed] [Google Scholar]
- 66.Hesser H, Weise C, Westin VZ, Andersson G. A systematic review and meta-analysis of randomized controlled trials of cognitive-behavioral therapy for tinnitus distress. Clin Psychol Rev. 2011;31(4):545–553. doi: 10.1016/j.cpr.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Andersson G. Psychological aspects of tinnitus and the application of cognitive-behavioral therapy. Clin Psychol Rev. 2002;22(7):977–990. doi: 10.1016/s0272-7358(01)00124-6. [DOI] [PubMed] [Google Scholar]
- 68.Lohnberg JA, Altmaier EM. A review of psychosocial factors in complex regional pain syndrome. J Clin Psychol Med Settings. 2013;20(2):247–254. doi: 10.1007/s10880-012-9322-3. [DOI] [PubMed] [Google Scholar]
- 69.Møller AR. Tinnitus and pain. Prog Brain Res. 2007;166:47–53. doi: 10.1016/S0079-6123(07)66004-X. [DOI] [PubMed] [Google Scholar]
- 70.Møller AR. Similarities between chronic pain and tinnitus. Am J Otol. 1997;18(5):577–585. [PubMed] [Google Scholar]
- 71.Moller AR. Similarities between severe tinnitus and chronic pain. J Am Acad Audiol. 2000;11(3):115–124. [PubMed] [Google Scholar]
- 72.Robinson P, Wicksell RKOGL. AT with chronic pain patients. In: Hayes SC, Strohsahl KD, editors. A Practical Guide to Acceptance and Commitment Therapy. New York, NY: Springer; 2004. pp. 315–345. [Google Scholar]
- 73.McCracken LM. Contextual Cognitive-Behavioral Therapy for Chronic Pain, Progress in Pain Research and Management. Seattle, WA: IASP Press; 2005. [Google Scholar]
- 74.Kabat-Zinn J. Full Catastrope Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Dell Publishing; 1990. [Google Scholar]
- 75.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–943. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 76.Harrop-Griffiths J, Katon W, Dobie R, Sakai C, Russo J. Chronic tinnitus: association with psychiatric diagnoses. J Psychosom Res. 1987;31(5):613–621. doi: 10.1016/0022-3999(87)90040-7. [DOI] [PubMed] [Google Scholar]