Abstract
Daytime sleepiness is a commonly reported adverse effect associated with psychotropic agents that may impair cognitive performance and functioning. The objective of this post-hoc analysis was to evaluate the long-term effects of lurasidone and quetiapine XR on daytime sleepiness and neurocognitive performance during a 6-month, double-blind continuation study, in subjects who completed an initial 6-week, randomized, placebo-controlled trial comparing these agents. Daytime sleepiness, cognitive performance, and health-related quality of life were assessed with the Epworth Sleepiness Scale (ESS), CogState computerized battery, and the Quality of Well-Being (QWB-SA) Scale, respectively. Treatment with flexible-dose lurasidone 40–160 mg/d, administered once daily in the evening, was associated with significantly reduced daytime sleepiness compared with flexibly dosed quetiapine XR 200–800 mg/d (p = 0.03, effect size = 0.36) at week 32 (month 6 of the continuation study endpoint). Incidence of markedly high sleepiness (ESS > 10) was significantly higher in the quetiapine XR (200–800 mg/d) group compared with the lurasidone (40–160 mg/day) group at both months 3 and 6 visits (p < 0.05). Lurasidone (40–160 mg/d) significantly improved neurocognitive performance compared to quetiapine XR (200–800 mg/d) before (effect size = 0.49) and after adjustment (effect size = 0.45) for sleepiness effect (p = 0.008 and 0.010, respectively). Increased daytime sleepiness was significantly associated with reduced neurocognitive performance (p = 0.019) and quality of well-being (p = 0.05). Our findings suggest that clinicians should actively monitor patients for the presence of daytime sleepiness due in part to its potential impact on neurocognitive performance and well-being.
Keywords: Daytime sleepiness, Cognitive performance, Lurasidone, Quetiapine XR, Long-term double-blind study
1. Introduction
Daytime sleepiness adversely impacts neurocognitive performance, which is often significantly impaired in patients with schizophrenia (Kane and Sharif, 2008, Leifker, 2009, Hawley et al., 2010, Wilson and Argyropoulos, 2012, Loebel et al., 2013a). Daytime sleepiness is a commonly reported adverse effect of pharmacologic agents with significant antihistaminergic and/or antiserotonergic effects, such as certain antipsychotic agents. The propensity to doze while performing daily activities such as driving or working can have serious consequences, including increased motor vehicle and work-related accidents, as well as compromised job performance (National Sleep Foundation, 2007, American Academy of Sleep Medicine, 2009). It has been estimated that driving while drowsy contributes to 100,000 police-reported crashes that resulted in 71,000 injuries and 1550 deaths each year in the United States (National Sleep Foundation, 2007). Daytime sleepiness, if persistent, can adversely impact patients' social and daily functioning, quality of life and can compromise treatment adherence (Kane and Sharif, 2008, McWhirter et al., 2007). Despite awareness of the health and economic burden posed by daytime sleepiness, the long-term effects of antipsychotic treatments on daytime sleepiness and their clinical consequences have not been adequately studied (Kane and Sharif, 2008, McWhirter et al., 2007).
Lurasidone is a novel benzisothiazol derivative with potent binding affinity for the D2, 5-HT2A and 5HT7 receptors (antagonist), and moderate affinity for 5HT1A (partial agonist) and α2C receptors (antagonist) (Ishibashi et al., 2010, Horiguchi et al., 2011). Lurasidone has no appreciable affinity for H1 and M1 receptors (Ishibashi et al., 2010). In contrast, quetiapine is associated with a high affinity at the H1 receptor where it acts as an antagonist (Baldwin and Scott, 2009); this mechanism is associated with sedation in both animal models and human studies (Witek et al., 1995). The short-term effects of lurasidone on neurocognitive performance were demonstrated in a 3-week double-blind, active-controlled study of lurasidone and ziprasidone (Harvey et al., 2011), as well as a 6-week, double-blind, placebo- and active-controlled study of lurasidone and quetiapine XR (Harvey et al., 2013). In a 6-month, double-blind, flexible-dose, continuation study, lurasidone (40–160 mg/d) was superior to quetiapine XR (200–800 mg/d) for cognitive performance at both months 3 and 6 of the extension study (Harvey et al., 2013, Harvey et al., 2015). The objective of this post-hoc analysis was to evaluate the long-term effects of flexible-dose lurasidone 40–160 mg/d and quetiapine XR 200–800 mg/d on daytime sleepiness, and the impact of daytime sleepiness on neurocognitive performance in a long-term continuation study that followed a 6-week acute trial in patients with schizophrenia.
2. Methods
The analysis reported here is based on data from a previously reported randomized, double-blind, 6-week, placebo- and active-controlled acute study (Loebel et al., 2013b), followed by a double-blind continuation study that continued up to 1 year (Loebel et al., 2013c). The design of these studies will therefore only be briefly summarized here. The study was approved by an institutional review board at each investigational site and was conducted in accordance with the International Conference on Harmonisation of Good Clinical Practices guidelines, and with the ethical principles of the Declaration of Helsinki. All patients signed an informed consent document explaining study procedures and potential risks before study entry.
2.1. Patients
Patients with a primary diagnosis of schizophrenia, who had recently been hospitalized for an acute exacerbation of psychotic symptoms, were randomly assigned to receive 6 weeks of double-blind treatment with once-daily fixed doses of lurasidone (80 mg or 160 mg), quetiapine XR (600 mg), or placebo. Upon completion of the initial 6-week study, eligible patients were enrolled in the 1-year, double-blind, flexible-dose, continuation study, where patients continued treatment with either lurasidone 40–160 mg/d (lurasidone-to-lurasidone) or quetiapine XR 200–800 mg/d (quetiapine XR-to-quetiapine XR). Cognitive and functional capacity assessments were obtained at baseline and up to 6 months in the continuation study.
Patients who had been treated with placebo in the initial 6-week study were switched in a blinded fashion to flexible-dose lurasidone treatment in the continuation study (placebo-to-lurasidone), but the present analyses focused primarily on those patients receiving lurasidone during both the acute and continuation study phases (lurasidone-to-lurasidone). Entry into the continuation study required patients to have completed all assessments on the final week 6 visit of the acute phase and to have been judged by the investigator as suitable for continuation study treatment in an outpatient setting.
2.2. Assessments
Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS), a validated self-reported measure of daytime sleepiness at baseline, 6 weeks, and 3 and 6 months in the continuation treatment phase (Johns, 1991, Johns, 2008). The total ESS score is the sum of all 8 items (each refers to level of anticipated sleepiness in various routine life situations) and ranges from 0 to 24. Each individual item ranges from 0 = ‘would never doze or sleep’ to 3 = ‘high chance of dozing or sleeping’. Higher ESS scores are associated with a greater severity of daytime sleepiness. If one or more items were missing at a visit, the total score was set to missing. The ESS has been validated in both case-controlled studies of normal patients and in a number of populations with various sleep disorders (Johns, 1991, Johns, 2008, Chervin, 2000).
Cognition was assessed by the CogState composite score (Pietrzak et al., 2009) at baseline, week 6 (end of acute treatment phase), week 19 (month 3 of the continuation study), and week 32 (month 6 of the continuation study). The composite measure of overall cognitive performance was calculated as an average of the 7 standardized domain scores over the tasks of: Detection Task, Identification Task, One Back Task, International Shopping List Task, One Card Learning Task, Groton Maze Learning Task, and the Social Emotional Cognition Task. These standardized domain scores were computed using the age stratified CogState normative mean and standard deviation according to the following equation: (raw domain score – norm mean)/norm SD. The sign of the standardized domain score was adjusted so that negative scores indicated performance worse than average, and positive scores indicated performance better than average. If three or more components were missing at a visit, the CogState composite score was set to missing. Health-related quality of life was measured using the Quality of Well-Being (QWB-SA) Scale (Anderson et al., 1989).
2.3. Statistical methods
The primary treatment group comparison was between patients continuing on flexibly dosed lurasidone 40–160 mg/d (lurasidone-to-lurasidone) or quetiapine XR 200–800 mg/d (quetiapine XR-to-quetiapine XR) at week 19 and week 32 (month 3 and 6 of the continuation study period). The change in neurocognitive composite score from pretreatment baseline (week 0) to week 19 and week 32 (primary endpoint) was analyzed using a mixed-effects longitudinal data analysis model (Fitzmaurice et al., 2004), with fixed effects for treatment, visit, baseline score, treatment-by-visit interaction, and study sites. In addition, we evaluated the change from the pretreatment baseline in the neurocognitive composite score after controlling for change in ESS total score from baseline (week 0) over time. Cohen’s d between-group effect size was calculated as least squares mean difference between LUR-to-LUR and QXR-to-QXR divided by the model estimate of the pooled SD (across treatment groups), adjusting for site effects and baseline at randomization. A Generalized Estimating Equation (GEE) model was applied to evaluate the incidence of markedly high sleepiness.
3. Results
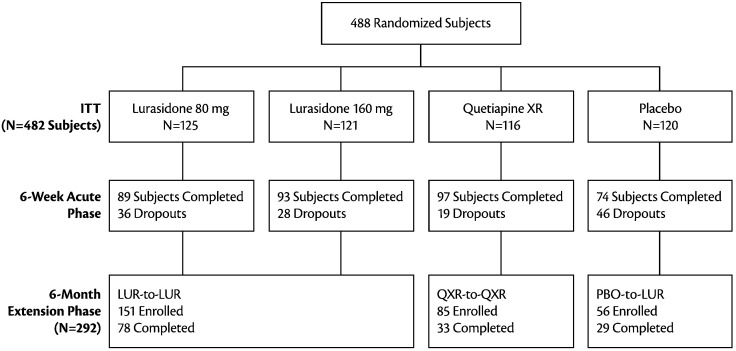
A total of 292 patients completed the 6-week acute phase and were enrolled in the double-blind continuation study (Fig. 1). Figure 1 depicts the disposition of patients. The analyses reported herein were based on full analysis sample, with 207 patients had cognitive assessment data at week 19 or week 32 or both visits (month 3 or 6 of the continuation study period). The median doses at the 6-month study endpoint in the continuation study for lurasidone (lurasidone-to-lurasidone, n = 151) and quetiapine XR (quetiapine XR-to-quetiapine XR, n = 85) were 120 mg/d and 600 mg/d, respectively. There were no significant differences between the treatment groups in demographics, baseline PANSS total, baseline neurocognitive composite score and baseline daytime sleepiness ESS score (Table 1).
Fig. 1.
Disposition of Subjects. LUR-to-LUR: Lurasidone 80 mg/d or 160 mg/d in the 6-week acute phase followed by flexible-dose lurasidone 40-160 mg/d in the 6-month continuation study; PBO-to-LUR: placebo in the 6-week acute phase followed by flexible-dose lurasidone 40-160 mg/d in the 6-month continuation study; QXR-to-QXR: Quetiapine XR 600 mg/d in the 6-week acute phase followed by flexible-dose quetiapine XR 200-800 mg/d in the 6-month continuation study.
Table 1.
Demographics and clinical characteristics.
| LUR80/160-LUR |
PBO-LUR |
QXR-QXR |
P-value | |
|---|---|---|---|---|
| n = 151 | n = 56 | n = 85 | ||
| Age, mean (SD) | 37.1 (12.3) | 37.5 (11.2) | 38.5(10.1) | 0.847 |
| Gender, n (%) | ||||
| Male | 108 (72%) | 35 (63%) | 52 (61%) | 0.248 |
| Female | 43 (28%) | 21 (38%) | 33 (39%) | |
| Race, n (%) | ||||
| White | 87 (58%) | 32 (57%) | 56 (66%) | 0.500 |
| Black | 22 (15%) | 8 (14%) | 13 (15%) | |
| Asian/other | 35 (23%) | 16 (29%) | 12 (14%) | |
| Pretreatment PANSS total score | 98.0 (10.6) | 96.6 (10.3) | 97.8 (10.6) | 0.685 |
| Mean (SD) | N = 151 | N = 56 | N = 85 | |
| Pretreatment CogState composite score | ||||
| Full analysis sample | − 3.90 (3.41) | − 4.54 (3.48) | − 4.30 (3.62) | 0.467 |
| Mean (SD) | N = 137 | N = 54 | N = 80 | |
| Pretreatment ESS | ||||
| Mean (SD) | 5.80 (4.66) | 5.89 (4.38) | 6.73 (3.92) | 0.304 |
| Pretreatment QWB-SA | ||||
| Mean (SD) | 0.57 (0.19) | 0.58 (0.21) | 0.57 (0.19) | 0.959 |
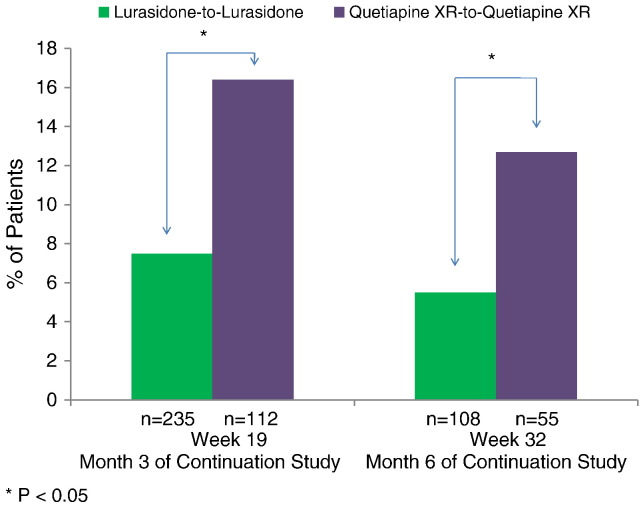
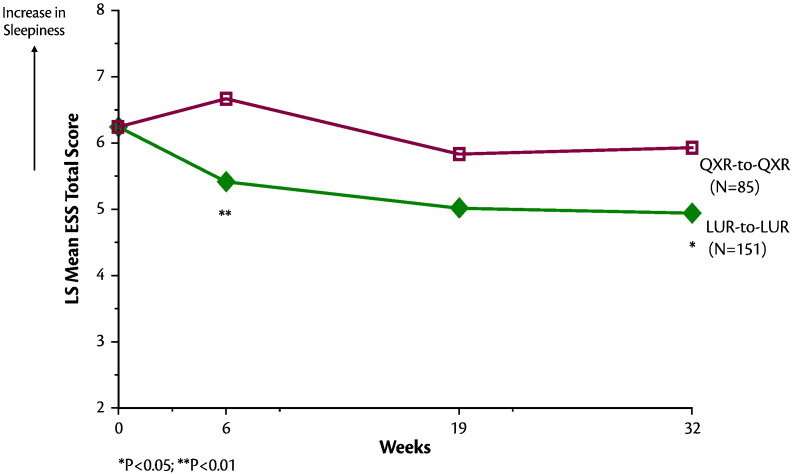
The proportion of patients who experienced markedly high sleepiness (as assessed by ESS total score > 10) was significantly greater in the quetiapine XR-to-quetiapine XR group compared to the lurasidone-to-lurasidone group at both month 3 (16.4% vs. 7.5%, z = 2.08, p = 0.038, NNT = 12) and month 6 (12.7% vs. 5.5%, z = 1.97, p = 0.049, NNT = 14) endpoints (Fig. 2). Change in ESS total score from baseline to week 32 (month 6 of the continuation study) significantly favored the lurasidone-to-lurasidone group (LS mean change − 1.10, SE 0.26) compared to the quetiapine XR-to-quetiapine XR group (LS mean change − 0.07, SE 0.40) (p = 0.03, t = − 2.2; df = 748; effect size = 0.36) (Fig. 3).
Fig. 2.
Rates of markedly high daytime sleepiness (ESS total score > 10).
Fig. 3.
Epworth Sleepiness Scale Total Score Over 32 Weeks of Follow-up (Mixed Effects Model). LUR-to-LUR: Lurasidone 80 mg/d or 160 mg/d in the 6-week acute phase followed by flexible-dose lurasidone 40-160 mg/d in the 6-month continuation study; QXR-to-QXR: Quetiapine XR 600 mg/d in the 6-week acute phase followed by flexible-dose quetiapine XR 200-800 mg/d in the 6-month continuation study.
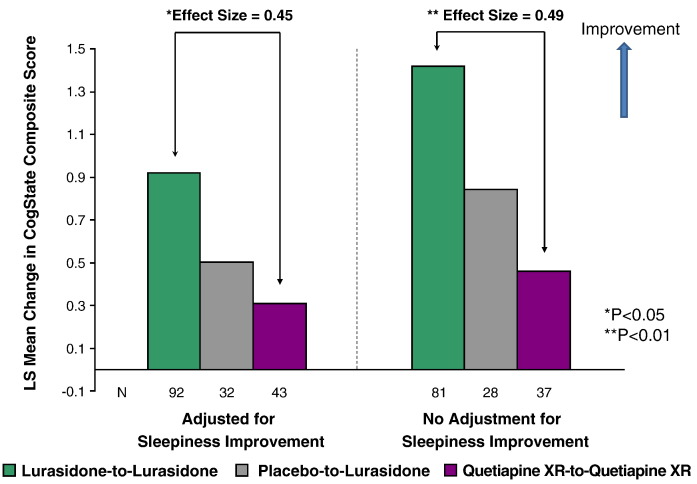
Subjects treated with flexible, once-daily doses of lurasidone (40–160 mg) showed significantly better cognitive performance (assessed by the CogState computerized cognitive battery) compared to quetiapine XR (200–800 mg) both before (effect size = 0.49) and after adjustment (effect size = 0.45) for daytime sleepiness effect (p = 0.008 and 0.010, respectively) (Fig. 4).
Fig. 4.
Longitudinal relationship between change in daytime sleepiness and cognitive performance at month 6 of the double-blind continuation study.
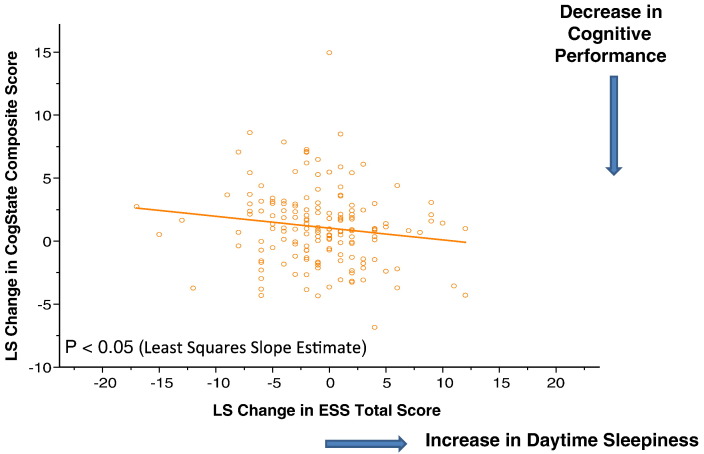
Reduction in daytime sleepiness (lower ESS total score) from baseline was significantly associated with improvement in neurocognitive performance over the double-blind, acute and continuation study periods (p = 0.019, chi-square = 5.53, df = 258), with an increase of 1.85 points in the ESS total score associated with a 0.1 (CogState composite score) worsening in neurocognitive performance (Fig. 5). Reduction in daytime sleepiness from baseline was also significantly associated with improvement of the QWB-SA score across the treatment groups (p = 0.05, chi-square = 3.83, df = 236).
Fig. 5.
Effect of Lurasidone on Change in CogState Composite Score from Baseline to Month 6 Endpoint of the Continuation Study. LUR-to-LUR: Lurasidone 80 mg/d or 160 mg/d in the 6-week acute phase followed by flexible-dose lurasidone 40-160 mg/d in the 6-month continuation study; PBO-to-LUR: placebo in the 6-week acute phase followed by flexible-dose lurasidone 40-160 mg/d in the 6-month continuation study; QXR-to-QXR: Quetiapine XR 600 mg/d in the 6-week acute phase followed by flexible-dose quetiapine XR 200-800 mg/d in the 6-month continuation study.
4. Discussion
In this post-hoc analysis of a long-term, active-controlled, double-blind, continuation study that followed a 6-week acute trial, treatment with flexibly dosed lurasidone 40–160 mg/d, administered once daily in the evening, was associated with significantly less daytime sleepiness compared with flexibly dosed quetiapine XR 200–800 mg/d. Incidence of markedly high sleepiness was higher at both weeks 19 (month 3 of the continuation study) and 32 (month 6 of the continuation study) in the quetiapine XR-to-quetiapine XR group compared with the lurasidone-to-lurasidone group. These results are consistent with findings reported from the acute phase, placebo-controlled study, where patients with schizophrenia were randomized to lurasidone and quetiapine XR (Loebel et al., 2013a), and we extend these results by demonstrating that the excess daytime sleepiness associated with quetiapine XR (vs. lurasidone) persisted in the subsequent long-term continuation study.
The findings that quetiapine XR was associated with greater incidence of markedly high sleepiness and reduced neurocognitive performance at week 32 (end of 6 month continuation study) compared with lurasidone suggest that tolerance and adaptation to this drug-induced adverse effect did not occur over time. Significantly greater improvement from baseline to week 32 (month 6 of the continuation study) in favor of lurasidone compared with quetiapine XR was observed in neurocognitive performance before and after adjustment for daytime sleepiness effects.
We note that while the current study evaluated quetiapine XR, similar comparative findings versus lurasidone would be expected for quetiapine IR, which has been shown to be associated with higher sedation intensity and less patient satisfaction compared to the XR formulation (Datto et al., 2009, Riesenberg et al., 2012, Riedel et al., 2015).
Across all treatment groups, we found that increased daytime sleepiness was significantly related to reduced neurocognitive performance and quality of well-being, assessed over the acute phase study and longer-term extension. Given that cognitive impairment is both pervasive and relatively severe in patients with schizophrenia (Kane and Sharif, 2008; Leifker et al., 2009), our findings suggest that patients may be vulnerable to even modest levels of drug-induced sedation, due to its negative impact on cognition and well-being, and that this should be avoided when possible.
Drug-induced sedation, including by antipsychotic agents, is among the most common causes of excessive daytime sleepiness (Kane and Sharif, 2008). However, this common clinical problem has not been well-studied, so that the magnitude of antipsychotic-induced daytime sleepiness and its impact on cognition and functioning are poorly understood. Our study contributes to further understanding of the comparative impact and longitudinal course of daytime sleepiness associated with antipsychotic agents.
The findings reported here that increased daytime sleepiness was significantly associated with worsening in neurocognitive performance and quality of well-being are consistent with previous reports that show excessive daytime sleepiness may adversely affect personal well-being, functional and cognitive performance, including concentration and alertness (McWhirter et al., 2007, Kane and Sharif, 2008, Wilson and Argyropoulos, 2012, Loebel et al., 2013a, Riedel et al., 2015).
Despite the significant health and economic burden associated with daytime sleepiness for both individuals and society, its evaluation is challenging due to the lack of consensus on systematic methods of assessment. This study confirms that the Epworth Sleepiness Scale, a validated, patient-reported assessment of daytime sleepiness, is sensitive to drug-related changes in daytime sleepiness in patients with schizophrenia. Our finding supports the use of the Epworth Sleepiness Scale as a practical monitoring tool to assess level of daytime sleepiness in patients with schizophrenia.
Several limitations of these findings should be noted. First, the primary analysis was focused on longitudinal changes from baseline to individual follow-up visits in patients who had completed an initial 6-week, randomized, placebo-controlled trial comparing fixed doses of lurasidone or quetiapine XR. Therefore, no long-term data were available for those patients who did not participate in the continuation study. However, demographics and baseline patient characteristics (age, gender, PANSS scores, neurocognitive composite score, and ESS scores) were comparable between the treatment groups in the continuation study (Table 1), and between the 292 patients entering the 6-month continuation study versus the original randomized ITT population (Harvey et al., 2013). These data suggest that the extension study population was representative of the randomized ITT population. Second, potential biases due to practice effects and regression to the mean may confound the results. These potential confounds were addressed through the use of active controls in the double-blind, acute and continuation study phases over an extended treatment period (up to 32 weeks) (Allison et al., 2009), permitting valid comparisons of outcomes in the continuation study. Pseudospecificity issues were also addressed by adjustment for clinical symptoms (Harvey et al., 2013) or daytime sleepiness effects with consistent results in all findings.
In summary, treatment with flexible-dose lurasidone 40–160 mg/d, administered once daily in the evening, was associated with significantly less daytime sleepiness and significantly improved neurocognitive performance compared with flexibly dosed quetiapine XR 200–800 mg/d in a long-term continuation study that followed a 6-week acute trial in patients with schizophrenia. Across all treatment groups, increased daytime sleepiness was significantly associated with reduced neurocognitive performance and quality of well-being over the acute and continuation study periods. Clinicians should therefore actively monitor patients for the presence of daytime sleepiness due in part to its potential for adverse impact on neurocognitive performance and well-being.
Role of Funding Source
This study was sponsored by Sunovion Pharmaceuticals Inc. The sponsor was involved in the study design and collection of data.
Contributors
All authors contributed to the design of the study and the development of the manuscript. Drs. Harvey and Siu undertook the analysis. All authors contributed to and approved the final manuscript.
Conflict of Interest
Dr. Harvey serves as a consultant/advisory board member for Boeheringer-Ingelheim, Forum Pharma, Lundbeck, Otsuka-America, Sanofi, Sunovion, and Takeda. Dr. Siu has received funding and consulting fees from Sunovion that support research and the use of lurasidone clinical trial databases for analyses and preparation of this manuscript. She has also received funding and consulting fees from Pfizer, Hong Kong Health and Medical Research Grant, and the Chinese University of Hong Kong that support research and the use of clinical trial and genetic databases for analyses, preparation of manuscripts, and data science activities. Dr. Loebel is an employee of Sunovion Pharmaceuticals.
Footnotes
ClinicalTrials.govIdentifier:NCT00790192.
ClinicalTrials.govIdentifier:NCT00789698.
References
- Allison DB, Loebel AD, Lombardo I. Understanding the relationship between baseline BMI and subsequent weight change in antipsychotic trials: Effect modification or regression to the mean? Psychiatry Res. 2009;170:172–176. doi: 10.1016/j.psychres.2008.10.007. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Driving fact sheet 2. 2009. http://www.aasmnet.org/Resources/Factsheets/DrowsyDriving.pdf; In. (accessed Jan 20, 2016])
- Anderson JP, Kaplan RM, Berry CC. Interday reliability of function assessment for health status measure: The Quality of Well Being Scale. Med. Care. 1989;27:1076–1083. doi: 10.1097/00005650-198911000-00008. [DOI] [PubMed] [Google Scholar]
- Baldwin CM, Scott LJ. Quetiapine extended release: In schizophrenia. CNS Drugs. 2009;23(3):261–269. doi: 10.2165/00023210-200923030-00007. [DOI] [PubMed] [Google Scholar]
- Chervin RD. The multiple sleep latency test and Epworth Sleepiness Scale in the assessment of daytime sleepiness. J. Sleep Res. 2000;9(4):397–400. doi: 10.1046/j.1365-2869.2000.0227a.x. [DOI] [PubMed] [Google Scholar]
- Datto C, Berggren L, Patel JB, Eriksson H. Self-reported profile of immediate-release quetiapine fumarate compared with extended-release quetiapine fumarate during dose initiation: A randomized, double-blind, crossover study in healthy adult subjects. Clin. Ther. 2009;31(3):492–502. doi: 10.1016/j.clinthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. John Wiley & Sons, Inc.; New Jersey: 2004. Applied Longitudinal Analysis. [Google Scholar]
- Harvey PD, Ogasa M, Cucchiaro J. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr. Res. 2011;127:188–194. doi: 10.1016/j.schres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Siu CO, Hsu J, Cucchiaro J, Maruff P, Loebel A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: A short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur. Neuropsychopharmacol. 2013;23:1373–1382. doi: 10.1016/j.euroneuro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Siu CO, Ogasa M, Loebel A. Effect of lurasidone dose on cognition in patients with schizophrenia: Post-hoc analysis of a long-term, double-blind continuation study. Schizophr. Res. 2015;166:334–338. doi: 10.1016/j.schres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Hawley CJ, Gale TM, Sivakumaran T. Excessive daytime sleepiness in psychiatric disorders: Prevalence, correlates and clinical significance. Psychiatry Res. 2010;175(1–2):138–141. doi: 10.1016/j.psychres.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Huang M, Meltzer HY. The role of 5-Hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition deficit in rats. J. Pharmacol. Exp. Ther. 2011;338(2):605–614. doi: 10.1124/jpet.111.180638. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Horisawa T, Tokuda K. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-HT7 and 5-HT1A receptor activity. J Pharmacol ExpTher. 2010;334:171–181. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johns MW. Epworth Sleepiness Scale (ESS) In: Rush A.J. Jr., First M.B., Blacker D., editors. Handbook of Psychiatric Measures. second ed. American Psychiatric Publishing; Washington, DC: 2008. [Google Scholar]
- Kane JM, Sharif ZA. Atypical antipsychotics: Sedation versus efficacy. J Clin Psychiatry. 2008;69(Suppl. 1):18–31. [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia: The influence of cognitive impairment, functional capacity and symptoms. Schizophr. Res. 2009;115:82–87. doi: 10.1016/j.schres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Loebel A, Siu CO, Cucchiaro J. Daytime sleepiness associated with lurasidone and quetiapine XR: A randomized, double-blind, placebo-controlled trial in patients with schizophrenia. CNS Spectr. 2013;19:197–205. doi: 10.1017/S1092852913000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Sarma K. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: A randomized, double-blind, placebo- and active-controlled trial. Schizophr. Res. 2013;145:101–109. doi: 10.1016/j.schres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Loebel A, Cucchiaro J, Xu J. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: A 12-month, double-blind, continuation study. Schizophr. Res. 2013;147:95–102. doi: 10.1016/j.schres.2013.03.013. [DOI] [PubMed] [Google Scholar]
- McWhirter D, Bae C, Budur K. Treatment of excessive sleepiness: Practical consideration for the psychiatrists. Psychiatry. 2007;4:26–35. [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation State of the states report on drowsy driving. 2007. http://www.drowsydriving.org/stateofthestatesreport Available at. (Accessed Jan 20, 2016)
- Pietrzak RH, Olver J, Norman T. A comparison of the CogState schizophrenia battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J. Clin. Exp. Neuropsychol. 2009;31(7):848–859. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- Riedel M, Schmitz M, Ostergaard PK. Comparison of the effects of quetiapine extended-release and quetiapine immediate-release on cognitive performance, sedation and patient satisfaction in patients with schizophrenia: A randomized, double-blind, crossover study (eXtRa) Schizophr. Res. 2015;162:162–168. doi: 10.1016/j.schres.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Riesenberg RA, Baldytcheva I, Datto C. Self-reported sedation profile of quetiapine extended-release and quetiapine immediate-release during 6-day initial dose escalation in bipolar depression: A multicenter, randomized, double-blind, phase IV study. Clin. Ther. 2012;34(11):2202–2211. doi: 10.1016/j.clinthera.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Wilson S, Argyropoulos S. Sleep in schizophrenia: Time for closer attention. Br. J. Psychiatry. 2012;200:273–274. doi: 10.1192/bjp.bp.111.104091. [DOI] [PubMed] [Google Scholar]
- Witek TJ, Jr., Canestrari DA, Miller RD. Characterization of daytime sleepiness and psychomotor performance following H1 receptor antagonists. Ann. Allergy Asthma Immunol. 1995;74(5):419–426. [PubMed] [Google Scholar]