Abstract
The addition of off-the-shelf cognitive measures to established prodromal criteria has resulted in limited improvement in the prediction of conversion to psychosis. Tests that assess cognitive processes central to schizophrenia might better identify those at highest risk. The latent inhibition paradigm assesses a subject's tendency to ignore irrelevant stimuli, a process integral to healthy perceptual and cognitive function that has been hypothesized to be a key deficit underlying the development of schizophrenia. In this study, 142 young people at ultra high-risk for developing psychosis and 105 controls were tested on a within-subject latent inhibition paradigm. Additionally, we later inquired about the strategy that each subject employed to complete the test, and further investigated the relationship between reported strategy and the extent of latent inhibition exhibited. Unlike controls, ultra high-risk subjects did not demonstrate a significant latent inhibition effect. This difference between groups became greater when controlling for strategy. The lack of latent inhibition effect in our ultra high-risk sample suggests that individuals at ultra high-risk for psychosis are impaired in their allocation of attentional resources based on past predictive value of repeated stimuli. This fundamental deficit in the allocation of attention may contribute to the broader array of cognitive impairments and clinical symptoms displayed by individuals at ultra high-risk for psychosis.
Keywords: Psychosis, Schizophrenia, Adolescence, High-risk, Cognition
1. Introduction
Efforts to establish criteria that identify individuals at highest risk for schizophrenia have had limited success (Yung et al., 2005, Cannon et al., 2008, McGorry et al., 2008). Recent work has attempted to improve predictive capability by supplementing current prodromal symptom criteria with neurocognitive measures (Brewer et al., 2005, Brewer et al., 2006, Keefe et al., 2006). However, many of the cognitive assessments explored to date have used off-the-shelf tests designed for measuring intelligence or brain damage. Methodologies that probe specific cognitive impairments characteristic of the at-risk state may yield greater risk prediction specificity.
Recent theories of brain function have suggested that a central role of the human brain is to encode hierarchical memories that emphasize the commonality of experiences across time and to use these “invariant” memories to continually predict the next moment of experience, a process we have termed learning-dependent predictive perception (LDPP) (Hawkins and Blakeslee, 2004, Krishnan et al., 2011b). LDPP employs regularities in past experience to guide the allocation of attentional and cognitive resources, thus facilitating efficient and appropriate interaction with the world. In fact, it has been postulated that the columnar circuitry present throughout the neocortex aids these processes (Hawkins and Blakeslee, 2004) and thus LDPP is a fundamental function of neocortical architecture. We have hypothesized that individuals with schizophrenia exhibit impaired LDPP function and that deficits in LDPP may be a key cognitive risk factor for developing psychosis (Keefe et al., 2011, Kraus et al., 2009, Krishnan et al., 2011a, Krishnan et al., 2009). As such, we believe that cognitive tests that assess aspects of LDPP may be especially sensitive predictors of conversion to psychosis (Keefe and Kraus, 2009).
We have been testing the hypothesis that LDPP is impaired in individuals at high risk for developing schizophrenia. The Longitudinal Youth at Risk Study (LYRIKS) is a prospective, observational, single-site project conducted in Singapore to identify the clinical, cognitive and biological factors that predict the development of psychosis (Chong et al., 2011). The study identified youth at ultra high-risk (UHR+) for developing psychosis and then followed these individuals for 2 years, assessing their neurocognitive function at 6 month intervals [For a complete description of the neuropsychological battery, see Lee et al. (2013)].
Here we report the results from one test of LDPP function, the Latent Inhibition (LI) test. Pre-exposure to a conditioned stimulus in the absence of an unconditioned stimulus inhibits conditioning when the stimuli are subsequently paired, a phenomenon termed latent inhibition. This paradigm tests the viability of the LDPP system by assessing a participant's tendency to ignore stimuli that had been irrelevant to task performance previously and focus on stimuli that are more likely to aid task performance. This ability to filter out irrelevant stimuli and focus on meaningful stimuli is crucial to the efficient allocation of perceptual and cognitive resources and impairment in this ability has been hypothesized to underlie the development of psychosis (Gray et al., 1991, Kapur, 2003). Reduced LI has been demonstrated in unmedicated and/or acute schizophrenia patients (Baruch et al., 1988a) and LI scores have exhibited strong correlations with schizotypal traits and degree of latent inhibition in the general population (Baruch et al., 1988b). In this study, we have for the first time assessed the latent inhibition effect in a group of young people at high-risk for developing psychosis.
2. Method
2.1. Overall study design
The design of the overall study is described in detail elsewhere (Lee et al., 2013). The study was approved by the National Healthcare Group's Domain Specific Review Board and all study procedures were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Recruitment adopted a hybrid approach that has been described in greater detail in an earlier publication (Mitter et al., 2014). Both help-seeking and non-help-seeking individuals in the community were approached for this study. The inclusion criteria for the study were (i) youths between 14 and 29 years old and (ii) English-speaking because of the neurocognitive measures. Participants were excluded if they (i) had a past or current history of psychosis or mental retardation, (ii) were currently using illicit substances, (iii) were taking antipsychotics or mood stabilizers, or (iv) had medical causes associated with their psychosis.
The CAARMS (Yung et al., 2005) positive symptoms subscale was used to determine the risk status of each participant. The CAARMS was administered by either psychiatrists or research psychologists. The administrators attended a 1-week intensive training at the Personal Assessment and Crisis Evaluation Clinic (PACE), in Melbourne, Australia. Inter-rater reliability was established at >0.9. Supervision was provided by two on-site research clinicians and at monthly rater meetings. The CAARMS composite score was computed, by weighting intensity (I) of symptoms by their frequency (F) within the four domains of the CAARMS Positive Scale — Unusual Thought Content (UTC), Non-Bizarre Ideas (NBI), Perceptual Abnormalities (PA) and Disorganized Speech (DS), according to the formula (Iutc ∗ Futc) + (Inbi ∗ Fnbi) + (Ipa ∗ Fpa) + (Ids ∗ Fds).
The LI task was included as part of the neurocognitive battery, consisting of standard neuropsychological tests, as well as experimental tests included to assess specific aspects of LDPP. Due to technical issues, the latent inhibition test was not ready at the start of the study, resulting in a large amount of missing data that prohibits its inclusion in the longitudinal analyses involving the rest of the neurocognitive battery, which will be reported elsewhere.
2.2. Latent inhibition test design
The LI task was programmed in MATLAB version 7.8.0.347 (R2009a), based on the design of Schmidt-Hansen et al. (2009) and the task was run on a 17 inch Dell laptop.
When the task was started, the screen displayed the instruction “Look for X”. Participants were then read the following instructions (as used by Schmidt-Hansen et al. (2009)): This is a reaction time test which lasts for about 7 minutes. In this task I want you to watch the sequence of letters. Your task is to try to predict when the letter “X” is going to appear. If you think that you know when the “X” will appear, then you can press the spacebar early in the sequence. Alternatively, press as quickly as you can when you see the “X”. There may be more than one rule that predicts the “X”. Please try to be as accurate as you can, but do not worry about the occasional error.
The LI test consisted of two phases, a pre-exposure phase and a test phase divided into two blocks. In the pre-exposure phase, the pre-exposed (PE) letter was presented 10 times, intermixed with 4 different filler letters, each presented a total of 14 times in a fixed pseudo-random sequence. The sequence was constrained by the rule that no stimulus should be presented in consecutive presentations. The test phase immediately followed the pre-exposure phase with no break in between.
In each test block, the target stimulus (the letter X) was presented 24 times. Filler letters were interspersed with the presentation of the target. Each filler letter was presented an average of 27 times per block, with the target letter immediately following on 2 of the trials (7.4% of trials) for each filler letter per block. During the test phase, a non pre-exposed (NPE) letter (a letter that was not presented during the pre-exposure phase) and the PE letter were presented 8 times per block and were always followed by the presentation of the target (100% of trials). Each letter was black, 1.2 cm high and presented on a white background for 1000 ms with no inter-stimulus interval. The rationale behind this method is that subjects will learn during the pre-exposure phase that pre-exposed letters do not precede the target letter, thus their reaction time to targets that are preceded by pre-exposed letters will be longer than targets preceded by non-pre-exposed letters. The key comparison for the test is the number of anticipatory responses and mean reaction time to targets following an NPE predictor compared to a PE predictor (Schmidt-Hansen et al., 2009). The presentation order of all stimuli was fixed across all participants. Also, the presentation order was fixed across assessments, but filler, PE and NPE letters were rotated across versions of the test. X was always used as a target and all consonants except K and Y (which were deemed to be too visually similar to X) were rotated such that none appeared in more than 2 non-consecutive versions and no letter was used as a PE or NPE on more than 1 version. The task lasted approximately 7 min.
Because early testing indicated a large range of reaction times for the PE and NPE trials in both UHR− and UHR+ subjects, we questioned whether these differences were due to different strategies employed to perform the task. Thus, we began explicitly asking subjects about the strategy they used. Immediately following completion of the test, the psychometrician asked the following two questions: 1) “What was your strategy for doing that task?” and 2) “Was there anything you did to try to respond more quickly when the X came up?” Participants' responses were then classified into one of five categories: 1) Optimal (participants mentioned that both the PE and NPE stimuli reliably predicted X, 2) Favoring the PE stimulus (participants specifically mentioned only that the PE stimulus reliably predicted X), 3) Favoring the NPE stimulus (participants specifically mentioned only that the NPE stimulus reliably predicted X), Irrelevant (participants reported using an irrelevant strategy such as counting letters following the appearance of an X), and 5) None (participants denied using any strategy beyond looking for the X and hitting the spacebar quickly when it appeared).
2.3. Statistical analyses
Although LI assessments were conducted at 6 month intervals, missing data and divergent participant strategy greatly reduced the power of our sample and precluded the longitudinal analysis of the data. Instead, we conducted cross-sectional analyses to investigate the differences between UHR− and UHR+ subjects. Three separate analyses with different subsamples were conducted: 1) To eliminate a possible effect of assessment time, we analyzed just the baseline assessments from all participants who completed the LI test at baseline, 2) To incorporate the greatest number of participants possible, we included the first assessment of all participants, regardless of the visit number at which it occurred. 3) To reduce the effect of strategy, we analyzed only the baseline assessments of subjects reporting the optimal strategy for completing the test.
Statistical analyses were conducted using SPSS Statistics 20.0 for PC. Group differences for age were analyzed using the Mann–Whitney test, while group differences for categorical demographic variables (gender, ethnicity and smoking) were analyzed using Pearson's chi-square test. For each subject, number of anticipatory responses (coming before the presentation of the target stimulus) and mean reaction time were calculated for correct trials in each exposure condition (random, pre-exposed and non pre-exposed) in each of the two blocks. Anticipatory response and mean reaction time data were analyzed using a 2×(3 × 2) mixed factorial ANOVA, with UHR status as the between groups variable and exposure (random, pre-exposed, and non-pre-exposed trials) and block as the within subjects variables.
3. Results
3.1. Anticipatory responses
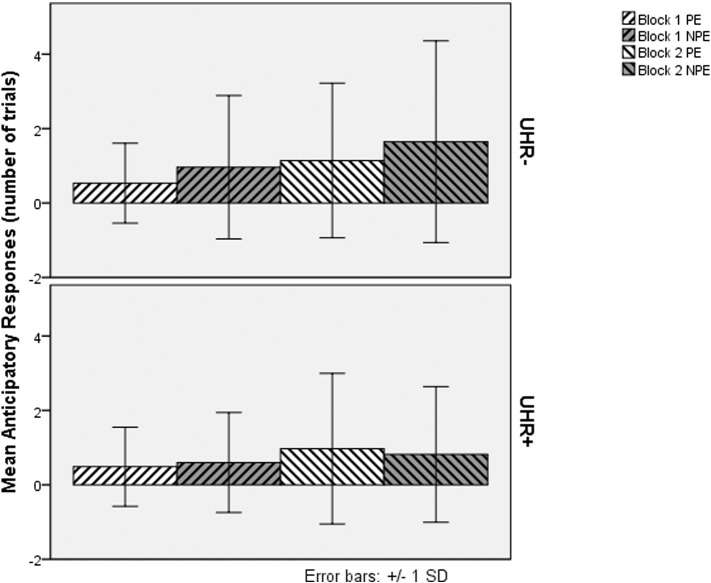
We analyzed anticipatory responses and mean reaction times of participants at their baseline visit (N = 142 UHR+, 105 UHR−). The demographic characteristics of this sample are reported in Table 1. The groups did not differ on any of the demographic variables, but clearly differed in their CAARMS positive symptom subscale scores. The mean number of correct anticipatory responses of UHR− and UHR+ participants at baseline is displayed in Fig. 1. ANOVA of the number of anticipatory responses indicated significant main effects for block (F(1, 245) = 35.84, MSE = 1.11, p < .001, η2p = .128) and exposure (F(1.85, 453.86) = 39.21, MSE = 2.84 (Greenhouse–Geisser correction for deviation from sphericity), p < .001, η2p = .138) as well as a marginally significant main effect of UHR status (F(1, 245) = 3.84, MSE = 5.70, p = .051, η2p = .015). Significant interactions were seen for exposure × group (F(2, 490) = 4.31, MSE = 2.63, p = .014, η2p = .017), block × exposure (F(2, 490) = 18.28, MSE = .578, p < .001, η2p = .069) and block × exposure × group (F(2, 490) = 3.34, MSE = 0.58, p = .036, η2p = .013). The interaction of block × group was not significant. Simple effects analysis indicated a difference between the UHR− and UHR+ groups in the number of anticipatory responses to NPE stimuli that approached significance in block 1 (F(1, 245) = 3.05, MSE = 2.61, p = .082, 95% confidence interval of difference −.05 to .77) and reached significance in block 2 (F(1, 245) = 8.28, MSE = 5.03, p = .004, 95% confidence interval of difference .26 to 1.40). Simple effects also indicated that the effect of exposure was significant in both UHR− (F(2, 243) = 27.08, p < .001, η2p = .182) and UHR+ (F(2, 243) = 22.26, p < .001, η2p = .155) groups. Pairwise comparisons indicated that the number of anticipatory responses for the UHR− group was larger for NPE trials than PE trials in both block 1 (p = .016) and block 2 (p = .021) but that differences were not significant in the UHR+ group. To summarize, this ANOVA of anticipatory responses at baseline suggests that UHR− participants exhibited LI in both blocks while UHR+ did not exhibit LI in either block.
Table 1.
Socio-demographic and clinical description of all subjects tested at baseline.
| Ultra High Risk |
|||
|---|---|---|---|
| Negative |
Positive |
P value | |
| (N = 105) | (N = 142) | ||
| Age in years, mean (SD) | 21.93 (3.76) | 21.39 (3.59) | 0.130 |
| Gender, n (%) | 0.845 | ||
| Male | 69 (65.7) | 95 (66.9) | |
| Female | 36 (34.3) | 47 (33.1) | |
| Ethnicity, n (%) | 0.969 | ||
| Chinese | 77 (73.3) | 103 (72.5) | |
| Malay | 16 (15.2) | 20 (14.1) | |
| Indian | 9 (8.6) | 14 (9.9) | |
| Others | 3 (2.9) | 5 (3.5) | |
| Smoking, n (%) | |||
| Yes | 32 (30.5) | 40 (28.2) | 0.693 |
| No | 73 (69.5) | 102 (71.8) | |
| CAARMS Pos, mean (SD) | 3.24 (5.12) | 23.68 (15.10) | <.001 |
| PANSS Pos, mean (SD) | 10.73 (2.71) | ||
| PANSS Neg, mean (SD) | 12.09 (4.22) | ||
| PANSS Gen, mean (SD) | 25.18 (6.26) | ||
| PANSS Total, mean (SD) | 48.00 (10.92) | ||
| CDSS, mean (SD) | 5.56 (4.71) | ||
| BAI, mean (SD) | 20.01 (12.93) | ||
| GAF, mean (SD) | 57.77 (10.81) | ||
Fig. 1.
Anticipatory Responses of UHR− and UHR+ Subjects to Pre-exposed and Non Pre-exposed Targets at Baseline. PE = Pre-exposed Trials, NPE = Non Pre-exposed Trials.
3.2. Reaction time
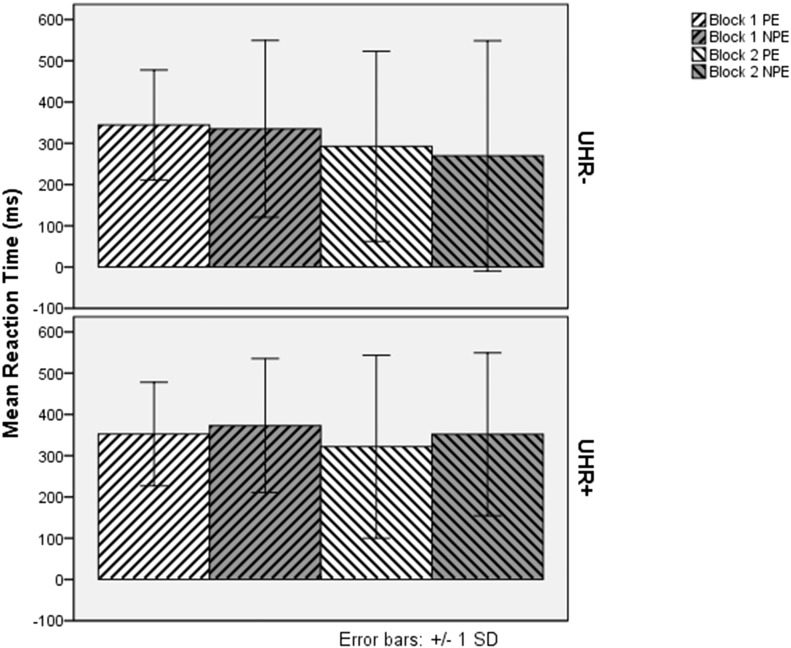
The mean reaction time of participants at baseline is presented in Fig. 2. ANOVA of mean reaction times at baseline revealed significant main effects for block (F(1, 244) = 10.13, MSE = 13,695.79, p = .002, η2p = .040) and exposure (F(1.92, 467.19) = 50.63, MSE = 34,264.54 (Greenhouse–Geisser correction for deviation from sphericity), p < .001, η2p = .172). The main effect for UHR status was marginally significant (F(1, 244) = 3.59, MSE = 71,002.86, p = .059, η2p = .015). A significant interaction was seen for block by exposure (F(2, 488) = 22.67, MSE = 8404.49, p < .001, η2p = .085). Marginally significant interactions were seen for block by group (F(1, 244) = 3.54, MSE = 13,695.79, p = .061, η2p = .014) and importantly, exposure by group (F(2, 488) = 2.82, MSE = 34,264.54, p = .061, η2p = .011), suggesting that the effect of exposure type differed between UHR+ and UHR− groups. The three-way interaction was not significant. Simple effects analysis indicated that the two UHR groups differed in their reaction time to non pre-exposed trials (F(1, 244) = 5.22, MSE = 38,995.70, p = .023, 95% confidence interval of difference 8.00 to 108.28), but not pre-exposed or random trials. Simple effects also indicated that the effect of exposure was significant in both UHR− (F(2, 243) = 27.08, p < .001, η2p = .182) and UHR+ (F(2, 243) = 22.26, p < .001, η2p = .155) groups. Pairwise comparisons indicated that both UHR− and UHR+ groups differed in their reaction times between random and preexposed trials, and random and non pre-exposed trials (all p < .001, Bonferroni-corrected for multiple comparisons), but not in their reaction time to pre-exposed and non pre-exposed trials. Thus, the reaction time data at baseline suggest that neither group is exhibiting latent inhibition, but that UHR+ individuals exhibit a selective impairment on NPE trials.
Fig. 2.
Reaction Time of UHR− and UHR+ Subjects to Pre-exposed and Non Pre-exposed Targets at Baseline. PE = Pre-exposed Trials, NPE = Non Pre-exposed Trials.
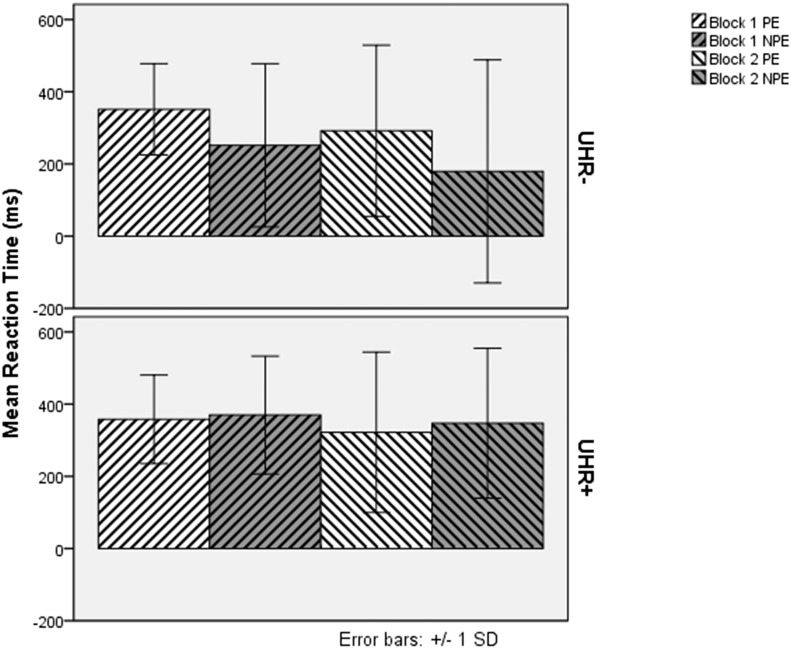
Due to difficulties with test development, the LI test was not ready at the start of the LYRIKS study. Therefore many subjects did not receive the LI test at their baseline visit. To incorporate the maximal data, we conducted a secondary analysis that included the first LI assessment from every participant, regardless of the time point at which it occurred. Demographic data are reported in Table 2. The groups differed in age and smoking status. Because smoking has been shown to reduce the latent inhibition effect (Della Casa et al., 1999, Evans et al., 2007), we included smoking status in the analyses and covaried for age. The results of anticipatory responses at first assessment did not differ substantially from those at baseline and thus are not presented here due to space constraints. The mean reaction times for UHR− and UHR+ subjects at their first assessment are displayed in Fig. 3. Key findings from the ANOVA of reaction time data included a significant main effect for UHR status (F(1, 534) = 9.15, MSE = 88,354.59, p = .003, η2p = .017) and significant interactions for block × UHR status (F(1, 534 ) = 6.33, MSE = 14,350.93, p = .012, η2p = .012), and importantly, exposure by UHR status (F(1.72, 919.59) = 20.93, MSE = 57,774.63, p < .001, η2p = .038) (Greenhouse–Geisser correction for deviation from sphericity). Although the main effect of smoking status was not significant (F(1, 534) = 2.22, MSE = 88,354.59, p = .137, η2p = .004), the interaction between smoking status and block was significant (F(1, 534) = 6.33, MSE = 14,350.93, p = .012, η2p = .012), and the interaction between smoking status and exposure (F(1.722, 919.59) = 3.23, MSE = 57,774.63, p = .073, η2p = .006), and the three way interaction between smoking status, exposure and UHR status (F(1.722, 919.59) = 2.97, MSE = 57,774.63, p = .085, η2p = .006) were marginally significant. Simple effects analysis indicated that the non-smokers in the two UHR groups differed in their reaction time to non pre-exposed trials in block 1 (F(1, 534) = 35.26, MSE = 43,602.84, p < .001, η2p = .062) and in block 2 (F(1, 534) = 32.32, MSE = 79,132.70, p < .001, η2p = .057), but not pre-exposed or random trials in either block. Smokers in the two UHR groups did not differ on their reaction time on any trial types in either block. Pairwise comparisons in the non-smokers indicated that while all three differences in reaction times were different in the UHR− group (all p < .001, Bonferroni-corrected for multiple comparisons), the UHR+ group differed in their reaction times between random and preexposed trials (p < .001), but not random and non pre-exposed trials (p = .083) or pre-exposed and non pre-exposed trials (p = .711) (all Bonferroni-corrected for multiple comparisons). This result indicates that the LI effect was evident in the UHR− subjects, but not the UHR+ subjects. The LI effect was not apparent in smokers as the difference in reaction time between the pre-exposed and non pre-exposed trials was not significant in either the UHR− (Block 1 p = .077, Block 2 p = .210) or UHR+ (Block 1 p = 1.000, Block 2 p = 1.000) groups. Taken together, these data suggest that in the larger sample that included everyone's first assessment, the LI effect was evident in the reaction time data of UHR− but not UHR+ subjects (analogous to the findings in anticipatory responses at baseline), and that these results were not due to differences in age or smoking status between the groups.
Table 2.
Socio-demographic description of all subjects with at least one LI assessment.
| Ultra High Risk |
|||
|---|---|---|---|
| Negative |
Positive |
P value | |
| (N = 381) | (N = 159) | ||
| Age in years, mean (SD) | 22.96 (3.33) | 21.42 (3.55) | <.005 |
| Gender, n (%) | 0.104 | ||
| Male | 228 (59.8) | 107 (67.3) | |
| Female | 153 (40.2) | 52 (32.7) | |
| Ethnicity, n (%) | 0.413 | ||
| Chinese | 301 (79.0) | 115 (72.3) | |
| Malay | 45 (11.8) | 24 (15.1) | |
| Indian | 26 (6.8) | 15 (9.4) | |
| Others | 9 (2.4) | 5 (3.1) | |
| Smoking, n (%) | 0.005 | ||
| Yes | 69 (18.2) | 46 (28.9) | |
| No | 311 (81.8) | 113 (71.1) | |
Fig. 3.
Reaction Time of UHR− and UHR+ Subjects to Pre-exposed and Non Pre-exposed Targets at first assessment. This analysis was conducted for each subject's first assessment on the latent inhibition task, regardless of what visit number at which it occurred. PE = Pre-exposed Trials, NPE = Non Pre-exposed Trials.
3.3. Effect of strategy
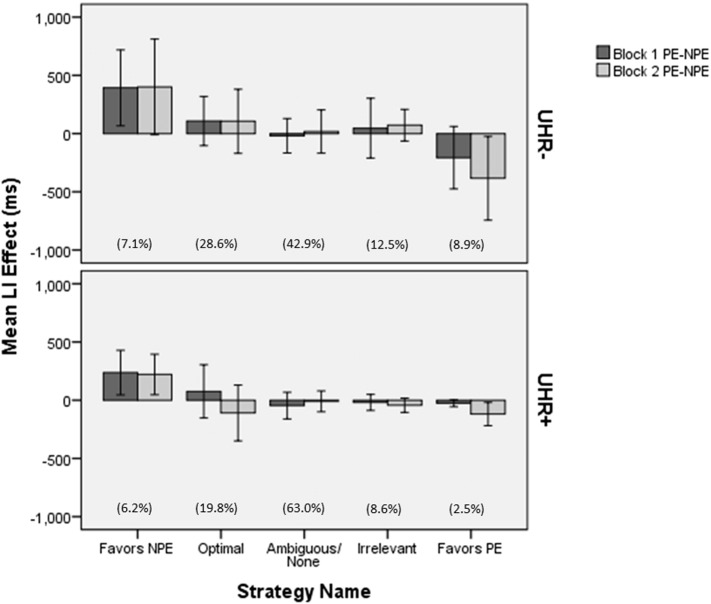
As mentioned previously, we suspected that participants might be employing different strategies to perform the task. In fact we found this to be the case, and as evident in Fig. 4, the strategy adopted influenced the LI effect observed. We thus completed an analysis of the baseline assessments for “optimal responders” alone. The groups did not differ on any of the demographic variables (Table 3). ANOVA results were largely in agreement with those from all subjects' first assessment and effect sizes were larger in this sample of individuals who reported adopting the optimal strategy. Significant main effects were found for block (F(1, 30) = 30.62, MSE = 20,877.23, p < .001, η2p = .286) and exposure (F(2, 60) =44.21, MSE = 51,836.29, p < .001, η2p = .596). The main effect for UHR was not significant when limiting the analysis to the optimal responders' baseline assessments. A significant interaction was seen for block by exposure (F(2, 60) = 24.30, d.f. = 2, MSE = 12,090.82, p < .001, η2p = .447). Importantly, both the two-way interactions of exposure by UHR (F(2, 60) = 3.00, MSE = not reported, p = .057, η2p = .091) and the three-way interaction of block by exposure by UHR (F(2, 60) = 2.92, MSE = not reported, p = .061, η2p = .089) were marginally significant, despite the small sample size. Pairwise comparisons indicated that while all three differences in reaction times were at least marginally significantly different in the UHR− group (random–NPE, p < .001, random–PE, p = .002, PE–NPE, p = .059, Bonferroni corrected for multiple comparisons), the UHR+ group differed in their reaction times between random and preexposed trials, and random and non pre-exposed trials (all p < .001, Bonferroni corrected for multiple comparisons), but not in their reaction times between pre-exposed and non pre-exposed trials (p = .738, Bonferroni corrected for multiple comparisons). This result suggests that the LI effect was evident in the UHR− subjects, but not the UHR+ subjects. An independent t-test of the LI score indicated that the latent inhibition effect was significantly smaller in UHR+ subjects than UHR− subjects in block 2 (p = .025), but that there was no significant difference between groups in block 1 (p = .686). Simple effects analysis indicated that the two UHR groups differed in their reaction time to pre-exposed trials (F(1, 30) = 4.38, MSE = 47,520.58, p = .045, 95% confidence interval of difference 3.923 to 318.73), but not non pre-exposed or random trials.
Fig. 4.
Latent Inhibition Effect at Baseline by Strategy. LI = Latent inhibition effect (mean pre-exposed reaction time − mean non pre-exposed reaction time). The percentage of subjects within the CAARMS status that reported each strategy is given in parentheses.
Table 3.
Socio-demographic description of subjects reporting an optimal response strategy at baseline.
| Ultra High Risk |
|||
|---|---|---|---|
| Negative |
Positive |
P value | |
| (N = 16) | (N = 15) | ||
| Age in years, mean (SD) | 20.81 (3.85) | 21.73 (3.58) | 0.401 |
| Gender, n (%) | 0.183 | ||
| Male | 8 (50.0) | 4 (26.7) | |
| Female | 8 (50.0) | 11 (73.3) | |
| Ethnicity, n (%) | 0.109 | ||
| Chinese | 9 (56.3) | 14 (93.3) | |
| Malay | 2 (12.5) | 0 (0.0) | |
| Indian | 3 (18.8) | 1 (6.7) | |
| Others | 2 (12.5) | 0 (0.0) | |
| Missing | 0 | 0 | |
| Smoking, n (%) | 0.916 | ||
| Yes | 12 (75.0) | 11 (73.3) | |
| No | 4 (25.0) | 4 (26.7) | |
| Missing | 0 | 0 | |
This effect of strategy on the LI effect impaired our ability to investigate the difference between LI scores of converters vs. non-converters. Overall, 17 UHR+ and 1 UHR− individuals converted to psychosis during the study. However, only 10 UHR+ individuals who converted to psychosis were assessed with the latent inhibition test at baseline and none of them reported employing an optimal strategy. We did not observe differences between the converters and non-converters in our analyses of reaction time or anticipatory responses.
4. Discussion
The results of this study demonstrate that the latent inhibition effect is absent in individuals at high-risk for psychosis. An impairment in the ability to adjust expectations of the next moment of experience based on past regularities, as evidenced by the UHR+ individuals in our study, may render an individual susceptible to developing schizophrenia (Corlett et al., 2007, Kraus et al., 2009). We have termed this the learning-dependent predictive perception (LDPP) hypothesis of schizophrenia.
If the process of predicting experience based on learned regularities is impaired in UHR+ individuals, as suggested by the latent inhibition reduction we observed in this study, they may be poorly equipped to efficiently and accurately interpret percepts. This breakdown may represent the primary cognitive vulnerability that puts an individual at risk for developing schizophrenia.
As deficits in learning-dependent predictive perception are proposed to lie more proximal to the biological causes of schizophrenia than deficits in standard cognitive constructs (Krishnan et al., 2011a), tests that more directly probe LDPP function may be especially sensitive predictors of conversion in individuals at high-risk for schizophrenia. Reduced latent inhibition in UHR+ individuals suggests that this group does exhibit deficits in LDPP and therefore tests that probe LDPP function are promising candidates to improve the prediction of conversion to psychosis. The ultimate test for these assessments will be to see if they can predict the UHR+ individuals who will go on to develop schizophrenia. Unfortunately, high rates of missing data and divergent participant strategies greatly reduced the power of our sample to investigate whether LI performance predicted conversion. However, we are currently investigating that question with the other tests of LDPP function from the cognitive battery.
A limitation of our study is that we did not remove outlier reaction times when calculating subjects' mean reaction time for a given trial type. Although the standard convention for this paradigm is to include all correct trials in determining a mean reaction time (Evans et al., 2007, Schmidt-Hansen et al., 2009), calculating a median reaction time would have reduced the effect of outlier trials. While our UHR− group exhibited the expected LI effect in anticipatory responses at baseline, the LI effect was not evident in their baseline reaction time means. It is possible that outlier reaction times obscured this effect. It is notable that the inclusion of additional subjects by analyzing each subject's first assessment of the LI test (regardless of the study time point at which that occurred) produced the expected reaction time LI effect in UHR− individuals but not in UHR+ individuals, much as was seen with anticipatory responses at baseline.
The current results in UHR+ individuals are largely in line with results from individuals in the acute stages of schizophrenia and individuals with high levels of schizotypy. However, some of our analyses conflict with previous work. The difference in LI effect between UHR+ and UHR− individuals in our full sample was primarily due to a significantly slower reaction time and fewer anticipatory responses in the UHR+ subjects during NPE trials, whereas previous studies had found that the impaired LI effect in patients and high schizotypy individuals was primarily due to decreased reaction time and more anticipatory responses in the PE trials (Baruch et al., 1988a, Gray et al., 1992, Gray et al., 1995, Rascle et al., 2001, Schmidt-Hansen et al., 2009). Two factors potentially confounded results of the current study: test version and participant strategy. Controlling for these factors produced findings in line with those from high schizotypy individuals. When the analysis was limited to the baseline assessments of individuals reporting an optimal strategy at baseline, there was a marginally significant difference in LI effect during block 2 only, which was due to reduced PE reaction time in the UHR+ subjects. This result is consistent with the decreased LI effect Schmidt-Hansen et al. (2009) observed in high schizotypy subjects in the second block, which was due to decreased PE reaction time in this group. While self-report is a sub-optimal means of exploring the effect of strategy on performance, this result suggests that subjects do adopt different strategies, which if not controlled for, can confound group differences.
In conclusion, the lack of LI effect in the high-risk sample indicates that individuals at high-risk for psychosis are impaired in their allocation of attentional resources based on past predictive value of repeated stimuli, a key aspect of healthy LDPP function. That a subject's strategy significantly affected LI suggests that care should be used in interpreting LI results and that improving test instructions to reduce strategic differences or controlling for strategy in statistical analysis may improve the power of the test to uncover differences between groups.
Role of Funding Source
The Singapore Translational and Clinical Research in Psychosis is supported by the National Research Foundation Singapore under the National Medical Research Council Translational and Clinical Research Flagship Programme (Grant No.: NMRC/TCR/003/2008). Dr. Lee is supported by the Singapore Ministry of Health's National Medical Research Council under its Transition Award (Grant No.: NMRC/TA/002/2012).
Contributors
Dr. Keefe and Dr. Chong designed the study and wrote the protocol. Mr. Kraus developed the task, analyzed the data and wrote the first draft of the manuscript. Dr. Chong and Dr. Subramaniam oversaw subject recruitment. Dr. Chong, Dr. Subramaniam, Dr. Lee and Dr. Rapisarda supervised data collection. Dr. Keefe, Dr. Rapisarda, Dr. Lee, Dr. Collinson, Mr. Lam and Mr. Thong assisted with data interpretation. All authors have contributed to and have approved the final manuscript.
Conflict of Interest
Dr. Richard Keefe currently or in the past 3 years has received investigator-initiated research funding support from the Department of Veteran’s Affair, Feinstein Institute for Medical Research, National Institute of Mental Health, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past 3 years has received honoraria, and served as a consultant, or advisory board member for Abbvie, Akebia, Asubio, Avanir, AviNeuro/ChemRar, BiolineRx, Biogen Idec, BiolineRx, Biomarin, Boehringer-Ingelheim, EnVivo/FORUM, GW Pharmaceuticals, Janssen, Johnson & Johnson, Lundbeck, Merck, Minerva Neurosciences, Inc., Mitsubishi, Neuralstem, Neuronix, Novartis, NY State Office of Mental Health, Otsuka, Pfizer, Reviva, Roche, Sanofi/Aventis, Shire, Sunovion, Takeda, Targacept, and the University of Texas South West Medical Center. Dr. Keefe receives royalties from the BACS testing battery, the MATRICS Battery (BACS Symbol Coding) and the Virtual Reality Functional Capacity Assessment Tool (VRFCAT). He is also a shareholder in NeuroCog Trials, Inc. and Sengenix.
Dr. Jimmy Lee has served as a consultant and received an honorarium from Roche in the past 3 years. Dr. Jimmy Lee is supported by the Singapore Ministry of Health’s National Medical Research Council under its Transition Award (Grant No.: NMRC/TA/002/2012).
Mr. Kraus, Dr. Rapisarda, Mr. Lam, Mr. Thong, Dr. Subramaniam, Dr. Collinson and Dr. Chong report no competing interests.
References
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. J. Nerv. Ment. Dis. 1988;176(10):598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Latent inhibition and "psychotic proneness" in normal subjects. Personal. Individ. Differ. 1988;9(4):777–783. ( http://www.sciencedirect.com/science/article/B6V9F-45X039R-B/2/d447ab591d3a5394accf3b834c7160a4) [Google Scholar]
- Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, Yung AR, Anderson VA, McGorry PD. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am. J. Psychiatry. 2005;162(1):71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, Cornblatt B, McGorry PD. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr. Bull. 2006;32(3):538–555. doi: 10.1093/schbul/sbj077. ( http://schizophreniabulletin.oxfordjournals.org/cgi/content/abstract/32/3/538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. ( http://archpsyc.ama-assn.org/cgi/content/abstract/65/1/28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S-A, Campbell A, Chee M, Liu J, Marx C, McGorry P, Subramaniam M, Yung A, Keefe RSE. The Singapore flagship programme in translational and clinical research in psychosis. Early Interv. Psychiatry. 2011;5(4):290–300. doi: 10.1111/j.1751-7893.2011.00304.x. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MRF, Shanks DR, Robbins TW, Bullmore ET, Dickinson A, Fletcher PC. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. 2007;130(9):2387–2400. doi: 10.1093/brain/awm173. ( http://brain.oxfordjournals.org/cgi/content/abstract/130/9/2387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Casa V, Höfer I, Weiner I, Feldon J. Effects of smoking status and schizotypy on latent inhibition. J. Psychopharmacol. (Oxf.) 1999;13(1):45–57. doi: 10.1177/026988119901300106. ( http://jop.sagepub.com/content/13/1/45.full.pdf) [DOI] [PubMed] [Google Scholar]
- Evans LH, Gray NS, Snowden RJ. A new continuous within-participants latent inhibition task: examining associations with schizotypy dimensions, smoking status and gender. Biol. Psychol. 2007;74(3):365–373. doi: 10.1016/j.biopsycho.2006.09.007. ( http://www.sciencedirect.com/science/article/B6T4T-4M81C90-1/2/2415b19347906b89b82adb077783204a) [DOI] [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlins J, Hemsley D, Smith A. The neuropsychology of schizophrenia. Behav. Brain Sci. 1991;14(01):1–20. [Google Scholar]
- Gray N, Hemsley D, Gray J. Abolition of latent inhibition in acute, but not chronic, schizophrenics. Neurol. Psychiatry Brain Res. 1992;1(2):83–89. [Google Scholar]
- Gray N, Pilowsky L, Gray J, Kerwin R. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr. Res. 1995;17(1):95–107. doi: 10.1016/0920-9964(95)00034-j. ( http://ac.els-cdn.com/092099649500034J/1-s2.0-092099649500034J-main.pdf?_tid=cb3a72de-217a-11e3-8530-00000aacb35e&acdnat=1379629883_2e2c13ce3ffe54091d2a247e9bd781f3) [DOI] [PubMed] [Google Scholar]
- Hawkins J, Blakeslee S. Times Books; New York: 2004. On Intelligence. [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. ( http://ajp.psychiatryonline.org/cgi/content/abstract/160/1/13) [DOI] [PubMed] [Google Scholar]
- Keefe RS, Kraus MS. Measuring memory-prediction errors and their consequences in youth at risk for schizophrenia. Ann. Acad. Med. Singap. 2009;38(5):414–416. [PubMed] [Google Scholar]
- Keefe RSE, Kraus MS, Krishnan RR. Failures in learning-dependent predictive perception as the key cognitive vulnerability to psychosis in schizophrenia. Neuropsychopharmacology. 2011;36(1):367–368. doi: 10.1038/npp.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr. Res. 2006;88(1–3):26–35. doi: 10.1016/j.schres.2006.06.041. ( http://www.sciencedirect.com/science/article/B6TC2-4KPP4D0-1/2/b4ed30e4e086da78b3d81f148234826f) [DOI] [PubMed] [Google Scholar]
- Kraus M, Keefe R, Krishnan R. Memory-prediction errors and their consequences in schizophrenia. Neuropsychol. Rev. 2009;19(3):336–352. doi: 10.1007/s11065-009-9106-1. [DOI] [PubMed] [Google Scholar]
- Krishnan RR, Fivaz M, Kraus MS, Keefe RSE. Hierarchical temporal processing deficit model of reality distortion and psychoses. Mol. Psychiatry. 2011;16(2):129–144. doi: 10.1038/mp.2010.63. [DOI] [PubMed] [Google Scholar]
- Krishnan RR, Keefe R, Kraus M. Schizophrenia is a disorder of higher order hierarchical processing. Med. Hypotheses. 2009;72(6):740–744. doi: 10.1016/j.mehy.2008.12.039. ( http://www.sciencedirect.com/science/article/B6WN2-4VP175C-9/2/9fa8156acd179785ef1e610ad77223c5) [DOI] [PubMed] [Google Scholar]
- Krishnan RR, Kraus MS, Keefe RSE. Comprehensive model of how reality distortion and symptoms occur in schizophrenia: could impairment in learning-dependent predictive perception account for the manifestations of schizophrenia? Psychiatry Clin. Neurosci. 2011;65(4):305–317. doi: 10.1111/j.1440-1819.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Rekhi G, Mitter N, Bong YL, Kraus MS, Lam M, Rapisarda A, Lee T-S, Subramaniam M, Chong SA. The longitudinal youth at risk study (LYRIKS)—an Asian UHR perspective. Schizophr. Res. 2013;151(1):279–283. doi: 10.1016/j.schres.2013.09.025. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Killackey E, Yung A. Early intervention in psychosis: concepts, evidence and future directions. World Psychiatry. 2008;7(3):148–156. doi: 10.1002/j.2051-5545.2008.tb00182.x. ( http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2559918/pdf/wpa030148.pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter N, Nah GQR, Bong YL, Lee J, Chong S.A. Longitudinal Youth‐At‐Risk Study (LYRIKS): outreach strategies based on a community‐engaged framework. Early intervention in psychiatry. 2014;8(3):298–303. doi: 10.1111/eip.12049. [DOI] [PubMed] [Google Scholar]
- Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, Thomas P. Clinical features of latent inhibition in schizophrenia. Schizophr. Res. 2001;51(2):149–161. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hansen M, Killcross AS, Honey RC. Latent inhibition, learned irrelevance, and schizotypy: assessing their relationship. Cogn. Neuropsychiatry. 2009;14(1):11–29. doi: 10.1080/13546800802664539. ( http://www.informaworld.com/10.1080/13546800802664539 November 17, 2009) [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust. N. Z. J. Psychiatr. 2005;39(11–12):964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]