Abstract
Cognitive impairment is a hallmark of schizophrenia; however, studies have not comprehensively examined such impairments in non-clinically ascertained schizotypic young adults. The present study employed a series of measures to assess episodic memory in high positive schizotypy, high negative schizotypy, and comparison groups (each group n = 25). Consistent with diminished cognitive functioning seen in negative symptom schizophrenia, the negative schizotypy group exhibited deficits on free recall, recognition, and source memory tasks. The positive schizotypy group did not demonstrate deficits on the above mentioned tasks. However, in contrast to the other groups, the positive schizotypy group showed an unexpected set-size effect on the cued-recall task. Set-size effect, which refers to the finding that words that have smaller networks of associates tend to have a memory advantage, is usually found in associative-cuing, but not cued-recall, tasks. The finding for the positive schizotypy group is consistent with heightened spreading activation and reduced executive control suggested to underlie psychotic symptoms. The findings support a multidimensional model of schizotypy and schizophrenia, and suggest that positive and negative schizotypy involve differential patterns of cognitive impairment.
Keywords: Episodic memory, Cognition, Semantic network, Schizotypy, Schizophrenia
1. Introduction
Cognitive impairment is a hallmark of schizophrenia. The vast literature on this topic indicates deficits in attention, memory, and executive functions (e.g., Aleman et al., 1999, Harvey, 2013, Heinrichs and Zakzanis, 1998, Reichenberg et al., 2008). However, challenges remain in distinguishing etiologically relevant cognitive impairment from sequelae of the disorder and determining whether individual areas of cognitive dysfunction simply represent generalized performance impairment. Episodic memory in schizophrenia appears to be impaired beyond deficits accounted for by generalized cognitive impairment (Dickinson et al., 2008, Mesholam-Gately et al., 2009). Episodic memory deficits in schizophrenia have been variously linked to deficits in encoding (i.e., organization of to be learned material), disruption of retrieval (i.e., conscious recollection), as well as deficits in working memory, inhibition, and context processing (e.g., Bonner-Jackson et al., 2005). Impaired memory in schizophrenia has significant real-world impact and is a strong predictor of poor functioning (Green, 1996, Green et al., 2000), even after accounting for generalized cognitive dysfunction (Laes and Sponheim, 2006). Memory deficits are found regardless of duration of illness prior to treatment and these deficits persist following treatment (Addington et al., 2005, Barnes et al., 2008, Goldberg et al., 2007). They also are independent of intelligence and executive functioning (Kopald et al., 2012).
The study of cognitive impairment in schizophrenia is complicated by the fact that it is difficult to disentangle whether the deficits are etiologically relevant, because the consequences of the disorder (e.g., medication, stress) may disguise influences that are specific to schizophrenia. Even when testing unmedicated, first-episode patients, acute symptoms may impair motivation and ability to perform cognitive tasks. Thus, schizotypy provides a promising vantage for studying these deficits relatively unaffected by the effects of schizophrenia. Schizotypy represents the expression of the underlying vulnerability for schizophrenia across a continuum of subclinical and clinical impairment (Kwapil and Barrantes-Vidal, 2015). Schizotypy, and by extension schizophrenia, is multidimensional with positive and negative dimensions the most commonly identified. Numerous studies have demonstrated that positive schizotypy and negative schizotypy are associated with differing patterns of impairment (e.g. Barrantes-Vidal et al., 2013, Ettinger et al., 2015, Gooding and Pflum, 2011, Kwapil et al., 2013).
Multiple processes determine memory performance, and impairment may not necessarily impact all processes comparably. Understanding which cognitive processes are affected by schizotypy and schizophrenia requires the use of different memory paradigms that measure specific processes (vs. broad neurocognitive assessments that are more useful for diagnostic purposes rather than detailed assessment of memory processes). The present study assessed episodic memory in positive and negative schizotypy using a combination of paradigms, including free recall, recognition, source memory, cued recall, and associative cuing, which assesses the influence of set-size effects in semantic networks on episodic memory.
Set-size effects are based on the notion that encoding a familiar word implicitly activates its related concepts from past experiences (e.g., Anderson, 1983, Kintsch, 1988, Nelson et al., 1992). Although implicitly activated associates are not consciously experienced, they nevertheless affect episodic memory. For example, words that produce fewer associates in free association (i.e., have smaller network of associates) have a memory advantage compared to words that produce many associates — known as the set-size effect (Nelson and Friedrich, 1980, Nelson et al., 1992, Nelson et al., 1998). The negative impact of set-size is contingent on the type of memory task. For example, in associative-cuing task, target words are studied in isolation (e.g., study DECORATION), and during the test, a meaningfully related word (e.g., CAKE) is presented as a cue to help retrieve the target. Associative-cuing tasks typically reveal the detrimental effect of set-size, with words having larger set size being poorly remembered. In contrast, set-size has no effect in a standard cued-recall test, when cues are studied simultaneously with the target (e.g., studying CAKE-DECORATION, and during the test receiving CAKE as a cue to retrieve DECORATION). The negative effect of set-size in associative cuing is attributed to interference from increasing number of competing associates that are activated in larger networks (DECORATION can have multiple meanings, and retrieving the appropriate meaning in response to CAKE becomes more challenging when there are multiple alternatives to select from given the large network size). In contrast, when cues are studied with targets, they constrain the meaning of the target, by down regulating or inhibiting the initial implicit activation of the target's competing associates. Thus, when CAKE is studied along with DECORATION, it prevents the alternative meanings of DECORATION from coming to mind. Therefore, set-size has a negative effect in associative cuing, but it does not affect cued recall.
The goals of the present study were to examine episodic memory deficits and set-size effect in positive and negative schizotypy. Previous studies have examined various forms of memory in schizotypy (see Ettinger et al., 2015, for a selective review); however, this is the first study to examine set-size effect. Gooding and Braun (2004) found reduced nonverbal memory performance in a negative schizotypy group relative to positive schizotypy and control groups. Stefaniak et al. (2015) reported that positive schizotypy was negatively related with controlled memory processes. LaPorte et al. (1994) failed to find associations between schizotypy and memory performance; however, their study was limited to a single logical memory task. Kaczorowski et al. (2009) reported that negative, but not positive, schizotypy was associated with memory recall deficits. However, the interpretation of other memory studies in schizotypy is often constrained by methodological limitations, including failure to examine schizotypy dimensions separately, use of problematic measures of schizotypy, failure to examine multiple memory processes, and the use of clinical screening measures of memory that are not sufficient for disentangling complex memory processes.
Given reports of cognitive impairment in negative symptom schizophrenia, it was expected that negative schizotypy would be associated with episodic memory impairment (Addington et al., 1991, Green and Walker, 1985), although the nature of the process that is disrupted in memory remains to be established. On the associative-cuing task, we expected all three groups to exhibit set-size effect, whereas obtaining larger set-size effect among schizotypy participants would suggest that they have larger and more expanded associative networks, indicating abnormalities with organization of their semantic system. Finally, consistent with previous memory research, we did not expect to obtain set-size effect in cued recall, whereas obtaining such an effect in the schizotypy groups would implicate disruption in processes that act on the semantic network (e.g., activation/inhibition).
2. Methods
2.1. Participants
Participants were 75 undergraduates from introductory psychology courses. They were invited to participate based on scores on the Wisconsin Schizotypy Scales—brief version (Winterstein et al., 2011) administered in mass-screening sessions. The positive and negative schizotypy groups included 25 participants each who scored at least 1.5 SD above the mean on the respective schizotypy dimension based on a sample of 6137 young adults (Gross et al., 2012). The comparison group contained 25 participants who scored within .5 SD of the mean on both the positive and negative schizotypy scores.
2.2. Materials and procedures
Participants completed brief versions of the Perceptual Aberration (Chapman et al., 1978), Magical Ideation (Eckblad and Chapman, 1983), Physical Anhedonia (Chapman et al., 1976), and Revised Social Anhedonia (Eckblad et al., 1982) Scales. Two factors, positive and negative schizotypy, underlie the original (Kwapil et al., 2008) and brief (Gross et al., 2015) versions of the scales. Positive and negative schizotypy factor scores were computed following formulae in Gross et al. (2015). Descriptive statistics for the schizotypy dimensions are in Table 1.
Table 1.
Mean standardized positive and negative schizotypy scores across the groups.
| Positive schizotypy Z-scores |
Negative schizotypy Z-scores |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Control group | −0.12 | 0.39 | −0.11 | 0.29 |
| Positive Schizotypy group | 2.65 | 0.67 | 0.07 | 0.48 |
| Negative Schizotypy group | −0.15 | 0.54 | 2.53 | 0.67 |
Positive and negative schizotypy factors scores are based upon formulae in Gross et al. (2015).
Participants completed a battery of verbal memory tests, and in between each test solved spatial tasks for 5 min. Memory was assessed with (A) a free recall test, (B) a recognition test along with source identification, (C) a cued-recall test, and (D) an associative-cuing test. To counterbalance the test order, forward (ABCD) and reverse (DCBA) order of administration was used. Stimuli for each test were unique and did not appear on the remaining tests.
Free recall test involved studying 12 unrelated nouns of medium frequency (based upon Kucera and Francis, 1967) presented at a rate of 5 s and completing math problems on the computer screen for 30 s, followed by a 60 s recall period, during which participants typed recalled words into the computer in any order. The procedure was repeated five times, with new words presented during each block.
Recognition-plus-source test involved encoding two lists of 20 unrelated words of medium frequency. During encoding, words were shown at a rate of 3 s. After the first list, instructions on the screen explained that participants were about to see another list, which should also be memorized. Following encoding of two lists, participants were shown 40 old words (Lists 1 and 2) intermixed with 40 new words, presented one at a time, and were asked to determine whether an item was old or new. If they identified an item as old, they were asked to indicate whether it was presented on List 1 or List 2.
Cued-recall test involved studying 24 meaningfully related pairs of words (e.g., CANOE–RIVER), presented at a rate of 3 s per pair. At test, participants received the first word of the pair as a cue to help them retrieve the second word. They were told that each cue was related only to one of the study words, and that they should guess if needed.
Associative-cuing test involved studying 24 unrelated words, presented at a rate of 3 s per item. Memory was tested by presenting a related cue word that did not appear on the study list, and instructing participants to use it to retrieve the related word from the study list (e.g., studying PLANET and being cued by SPACE). Participants were told that each cue was related only to one of the study words, and that they should guess if needed.
The stimuli for cued-recall and associative-cuing tests were selected from University of South Florida Free Association Norms (Nelson et al., 1999). Specifically, 48 words served as targets for two unrelated 24-item study lists (List A and B). For half of the participants, List A was used for associative cuing and List B for the cued-recall test (and vice versa). Within each list, 12 targets had a large set-size (M = 19.88 associates, SD = 3.05 in their set), whereas 12 targets had a small set-size (M = 6.56, SD = 1.59). An additional 48 words were selected to serve as test cues, one per target item. Targets were unrelated to each other and cues were unrelated to each other. Each cue was related only to a single target. While manipulating target set-size, we controlled remaining variables known to affect memory, including direct and indirect strength between cue and target, cue set-size, and frequency and concreteness of cues and targets (Table 2).
Table 2.
Mean strengths and standard deviations of controlled variables across set size manipulation for items used in associative-cuing test and cued-recall test.
| Controlled variables across set size manipulation | M | SD |
|---|---|---|
| direct Target-to-Cue strength | .02 | .02 |
| direct Cue-to-Target strength | .06 | .03 |
| indirect strength between cue and target: shared associate strength | .06 | .07 |
| indirect strength between cue and target: mediated strength | .04 | .05 |
| cue set size | 13.28 | 5.36 |
| number of Associate-to-Associate connections in network | 1.36 | .65 |
| sum of Associate-to-Associate link strengths | 2.37 | .84 |
| number of Associates-to-Target connections in network | .49 | .22 |
| sum of Associates-to-Target link strengths | 1.78 | .69 |
| target printed frequency (Kucera and Francis, 1967) | 89.94 | 79.22 |
| target concreteness (on a scale from 1 to 7) | 5.00 | 1.19 |
| cue printed frequency (Kucera and Francis, 1967) | 38.63 | 45.94 |
| cue concreteness (on a scale from 1 to 7) | 4.88 | 1.18 |
Values are based on the USF Free Association Norms, unless otherwise noted.
The testing session lasted 90 min. The project was approved by the UNCG IRB and participants provided informed consent.
3. Results
Effect sizes (η2) are reported for ANOVAs. Following Cohen (1988), η2 values of .02 are considered small, .13 are medium, and .26 are large effect sizes.
3.1. Free recall
Fig. 1 presents recall accuracy and errors on the free recall task. Given that recall and errors did not differ across the blocks, we report average performance across the five blocks. The groups differed significantly in recall accuracy, F(2,72) = 3.76, p < .05, η2 = .10. The negative schizotypy group recalled fewer words than either the positive schizotypy, t(48) = 2.44, p < .05, or control, t(48) = 2.29, p < .05, groups, which did not differ from each other, t < 1. During recall, participants occasionally made errors, which we separated into intrusions from previous study lists (prior-list errors) or pre-experimental intrusions (extra-list errors). However, there was no difference between the groups on either type of error. Furthermore, the groups did not differ on the number of attempted or accurately solved filler math problems.
Fig. 1.

Free recall performance for the schizotypy and control groups. Note that the Negative Schizotypy group performed worse on recall accuracy than the other two groups.
3.2. Recognition memory
Recognition memory was assessed using d’, which is a measure of discrimination accuracy. The groups differed significantly in accuracy, F(2,72) = 3.43, p < .05, η2 = .09 (see Fig. 2). The negative schizotypy group had worse accuracy than the positive schizotypy, t(48) = 2.26, p < .05, and control, t(48) = 2.48, p < .05, groups, which did not differ from each other. Raw measures of hits and false alarms are shown in Table 3.
Fig. 2.

Recognition accuracy for the schizotypy and control groups. Note that the Negative Schizotypy group performed worse than the other two groups.
Table 3.
Hits and false alarms in recognition memory.
|
Hits |
False alarms |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Control group | .76 | .19 | .14 | .11 |
| Positive Schizotypy group | .78 | .15 | .15 | .12 |
| Negative Schizotypy group | .67 | .15 | .17 | .12 |
Raw measures of hits and false alarms for the schizotypy and control groups.
3.3. Source memory
Source memory was assessed using conditional source identification measure (CSIM). This is computed as the proportion of items which were correctly attributed to its list out of total number of items from that list that were correctly recognized (Murnane and Bayen, 1996). After computing CSIM scores for each list, we took their average (ACSIM) to indicate overall list source memory, with .50 indicating chance source memory. One-way ANOVA on ACSIM scores revealed significant group differences, F(2,72) = 4.52, p < .05, η2 = .11 (see Fig. 3). Although all three groups had above chance source memory, the negative schizotypy group was worse at remembering the source of items than the positive schizotypy, t(48) = 2.20, p < .05, and control t(48) = 3.09, p < .05, groups, which did not differ from each other.
Fig. 3.

Source memory performance for the schizotypy and control groups. Note that the Negative Schizotypy group performed worse on source identification than the other two groups.
3.4. Associative cuing
We analyzed the proportion of items correctly recalled on the associative-cuing test using a set-size (small vs. large) × group (positive, negative, control) mixed ANOVA. There was neither a main effect of group, nor a group × set-size interaction. However, there was a main effect of set-size, F(1,72) = 65.78, p < .001, η2 = .48. On average, targets from small sets were better recalled (M = .72, SD = .18) than targets from large sets (M = .57, SD = .18), revealing the typical set-size effect. The size of this effect was invariant across the groups (see Fig. 4, top panel).
Fig. 4.
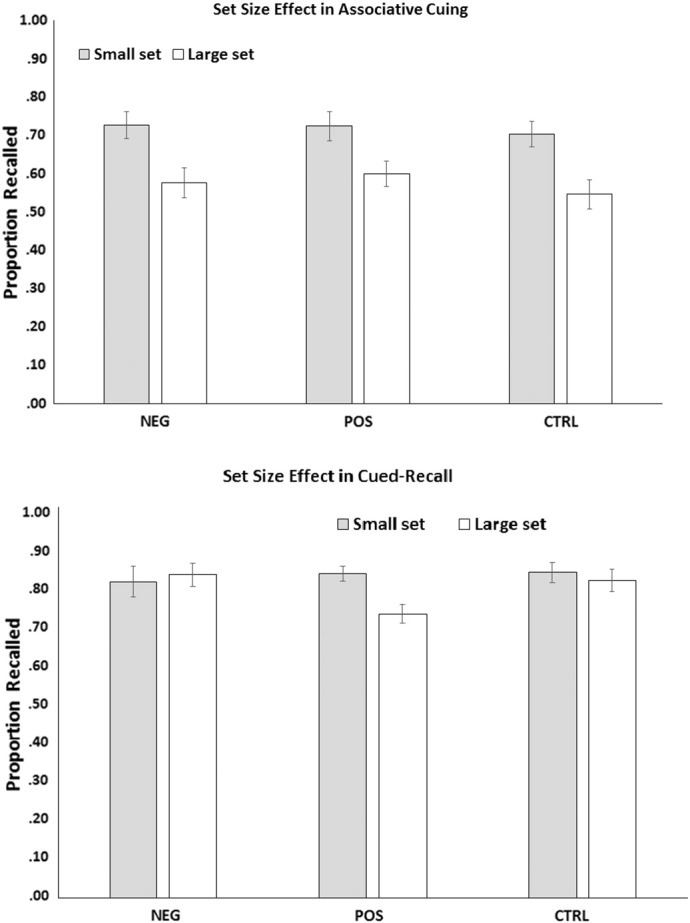
All groups demonstrated the expected set size effect in the associative-cuing task. Only the positive schizotypy group demonstrated set size effect in the cued-recall task.
3.5. Cued recall
The same analyses performed on the proportion of items recalled on the cued-recall test surprisingly revealed a main effect of set-size, F(1,72) = 6.10, p < .05, η2 = .08. However, as shown by a significant interaction with group, F(1,72) = 6.08, p < .01, η2 = .15, the set-size effect was obtained only in positive schizotypy. Specifically, there was a recall disadvantage for large set items compared to small set items in the positive schizotypy group, t(24) = 3.52, p < .01, but not in the negative schizotypy or control groups, in which recall of small sets and large sets was comparable (see Fig. 4, bottom panel). Another way of decomposing the interaction is examining the effect of group separately on the recall of large and small set items. There was no effect of group in small set recall (F < 1), however, there was a significant group effect in large set recall (F(2, 72) = 3.72, p < .05), with positive group showing a deficit in recall compared to the control (t(48) = 2.24, p < .05) or the negative group (t(48) = 2.57, p < .05), which did not differ from each other, t < 1.
4. Discussion
Schizotypy and schizophrenia are characterized by heterogeneity in etiology and expression. This heterogeneity appears to be captured in a multidimensional structure that includes positive and negative symptom dimensions. The finding that cognitive deficits, like many other impairments in schizotypy and schizophrenia, are differentially associated with these dimensions holds promise for understanding etiology and development of such impairments and their role as possible endophenotypes. The present study involves the first comprehensive demonstration of the differential association of episodic memory impairments with schizotypy dimensions.
The negative schizotypy group had deficits in free recall as evidenced by lower recall accuracy compared to the positive schizotypy and control groups, consistent with Gooding and Braun (2004). Similar results were also obtained in recognition memory, indicating that their deficits were not restricted to retrieval processes. The negative schizotypy group also had impaired source memory, compared to the positive schizotypy and control groups. Given that all participants attempted and solved a similar number of math problems during the distractor task, simple motivational differences or ability differences are unlikely to explain these findings. It is also unlikely that generalized cognitive deficit is responsible for these results, as the negative schizotypy group performed comparably to the remaining participants on the associative-cuing test. All three groups showed similar magnitude of set-size effect on the associative-cuing task, indicating that the semantic network is unlikely to be organized differently across the control and schizotypy participants. In other words, the underlying knowledge organization and pre-existing associations between language concepts appeared to be comparable across the groups. Thus, negative, but not positive, schizotypy appears to involve deficits in episodic memory. This is consistent with meta-analytic findings that negative symptoms are associated with heightened memory impairment (Aleman et al., 1999).
In contrast, we observed a set-size effect in the positive schizotypy group on cued recall, despite the fact that the targets were studied simultaneously with meaningful cues during encoding. This is an unusual but theoretically informative finding. Typically, the presence of cue words during encoding inhibits the activation of competing associates of the target, thereby constraining the meaning of the target and eliminating the set-size effect that emerges when targets are studied without the test cues. This was the case in the control and the negative schizotypy groups, which did not show a set-size effect in cued recall, despite showing the expected set-size effect in associative cuing. In contrast, the positive schizotypy group showed a set-size effect in both types of memory tests. The results cannot be attributed to test difficulty, because performance was higher in cued recall than in associative-cuing test, indicating that the former was an easier test. The unexpected set-size effect for the positive schizotypy group on cued recall is consistent with excessive spreading activation and interference from pre-existing associates in large networks — well-documented in schizophrenia (e.g., Spitzer et al., 1993). It is also consistent with reduced controlled processes in positive schizotypy (Neill et al., 2014, Stefaniak et al., 2015) and higher levels of automatic processes (Burch et al., 2006). If positive schizotypy is associated with weakened inhibitory processes, which would otherwise act to suppress the initial activation of competing associates in the presence of meaningful cue words, this could lead to the set-size effect seen in cued recall.
These results demonstrate that there are long-term memory deficits in a non-clinical schizotypy sample. On some tests, memory deficits emerged only in negative schizotypy, on other tests, the deficits were specific to positive schizotypy, and yet on some tests, all three groups performed comparably. The findings suggest that the results did not reflect generalized performance deficits in either schizotypy group. The present results support the model (Kwapil and Barrantes-Vidal, 2015) that the underlying vulnerability for schizophrenia is expressed across a broad continuum of clinical and subclinical symptoms and impairment. Studying non-psychotic schizotypes provides a powerful method for examining the expression and development of schizophrenia-spectrum disorders. Furthermore, the finding that cognitive deficits are present in subclinical schizotypes lends support to the notion that cognitive impairment may be a useful endophenotype for schizophrenia (Alle et al., 2009). Finally, the differential findings for positive and negative schizotypy are consistent with recent interview, laboratory, and daily life findings (e.g., Kwapil et al., 2012) and highlight the importance of considering the multidimensionality of schizotypy and schizophrenia.
Role of funding source
This project was not supported by external funding sources.
Contributors
Lili Sahakyan, Ph.D., designed the study, oversaw data collection, and was lead author of the manuscript. Thomas R. Kwapil, PhD, contributed to the data analyses and writing of the manuscript.
Conflict of interest statement
Neither author had a conflict of interest.
References
- Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr. Res. 1991;5:123–134. doi: 10.1016/0920-9964(91)90039-t. [DOI] [PubMed] [Google Scholar]
- Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr. Res. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hij R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am. J. Psychiat. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Alle AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD. Endophenotypes in schizophrenia: a selective review. Schizophr. Res. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR. Harvard University Press; Cambridge, MA: 1983. The Architecture of Cognition. [Google Scholar]
- Barnes TRE, Leeson VC, Mutsatsa SH, Watt HC, Hutton SB, Joyce EM. Duration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophrenia. Br. J. Psychiat. 2008;193:203–209. doi: 10.1192/bjp.bp.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Gross G, Sheinbaum T, Mitjavila M, Ballespi S, Kwapil TR. Positive and negative schizotypy are associated with prodromal and schizophrenia-spectrum symptoms. Schizophr. Res. 2013;145:50–55. doi: 10.1016/j.schres.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol. Psychiat. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch SJB, Hemsley DR, Corr PJ, Gwyer P. The relationship between incidental learning and multi-dimensional schizotypy as measured by the Oxford–Liverpool inventory of feelings and experiences (O-LIFE) Pers. Indiv. Dif. 2006;40:385–394. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976;85:374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body image aberration in schizophrenia. J. Abnorm. Psychol. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. Erlbaum; Hillsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol. Psychiat. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Eckblad, M.L., Chapman, L.J., Chapman, J.P., Mishlove, M., 1982. The Revised Social Anhedonia Scale. Unpublished test copies available from T.R. Kwapil, UNC-Greensboro, Greensboro, NC 27402.
- Ettinger U, Mohr C, Gooding DC, Cohen AS, Rapp A, Haenschel, Park S. Cognition and brain function in schizotypy: a selective review. Schiz. Bull. 2015;(Suppl. 2):S417–S426. doi: 10.1093/schbul/sbu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC.…Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiat. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Braun JG. Visuoconstructive performance, implicit hemispatial inattention, and schizotypy. Schizophr. Res. 2004;68:261–269. doi: 10.1016/S0920-9964(03)00157-9. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. Theory of mind and psychometric schizotypy. Psychait. Res. 2011;188:217–223. doi: 10.1016/j.psychres.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiat. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green M, Walker E. Neuropsychological performance and positive and negative symptoms in schizophrenia. J. Abnorm. Psychol. 1985;94:460–469. doi: 10.1037//0021-843x.94.4.460. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophr. Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Gross GM, Silvia PJ, Barrantes-Vidal N, Kwapil TR. Psychometric properties and validity of short forms of the Wisconsin schizotypy scales in two large samples. Schizophr. Res. 2012;134:267–272. doi: 10.1016/j.schres.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Gross GM, Silvia PJ, Barrantes-Vidal N, Kwapil TR. The dimensional structure of short forms of the Wisconsin schizotypy scales. Schizophr. Res. 2015;166:80–85. doi: 10.1016/j.schres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Harvey P. University Press; Cambridge: 2013. Cognitive Impairment in Schizophrenia: Characteristics, Assessment and Treatment. [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Kaczorowski JA, Barrantes-Vidal N, Kwapil TR. Neurological soft signs in psychometrically identified schizotypy. Schizophr. Res. 2009;115:293–302. doi: 10.1016/j.schres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Kintsch W. The role of knowledge in discourse comprehension: a construction–integration model. Psychol. Rev. 1988;95:163–182. doi: 10.1037/0033-295x.95.2.163. [DOI] [PubMed] [Google Scholar]
- Kopald BE, Mirra KM, Egan MF, Weinberger DR, Goldberg TE. Magnitude of impact of executive functioning and IQ on episodic memory in schizophrenia. Biol. Psychiat. 2012;71:545–551. doi: 10.1016/j.biopsych.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis W. Brown University Press; Providence, RI: 1967. Computational Analyses of Present-Day American English. [Google Scholar]
- Kwapil TR, Barrantes-Vidal N. Schizotypy: looking back and moving forward. Schiz. Bull. 2015;(Suppl. 2):S366–S373. doi: 10.1093/schbul/sbu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin schizotypy scales: factor identification and construct validity. Schizophr. Bull. 2008;34:444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Brown LH, Silvia PJ, Myin-Germeys I, Barrantes-Vidal N. The expression of positive and negative schizotypy in daily life: an experience sampling study. Psychol. Med. 2012;42:2555–2566. doi: 10.1017/S0033291712000827. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans' ten-year longitudinal study. J. Abnorm. Psychol. 2013;122:807–815. doi: 10.1037/a0033759. [DOI] [PubMed] [Google Scholar]
- Laes JR, Sponheim SR. Does cognition predict community function only in schizophrenia? A study of schizophrenia patients, bipolar affective disorder patients, and community control subjects. Schizophr. Res. 2006;84:121–131. doi: 10.1016/j.schres.2005.11.023. [DOI] [PubMed] [Google Scholar]
- LaPorte DL, Kirkpatrick B, Thaker GK. Psychosis-proneness and verbal memory in a college student population. Schizophr. Res. 1994;12:237–245. doi: 10.1016/0920-9964(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Murnane K, Bayen UJ. An evaluation of empirical measures of source identification. Mem. Cognition. 1996;24:417–428. doi: 10.3758/bf03200931. [DOI] [PubMed] [Google Scholar]
- Neill E, Rossell SL, Kordzadze M. Investigating word associations in a schizotypy sample: contrasting implicit and explicit processing. Cog. Neuropsychiatry. 2014;19:134–148. doi: 10.1080/13546805.2013.807727. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Friedrich MA. Encoding and cuing sounds and senses. J. Exp. Psychol-Hum. L. 1980;6:717–731. [PubMed] [Google Scholar]
- Nelson DL, Schreiber TA, McEvoy CL. Processing implicit and explicit representations. Psychol. Rev. 1992;99:322–348. doi: 10.1037/0033-295x.99.2.322. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McKinney VM, Gee NR, Janczura GA. Interpreting the influence of implicitly activated memories on recall and recognition. Psychol. Rev. 1998;105:299–324. doi: 10.1037/0033-295x.105.2.299. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme and word fragment norms. 1999. http://www.usf.edu/FreeAssociation/ Retrieved from. [DOI] [PubMed]
- Reichenberg A, Harvey PD, Bowie CR. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2008;133:833–858. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol. Psychiat. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- Stefaniak N, Giota C, Terriena S, Besche-Richard C. Impaired conscious memory in non-clinical schizotypy. Cog. Neuropsychiatry. 2015;20:243–253. doi: 10.1080/13546805.2015.1020147. [DOI] [PubMed] [Google Scholar]
- Winterstein BP, Silvia PJ, Kwapil TR, Kaufman JC, Reiter-Palmon R, Wigert B. Brief assessment of schizotypy: developing short forms of the Wisconsin schizotypy scales. Pers. Indiv. Differ. 2011;51:920–924. [Google Scholar]


