Abstract
Background
Atherosclerosis is a systemic vascular disorder, involving multiple arterial territories. This project sought to investigate the relationship between lower extremity peripheral artery disease (PAD) and carotid artery stenosis (CAS) in a large self-referred population.
Methods
Data from the ankle brachial index (ABI) and carotid duplex ultrasound were analyzed from 3.67 million US subjects in the Life Line Screening program between 2004 and 2008. PAD was defined by ABI<0.9 and CAS was defined by greater than 50% stenosis. Multivariate logistic regression analysis was used to estimate odds of CAS by PAD status and severity.
Results
Mean age of the population was 63.7± 10.6 years and 64% were women. The prevalence of PAD and CAS was 4.1% and 3.9%, respectively. Subjects with PAD had a higher prevalence of CAS than those without PAD (18.8% vs. 3.3%, P<0.0001). In multivariate analysis, PAD was associated with greater odds of CAS (OR 3.28, 95% CI 3.22–3.34). Both symptomatic (OR 3.66, 95% CI 3.58–3.75) and asymptomatic PAD (OR 2.91, 95% CI 2.84–2.98) was associated with CAS. Increasing severity of PAD was associated with greater odds of CAS (OR 2.32, 3.61, 4.19, 5.14, and 7.59 for ABI categories 0.81–0.90, 0.71–0.80, 0.61–0.70, 0.41–0.60, ≤0.40, respectively).
Conclusion
Presence and severity of PAD was associated with prevalence of CAS in a large community-based cohort, regardless of lower extremity symptoms.
Keywords: Peripheral artery disease, carotid artery stenosis
Atherosclerosis is a systemic disease that affects different arterial beds of the circulation. Risk factors for atherosclerosis are similar across different vascular territories.1, 2 Increasing age, smoking, diabetes and hypertension significantly increase the risk for coronary artery disease, peripheral artery disease and cerebrovascular disease. Concomitant atherosclerotic disease of different vascular territories portends an adverse prognosis.3
The ankle brachial index (ABI) is used in clinical practice to screen for the presence of PAD. Abnormal ABI is associated with cardiovascular morbidity and mortality.4 Although the ABI is used in clinical practice to diagnose PAD, its association with asymtomatic cerebrovascular disease is not fully understood. The Atherosclerosis Risk in Communities (ARIC) Study found that ABI <0.9 was associated with carotid plaque and carotid intima media thickening.5 However, after multivariate adjustment this association was mostly attenuated. Moreover, the association between the severity of PAD as quantified by different levels of the ABI with the prevalence of asymptomatic cerebrovascular disease is uncertain.
The goal of this study was to 1) evaluate the association between PAD and carotid artery stenosis (CAS), a well-defined asymptomatic measure of cerebrovascular disease;6 2) investigate the burden of CAS in symptomatic and asymptomatic PAD subjects; and 3) analyze the correlation between PAD severity and CAS severity in a large self-referred population.
Methods
Study population
The database was provided by Life Line Screening Inc (LLS, Independence, Ohio) to the Society of Vascular Surgery (SVS) for research purposes, and consists primarily of self-referred individuals who self-paid. Screenings were performed in over 20,000 screening sites across the United States between 2003–3008, and all subjects were evaluated by carotid duplex scan, abdominal aortic ultrasound and ankle-brachial indices. All Life Line sites do utilize identical protocols and are subject to a quality control program.7 Demographic information and cardiovascular risk factors, family and medical history, exercise routine and smoking status were obtained for each participant through a questionnaire administered prior to screening. As noted previously,7 the prevalence of different cardiovascular risk factors in this population database are similar to those of the general US population.
Diagnoses of PAD and CAS
The presence and severity of PAD in this study was quantified with the degree of ankle-brachial index (ABI) abnormality. Systolic blood pressure was measured in bilateral brachial arteries and bilateral posterior tibial (PT) arteries (if a PT signal was not audible, then a dorsalis pedis artery signal was measured). Bilateral ABI measurements were obtained by dividing the ankle systolic blood pressure by the highest arm pressure. PAD was defined by an ABI lower than 0.90 in either leg4. PAD was defined as symptomatic if a subject responded yes to “Do you have aching or pain in the legs that is worse with walking or running” or “relieved within a few minutes by rest”.
CAS was defined by carotid artery stenosis ≥ 50% in an internal carotid artery. The correlation between percent stenosis and peak systolic velocity (PSV) varies based on different vascular laboratories and different society guidelines. The normal range in this study was defined as a peak systolic velocity (PSV) < 110 cm/sec, moderate carotid artery disease was defined as a 110 ≤ PSV ≤ 139 cm/sec, and severe disease was defined as a PSV > 140 cm/sec or occlusion on ultrasound imaging or a dampened waveform consistent with excessive plaque.8 CAS was defined as symptomatic if a subject reported prior hospitalization for a stroke, transient ischemic attack or prior revascularization of a carotid artery.
Quality Control
All Life Line sites utilize identical protocols and are subject to the quality control program. Included in this program are random monthly audits where ultrasonography images are graded for each team and individual performance is tracked. Physician audits are performed on a quarterly basis. All results are processed by the results center and outliers are all reviewed. Percentage of abnormal findings is tracked per team to identify groups finding abnormally high or low number of disease cases. A clinical leadership team holds monthly review meetings to evaluate performance of teams and individual members. For imaging modalities, annual competencies are performed and all new employees must have demonstrated initial competencies before being allowed to perform testing without supervision.
Statistical analysis
Baseline characteristics are presented as mean ± standard deviation for continuous variables and proportions for categorical variables. The prevalence of CAS was presented by prevalence and severity of PAD. Multivariable logistic regression models were used to evaluate the association between PAD and CAS. Multivariable models were adjusted for age, sex, race/ethnicity, body mass index, smoking status, hypertension, hyperlipidemia, diabetes, physical activity and family history of vascular disease. Odds ratios (ORs) and 95 percentage confidence intervals (CIs) were reported to examine the strength of the associations. Sensitivity analyses were performed by excluding patients with symptomatic CAS, defined as prior history of stroke, TIA or carotid revascularization.
For all analyses, a 2-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed with SAS (version 9•3, SAS Institute Inc.), and the R package (R Development Core Team).
Results
Baseline characteristics
Among 3,666,938 participants included in the analysis, mean age was 63.7 ± 10.6 years, 64% were female, and 89% were self-identified whites. Baseline characteristics are presented in Table 1. Peripheral artery disease was prevalent in 4.1% of the population, of whom 54% were asymptomatic.
Table 1.
Baseline Characteristics of the Overall Population
| Study participants (N = 3,696,778) |
|
|---|---|
| Age (years ± SD) | 63.7 ± 10.6 |
| Male sex (%) | 35.8 |
| Race or ethnic group (%) | |
| Caucasian | 88.9 |
| African American | 3.1 |
| Hispanic | 2.5 |
| Asian | 2.0 |
| Native American | 2.9 |
| Other | 0.6 |
| Body mass index (kg/m2 ± SD) | 27.7 ± 5.8 |
| Obesity (%) | 27.8 |
| Smoking status, % | |
| Never smoked | 51.0 |
| Former smoker | 24.1 |
| Current smoker | 24.9 |
| Hypertension (%) | 48.2 |
| Hyperlipidemia (%) | 53.0 |
| Diabetes mellitus (%) | 10.8 |
| Sedentary lifestyle (%) | 37.6 |
| Family History of CVD (%) | 25.5 |
SD, standard deviation; CVD, cardiovascular disease
Prevalence of CAS based on PAD
Overall, moderate or severe CAS was present in 3.9% of the population. The prevalence of CAS in subjects with PAD was 18.8% compared with 3.3% in subjects without PAD (P<0.0001; Figure 1). After multivariate regression analysis, subjects with PAD had significantly higher odds of CAS (OR 3.28, 95% CI 3.22–3.34) (Figure 2a). Both symptomatic and asymptomatic PAD had increased odds of prevalent CAS (Figure 2b). The increased CAS prevalence was observed in both asymptomatic and symptomatic PAD (Figure 1 and Supplementary Appendix Table 1). A similar relationship was observed for the endpoints of moderate CAS and severe CAS, respectively. After excluding subjects with symptomatic CAS (prior history of stroke, TIA or prior carotid revascularization), PAD was still associated with increased odds of CAS (data not shown).
Figure 1. Frequencies of CAS severity by the presence of PAD and symptomatic status of subjects with PAD.
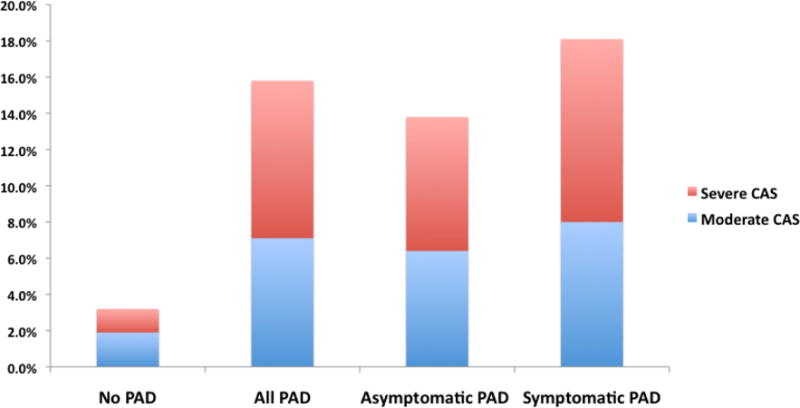
CAS, carotid artery stenosis; PAD, peripheral artery disease
Figure 2. Multivariate logistic regression analyses of moderate to severe CAS by (a) presence of PAD and (b) symptomatic status of subjects with PAD.

CAS, carotid artery stenosis; PAD, peripheral artery disease
Prevalence of CAS based on PAD severity
Severity of PAD (as determined by ABI) was associated with prevalence of CAS, demonstrating a reverse J-curve with a significantly higher proportion of severe CAS in individuals with lower ABI (Figure 3 and Supplementary Appendix Table 2). Moderate or severe CAS was found in 3.8%, 3.2%, 11.1%, 17.9%, 22.4%, 27.5%, and 36.8% among subjects with an ABI category of >1.4, 0.91–1.4, 0.81–0.9, 0.71–0.8, 0.61–0.7, 0.41–0.6, and ≤0.4, respectively (Figure 4). After multivariate adjustment, the odds ratios associated with ABIs of >1.4, 0.91–1.4, 0.81–0.9, 0.71–0.8, 0.61–0.7, 0.41–0.6, and ≤0.4 for the outcome of CAS were 1.0, 1.0 (reference), 2.35, 3.53, 4.13, 5.12, and 7.54, respectively. A similar relationship was observed for the endpoints of moderate CAS and severe CAS, respectively. After excluding subjects with symptomatic CAS (prior history of stroke, TIA or prior carotid revascularization), severity of ABI was still associated with increased odds of CAS (data not shown).
Figure 3. Frequencies of CAS Severity by the degree of PAD severity.

CAS, carotid artery stenosis; PAD, peripheral artery disease; ABI, ankle-brachial index
Figure 4. Multivariate logistic regression analyses of moderate to severe CAS by the degree of PAD severity.

CAS, carotid artery stenosis; ABI, ankle-brachial index
Discussion
This large-scale cross-sectional study of over 3.6 million adults in the United States demonstrates the significant prevalence of moderate to severe CAS in subjects with PAD. There are several important findings in this study. First, the prevalence of CAS in subjects with PAD was as common as 1 in 5 individuals. Next, while the prevalence of CAS is higher in subjects with symptomatic PAD than asymptomatic PAD, there is still a significant proportion (≈ 1 of 7 individuals) with asymptomatic PAD who also have CAS. Finally, increasing severity of PAD as quantified by the ABI is associated with increasing prevalence of CAS.
Abnormal ABI, as an indicator of PAD regardless of symptomatic status, has been associated with a significant increase in the relative risk of cardiovascular morbidity and mortality.9 ABI measurement has incremental value in predicting cardiovascular events when combined with the Framingham Risk Score.10 With regards to cerebrovascular disease, some reports have linked the presence of an abnormal ABI with a doubling of the relative risk of stroke or TIA in the elderly.11 The Honolulu Heart Program further demonstrated that ABI was inversely related to the incidence of stroke in a select group of Japanese-American men, even after adjustment for atherosclerotic risk factors.12
While these data support the association between an abnormal ABI and incident cerebrovascular events, there is less evidence regarding the association between ABI values and pre-clinical cerebrovascular disease. With stenosis of internal carotid arteries as a well-described risk factor for ischemic cerebrovascular events,6 one can postulate that identification of CAS may play a role in early preventive cardiovascular strategies. Resting ABI was investigated in the Atherosclerosis Risk in Communities (ARIC) longitudinal study of middle-aged adults (45 – 64 years), and subjects (both whites and blacks) with an ABI <0.9 had a significantly higher prevalence of pre-clinical carotid plaque, as well as symptomatic cerebrovascular disease.5 However, the association was attenuated after adjustment for cardiovascular risk factors. Limitations of the ARIC study include the measurement of ankle blood pressure in only one leg, combining asymptomatic and symptomatic PAD, and small sample size precluding the ability to look at PAD severity with pre-clinical cerebrovascular disease. The current study provides important data confirming the significant association between any PAD and CAS. Moreover, the current analysis demonstrates a significant association between PAD severity and CAS severity. In addition, the significant association between PAD and CAS was observed in asymptomatic as well as symptomatic subjects.
Non compressible PAD as determined by an ABI >1.4 was associated with a higher prevalence of CAS. However, after multivariable adjustment there was no significant association between this cut-off and CAS. Nonetheless, prior data from our group demonstrated a significant association between ABI >1.4 and prior stroke. Non-compressible ABI may be a better surrogate for symptomatic cerebrovascular disease (e.g. stroke) than asymptomatic disease, such as CAS. Whether higher ABI values would be associated with CAS remains uncertain.
Some of the strengths of the current study are the measurement of bilateral ABI, and the focus on the presence of moderate or severe CAS as detected by Duplex sonography, a screening method for carotid artery disease with high sensitivity and specificity8. Given the large sample size of the current study, all of the associations of CAS with PAD remained statistically significant after multivariate adjustment, regardless of PAD symptomatic status.
What would the diagnosis of asymptomatic cerebrovascular disease add to the management of patients with PAD? Prior studies have shown that patients with PAD are less intensively treated for their atherosclerosis risk factors than patients with a prior diagnosis of coronary artery disease.13, 14 Therefore, the diagnosis of atherosclerosis in a new vascular bed (CAS) in addition to PAD may in fact prompt more intensive treatment. Importantly, the current study is unable to determine whether earlier identification of CAS improves cerebrovascular outcomes. Guidelines from the joint vascular societies in 2011 provided a class IIb recommendation for carotid screening in patients with symptomatic PAD.15 The results from the current study demonstrate the significantly higher prevalence of moderate and severe CAS in subjects with PAD, regardless of PAD symptom status. Among subjects with mild (ABI 0.8–0.9), moderate (ABI 0.6–0.8), or severe (ABI <0.6) PAD, CAS was observed in 10%, 20%, and 29%, respectively.
While the large number of participants and quantitative measurements of both ABI and carotid artery duplex ultrasound represent some of the strengths of this study, the cross-sectional nature represents a significant limitation to inferences of causality and prognostication. The current study reports ABI and carotid Duplex ultrasound measurements at a single point in time, which precludes our ability to comment on trends or evolution of disease. Furthermore, the current study represents a self-selected group of individuals who were willing to pay for the diagnostic tests out of pocket, which creates a selection bias. Nonetheless, as reported previously, the prevalence of different cardiovascular risk factors in this population database were similar to those of the general US population.7 Moreover, the prevalence of PAD and CAS was similar to other representative cohorts demonstrating excellent external validity.16 In the current study, the ABI was measured using the posterior tibial artery; the dorsalis pedis artery was only used only when the posterior tibial artery was inaudible. Traditionally, the highest of the dorsalis pedis or posterior tibial artery pressures is used to form the calculation. This measurement of the ABI would likely underestimate the true prevalence of PAD and would likely bias the results towards the null. The higher proportion of women in the study and an uneven distribution of minorities may limit the generalizability of the findings and reflect this selection bias. Nonetheless, this study contains the largest number of women and men as well as whites and minorities for the investigation of ABI screening and prevalent CAS.
In the current study, the ABI was measured using the posterior tibial artery, and the dorsalis pedis artery was only used when the posterior tibial artery was inaudible. Traditionally, the highest of the dorsalis pedis or posterior tibial artery pressures at the ankle is used for the ABI calculation.4 However, when comparing this traditional method with an alternative method which utilizes the lower of the 2 ankle artery pressures, both methods have similar diagnostic and predictive accuracy for all-cause and cardiovascular mortality, suggesting the utility of the alternate method to identify a clinically-meaningful population with PAD.17 Moreover, we would expect any resulting ABI misclassification to bias toward a null result.
In conclusion, this is a large study of over 3.6 million self-referred individuals that demonstrates a high prevalence of significant CAS in subjects with PAD. The prevalence and severity of CAS was significantly increased in subjects with PAD regardless of their symptomatic status, but was proportional to the severity of PAD as measure by ABI’s. Current guidelines do not recommend screening for CAS in asymptomatic subjects. The high prevalence of moderate and severe CAS in subjects without symptoms and with PAD, in this self-selected large population, may be an argument for CAS screening in this population with a high cardiovascular disease burden. Future clinical trials investigating the effect of CAS screening in subjects with PAD are certainly required.
Supplementary Material
Acknowledgments
This work has utilized computing resources at the High Performance Computing Facility of the Center for Health Informatics and Bioinformatics at New York University Langone Medical Center.
Funding/Support
Dr Berger was partially funded by the National Heart and Lung Blood Institute of the National Institutes of Health (RO1 HL114978), American Heart Association Clinical Research Program (13CRP14410042) and Doris Duke Charitable Foundation (2010055). Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest Disclosures
There are no potential conflicts of interest. The authors gratefully acknowledge the participation and generosity of Life Line Screening (Cleveland, OH), who provided these data free of charge for the purposes of research and with no restrictions on its use for research or resultant publications.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger JS, Hochman J, Lobach I, Adelman MA, Riles TS, Rockman CB. Modifiable risk factor burden and the prevalence of peripheral artery disease in different vascular territories. Journal of vascular surgery. 2013;58:673–681. doi: 10.1016/j.jvs.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: Morbidity and mortality implications. Circulation. 2006;114:688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the american heart association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 5.Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: The atherosclerosis risk in communities (aric) study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/s0021-9150(97)06089-9. [DOI] [PubMed] [Google Scholar]
- 6.Mackey AE, Abrahamowicz M, Langlois Y, Battista R, Simard D, Bourque F, et al. Outcome of asymptomatic patients with carotid disease. Asymptomatic cervical bruit study group. Neurology. 1997;48:896–903. doi: 10.1212/wnl.48.4.896. [DOI] [PubMed] [Google Scholar]
- 7.Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. Association between advanced age and vascular disease in different arterial territories: A population database of over 3.6 million subjects. Journal of the American College of Cardiology. 2013;61:1736–1743. doi: 10.1016/j.jacc.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: Gray-scale and doppler us diagnosis–society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 9.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–2061. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 10.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA : the journal of the American Medical Association. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: The framingham study. Archives of internal medicine. 2003;163:1939–1942. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 12.Abbott RD, Rodriguez BL, Petrovitch H, Yano K, Schatz IJ, Popper JS, et al. Ankle-brachial blood pressure in elderly men and the risk of stroke: The honolulu heart program. Journal of clinical epidemiology. 2001;54:973–978. doi: 10.1016/s0895-4356(01)00373-0. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. Journal of general internal medicine. 1997;12:209–215. doi: 10.1046/j.1525-1497.1997.012004209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subherwal S, Patel MR, Kober L, Peterson ED, Jones WS, Gislason GH, et al. Missed opportunities: Despite improvement in use of cardioprotective medications among patients with lower-extremity peripheral artery disease, underuse remains. Circulation. 2012;126:1345–1354. doi: 10.1161/CIRCULATIONAHA.112.108787. [DOI] [PubMed] [Google Scholar]
- 15.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 asa/accf/aha/aann/aans/acr/asnr/cns/saip/scai/sir/snis/svm/svs guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary a report of the american college of cardiology foundation/american heart association task force on practice guidelines, and the american stroke association, american association of neuroscience nurses, american association of neurological surgeons, american college of radiology, american society of neuroradiology, congress of neurological surgeons, society of atherosclerosis imaging and prevention, society for cardiovascular angiography and interventions, society of interventional radiology, society of neurointerventional surgery, society for vascular medicine, and society for vascular surgery developed in collaboration with the american academy of neurology and society of cardiovascular computed tomography. Journal of the American College of Cardiology. 2011;57:1002–1044. doi: 10.1016/j.jacc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Stein RA, Rockman CB, Guo Y, Adelman MA, Riles T, Hiatt WR, et al. Association between physical activity and peripheral artery disease and carotid artery stenosis in a self-referred population of 3 million adults. ATVB. 2014 doi: 10.1161/ATVBAHA.114.304161. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle-brachial index method identifies additional at-risk individuals. Journal of the American College of Cardiology. 2013;62:553–559. doi: 10.1016/j.jacc.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


