Abstract
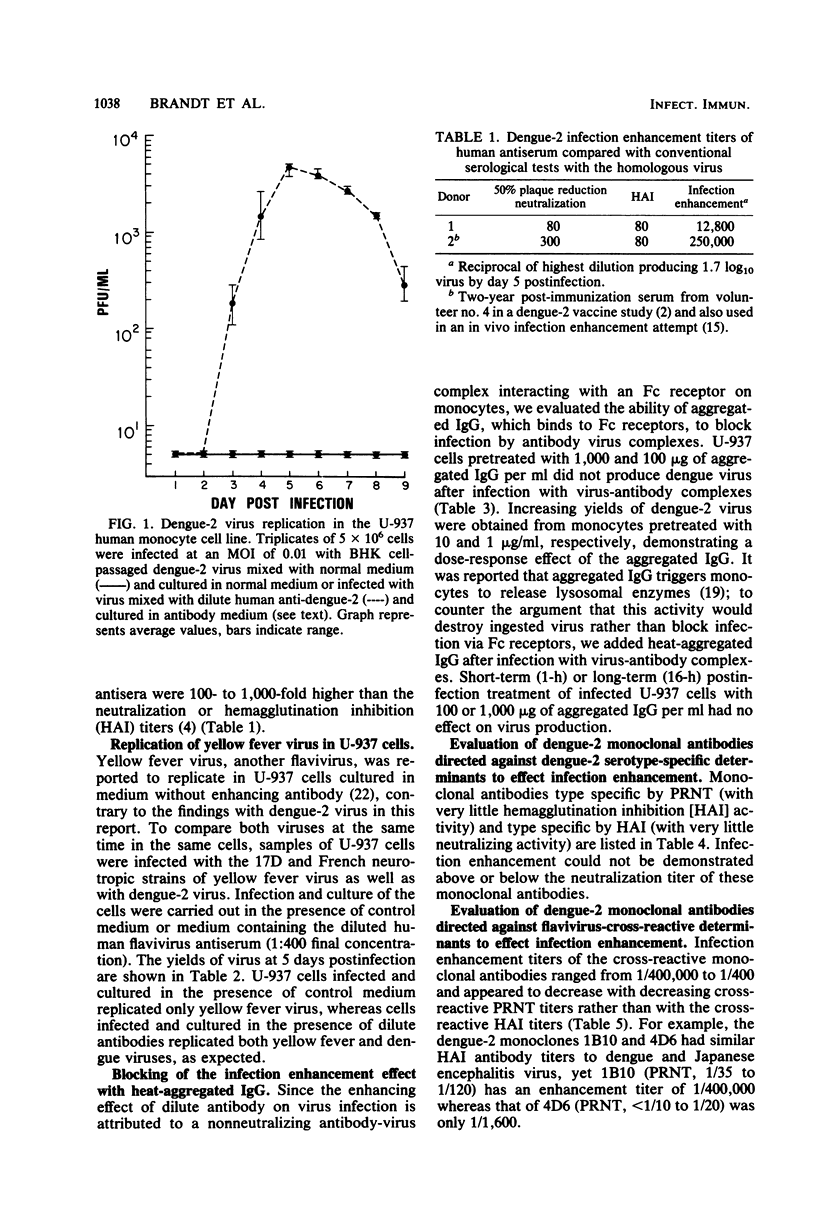
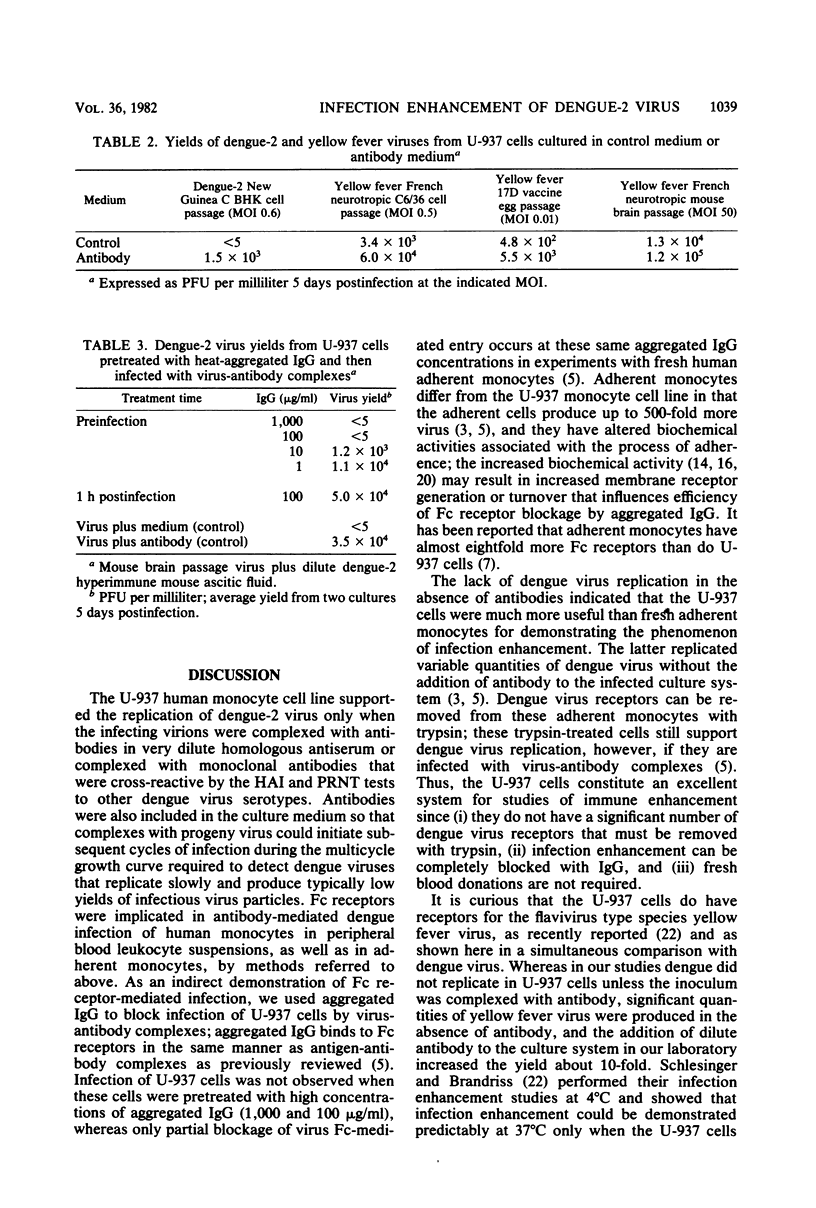
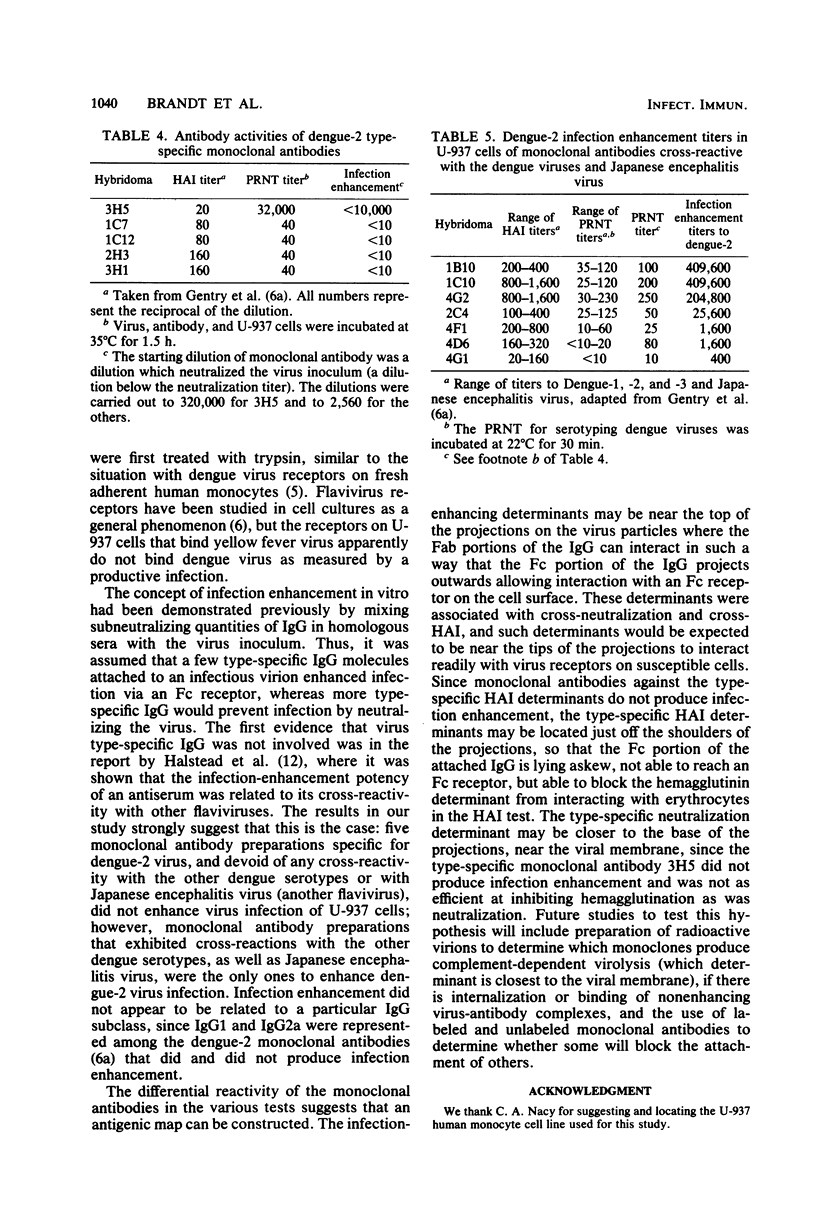
Dengue type 2 virus replication was detected in the U-937 human monocyte cell line when the virus inoculum and the culture medium contained flavivirus antibodies diluted beyond their neutralizing titers. This was in marked contrast to yellow fever virus, which replicated very well in the absence of antibodies; however, 10-fold-higher yields of yellow fever virus could be obtained in the presence of flavivirus antibodies. These infection-enhancing antibodies were obtained from either a dengue type 2 human antiserum or reference hyperimmune obtained from either a dengue type 2 human antiserum or reference hyperimmune mouse ascitic fluid. The infection enhancement phenomenon, previously shown to be due to infection of Fc receptor-bearing cells with virus-antibody complexes, was completely blocked by preincubation of the cells with aggregated gamma globulin. The blocking results suggested an Fc receptor-mediated infection of the U-937 cells as well. A panel of monoclonal antibodies, previously characterized as either virus type specific or flavivirus cross-reactive and with mouse immunoglobulin subclasses G1 and G2a in both categories, were tested for their infection enhancement characteristics. A type-specific neutralizing monoclonal antibody preparation that was diluted beyond its neutralization titer did not cause infection enhancement, nor did low-level neutralizing monoclonal antibodies that were dengue serotype specific by the hemagglutination inhibition test. Only flavivirus cross-reactive monoclonal antibodies caused infection enhancement, irrespective of whether the immunoglobulins were G1 or G2a. These cross-reactive flavivirus determinants may reside at the tips of the glycoprotein projections on the virus particles, enabling the Fc ends of the cross-reactive antibodies attached to these determinants to interact with Fc receptors on susceptible cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft W. H., Top F. H., Jr, Eckels K. H., Anderson J. H., Jr, McCown J. M., Russell P. K. Dengue-2 vaccine: virological, immunological, and clinical responses of six yellow fever-immune recipients. Infect Immun. 1981 Feb;31(2):698–703. doi: 10.1128/iai.31.2.698-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt W. E., McCown J. M., Top F. H., Jr, Bancroft W. H., Russell P. K. Effect of passage history on dengue-2 virus replication in subpopulations of human leukocytes. Infect Immun. 1979 Nov;26(2):534–541. doi: 10.1128/iai.26.2.534-541.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Daughaday C. C., Brandt W. E., McCown J. M., Russell P. K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981 May;32(2):469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch-Niggemeyer W. Polyphosphoinositides as receptor substances for certain groups of arboviruses. Acta Virol. 1971 Mar;15(2):119–125. [PubMed] [Google Scholar]
- Gentry M. K., Henchal E. A., McCown J. M., Brandt W. E., Dalrymple J. M. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):548–555. doi: 10.4269/ajtmh.1982.31.548. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Crabtree G. R., Bodwell J. E., Munck A. MLC-conditioned media stimulate an increase in Fc receptors on human macrophages. J Immunol. 1981 Feb;126(2):666–668. [PubMed] [Google Scholar]
- Halstead S. B., Chow J. S., Marchette N. J. Immunological enhancement of dengue virus replication. Nat New Biol. 1973 May 2;243(122):24–26. [PubMed] [Google Scholar]
- Halstead S. B., Marchette N. J., Sung Chow J. S., Lolekha S. Dengue virus replication enhancement in peripheral blood leukocytes from immune human beings. Proc Soc Exp Biol Med. 1976 Jan;151(1):136–139. doi: 10.3181/00379727-151-39160. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977 Feb 24;265(5596):739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., Porterfield J. S., O'Rourke E. J. Enhancement of dengue virus infection in monocytes by flavivirus antisera. Am J Trop Med Hyg. 1980 Jul;29(4):638–642. doi: 10.4269/ajtmh.1980.29.638. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Kraiselburd E. N., Lavergne J. A., Woodall J. P., Kessler M. J., Meier G., Chiriboga J., Moore C. G., Sather G. E., Pomales A., Maldonado E. Lack of greater seroconversion of rhesus monkeys after subcutaneous inoculation of dengue type 2 live-virus vaccine combined with infection-enhancing antibodies. Infect Immun. 1981 Aug;33(2):389–394. doi: 10.1128/iai.33.2.389-394.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins J. K., Koech D. K., Karnovsky M. L. Oxidation of glucose by mouse peritoneal macrophages: a comparison of suspensions and monolayers. J Cell Physiol. 1980 Nov;105(2):191–196. doi: 10.1002/jcp.1041050202. [DOI] [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M. Cultivation of dengue virus type 2 in baby hamster kidney cells in serum-free medium. Am J Trop Med Hyg. 1979 Sep;28(5):918–920. [PubMed] [Google Scholar]
- Peiris J. S., Gordon S., Unkeless J. C., Porterfield J. S. Monoclonal anti-Fc receptor IgG blocks antibody enhancement of viral replication in macrophages. Nature. 1981 Jan 15;289(5794):189–191. doi: 10.1038/289189a0. [DOI] [PubMed] [Google Scholar]
- Pestel J., Joseph M., Dessaint J. P., Capron A. Macrophage triggering by aggregated immunoglobulins. I. Delayed effect of IgG aggregates or immune complexes. J Immunol. 1981 May;126(5):1887–1891. [PubMed] [Google Scholar]
- Pofit J. F., Strauss P. R. Membrane transport by macrophages in suspension and adherent to glass. J Cell Physiol. 1977 Aug;92(2):249–255. doi: 10.1002/jcp.1040920213. [DOI] [PubMed] [Google Scholar]
- Russell P. K., McCown J. M. Comparison of dengue-2 and dengue-3 virus strains by neutralization tests and identification of a subtype of dengue-3. Am J Trop Med Hyg. 1972 Jan;21(2):97–99. doi: 10.4269/ajtmh.1972.21.97. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Brandriss M. W. Growth of 17D yellow fever virus in a macrophage-like cell line, U937: role of Fc and viral receptors in antibody-mediated infection. J Immunol. 1981 Aug;127(2):659–665. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]


