Abstract
Marginating-hepatic (MH) leukocytes (leukocytes adhering to the sinusoids of the liver), were shown to exhibit unique composition and characteristics compared to leukocytes of other immune compartments. Specifically, evidence suggests a distinct pro- and anti-inflammatory profile of the MH-leukocyte population and higher cytotoxicity of liver-specific NK cells (namely, pit cells) compared to circulating or splenic immunocytes in both mice and rats. The method presented herein enables selective harvesting of MH leukocytes by forced perfusion of the liver in mice and rats. In contrast to other methods used to extract liver-leukocytes, including tissue grinding and biological degradation, this method exclusively yields leukocytes from the liver sinusoids, uncontaminated by cells from other liver compartments. In addition, the perfusion technique better preserves the integrity and the physiological milieu of MH leukocytes, sparing known physiological responses to tissue processing. As many circulating malignant cells and infected cells are detained while passing through the liver sinusoids, physically interacting with endothelial cells and resident leukocytes, the unique MH leukocyte population is strategically located to interact, identify, and react towards aberrant circulating cells. Thus, selective harvesting of MH-leukocytes and their study under various conditions may advance our understanding of the biological and clinical significance of MH leukocytes, specifically with respect to circulating aberrant cells and liver-related diseases and cancer metastases.
Keywords: Immunology, Issue 113, Liver, leukocytes, pit cells, mouse, rat, marginating-hepatic, natural killer, liver-specific NK cells, sinusoids, immunity, cancer metastases, perfusion
Introduction
The liver sinusoids contain numerous leukocyte subtypes of various immune activities critical to the organism. For example, marginating hepatic (MH) natural killer (NK) cells, also known as pit cells, are characterized morphologically as large granular lymphocytes (LGLs) and functionally as leukocytes with spontaneous cytotoxic capacity, enabling hepatic-resistance against the establishment of blood-borne tumor metastases. The goal of the method presented herein is to enable selective harvesting of MH leukocytes, in order to study this important and unique cell population (and immune compartment), and to elucidate the impact of various manipulations (e.g. immune activation) on these specific cells.
Despite the failure of immunity to eliminate a developing primary tumor, evidence in cancer patients and animal models indicate that the immune system can control circulating tumor cells, micrometastases, and residual disease through cell-mediated immunity (CMI). However, there is an apparent inconsistency between these in vivo capabilities and in vitro studies in humans and in animals, which demonstrate that most autologous tumor cells are resistant to cytotoxicity by circulating or splenic leukocytes1,2. This inconsistency may be ascribed, at least in part, to the in vivo existence of a distinct leukocyte subpopulation, namely the marginating-hepatic (sinusoids) leukocytes and their subpopulation of activated NK cells, namely pit cells3. Indeed, unpublished data from our laboratory indicated that in F344 rats syngeneic tumor cells (MADB106), which were found resistant to circulating and splenic leukocytes, were lysed by MH-NK cells4. Thus, tumor cells that are allegedly “NK-resistant” to circulating leukocytes may be controlled by MH-NK cells. Noteworthy, in mice the enhanced activity of MH-NK is evident only following in vivo immune stimulation (e.g. through the use of Poly I:C or CpG-C)5.
Liver-specific NK cells (MH-NK) are situated inside the sinusoidal lumen, adhering to the endothelial cells and Kupffer cells. MH-NK cells are exclusively characterized by spherical dense granules and rod-cored vesicles6, which contain acid phosphatase as lysosomal enzymes, and perforin and granzymes as bioactive substances7,8. Compared with circulating NK cells, MH-NK cells exhibit a higher number and size of granules and vesicles9–11. Under inflammatory conditions, MH-NK cells were shown to exhibit higher expression of LFA-112, as compared to circulating NK cells. This enhanced expression might constitute a mechanism by which MH-NK cells are more cytotoxic against certain tumor cells than circulating NK cells13,14. nterestingly, following in vitro incubation with interleukin (IL)-2, MH-NK cells become enlarged, and their number and size of granules increase, all of which are consistent with a profile of lymphokine-activated killer (LAK) cells15.
Active MH leukocytes cannot be exclusively obtained through the standard liver leukocyte harvesting methods, which are based on grinding and biological degradation of the tissue. Our perfusion approach described herein has two major advantages compared to the standard approaches. First, the perfusion approach selectively harvests MH leukocytes, preventing contamination by other leukocytes from other liver compartments. Second, the perfusion technique better preserves the integrity, the activity, and the physiological milieu of MH leukocytes, unlike the tissue processing approaches that damage cells or alter their morphology, and due to tissue damage, induce the release of various factors that markedly modulate immune activity.
The liver is a major target organ for cancer metastasis and for various infections16. As MH cells exhibit unique characteristics it is important to study this specific population under various conditions with respect to these pathologies. For example, it is worthy to note that systemic immune activation by various BRMs (e.g. poly I:C or CpG-C) have been shown to activate MH-leukocytes more than circulating leukocytes5.
Protocol
Ethics Statement: Procedures involving animal subjects have been approved by the Institutional Animal Care and Use Committee (IACUC) at Tel-Aviv University.
1. Rats Protocol
- Preparations
- Prepare heparinized PBS (30 units/ml) solution to be used at room temperature (RT) by adding 30 units of preservative free heparin per ml of Phosphate buffered saline (PBS) 1× solution. Calculate 35 ml per animal.
- Pass heparinized PBS through the peristaltic pump lines and the butterfly needles to eliminate air bubbles.
- Sterilize surgical tools – 2 pairs of scissors, blunted-edged forceps, 2 hemostats, and tooth-tissue forceps (autoclave at 121 °C for at least 30 min on gravity (dry) setting).
- Perfusion of the Liver and Collection of MH-leukocytes
- Euthanize the animal by an overdose of 8% isoflurane. As a precaution, place a 50 ml tube containing an isoflurane-soaked pad around the rat head until opening its chest cavity.
- Open the peritoneal and chest cavities to expose the liver and the cardiopulmonary complex immediately upon cessation of respiration. Avoid bleeding through this process.
- Specifically, start the incision at the lower abdominal midline point, without damaging internal organs, and progress to both sides diagonally toward the ribs, cutting through them to upper chest-cavity levels.
- Within approximately a min, collect as much blood as possible (~6 ml from a 250 g animal) from the right ventricle into a syringe.
- Clamp the caudal vena cava with a hemostat as close as possible to the heart, to enable the collection of the perfusate.
- Pull out the intestines and place outside the animal to the experimenter’s right, to expose the portal vein.
- Insert a 25 G IV catheter, connected to a peristaltic pump, into the portal vein, as caudal as possible, but rostral to the splenic vein.
- Insert a 25 G butterfly needle, connected to a 5 ml syringe, into the inferior vena cava, caudal to the hemostat.
- Turn on the peristaltic pump at a speed of approximately 3 ml/min and gently collect the first ml of perfusate that are contaminated with blood into the syringe. Continue until the perfusate color is turns pale red (approximately 3–5 ml). Notice that the color of the liver is changing towards light brown.
- Without stopping the peristaltic pump, replace the 5 ml harvesting syringe with a 20 ml harvesting syringe and collect at least 20 ml of liver perfusate employing a higher speed of perfusion (up to 4 ml/min). Continuously monitor the perfusate flow into the collecting syringe, and avoid vacuum that is too strong (which may clog the needle through collapsing the vena cava walls).
- Terminate perfusion when the color of the liver turns to light brown.
- Leukocytes Extraction
- Centrifuge the perfusate for 10 min at 400 × g, 24 °C.
- Aspirate the supernatant
- Add 10 ml PBS, centrifuge at 400 × g for 10 min, and aspirate the supernatant. Based on study goals, use preparation as is, or conduct additional purifications (e.g., density-gradient separation).
- Specifically, layer 4 ml of perfusate over 4 ml of density-gradient separation mass in a 50 cc polypropylene centrifuge tube. Spin the tubes at 754 × g, RT, for 30 min with the break off.
- Obtain the mononuclear layers at the density-gradient separation-supernatant interface by manual pipetting, wash in PBS, and insert into a 5 ml tube.
2. Mouse Protocol
- Preparations
- Prepare heparinized PBS (30 units/ml) solution to be used at room temperature (RT) by adding 30 units of preservative free heparin per ml of Phosphate buffered saline (PBS) 1× solution. Calculate 20 ml per animal.
- Pass heparinized PBS through the peristaltic pump lines and the butterfly needles to eliminate air bubbles.
- Sterilize surgical tools – 2 pairs of scissors, blunted-edged forceps, 2 hemostats, and tooth-tissue forceps (autoclave at 121 °C for at least 30 min on gravity (dry) setting).
- Perfusion of the Liver and Collection of MH-Leukocytes
- Euthanize the animal by an overdose of 8% isoflurane. As a precaution, place a 15 ml tube containing an isoflurane-soaked pad around the mouse head until opening its chest cavity.
- Fix the limbs of the mouse to a bed.
- Open the peritoneal and chest cavities to expose the liver and the cardiopulmonary complex immediately upon cessation of respiration, keeping the lower aspect of the diaphragm intact (to create a draining chest cavity pool). Avoid bleeding through this process.
- Specifically, start the incision at the lower abdominal midline point, without damaging internal organs, and progress to both sides diagonally toward the ribs, cutting through them to upper chest-cavity levels.
- Pullout the intestines and place outside the animal to the experimenter’s right, to expose the portal vein.
- Insert a 30 g needle, connected to a peristaltic pump into the portal vein rostral to the splenic vein.
- Cut the inferior vena cava above the diaphragm to allow drainage and collection of the perfusate from the chest cavity.
- Turn on the peristaltic pump at a speed of approximately 3 ml/min and gently collect the first 3 ml of perfusate that are contaminated with blood from the chest cavity pool.
- Discard this perfusate. Repeat such a washing again if needed, by using saline, and only then start collecting liver perfusate.
- Re-initiate the perfusion at a speed of up to 4 ml/min, and collect 10 ml of perfusate from the chest cavity using a butterfly connected to a syringe.
- Terminate perfusion when the color of the liver turns to light brown.
- Leukocytes Extractio
- Centrifuge the perfusate for 10 min at 400 × g.
- Aspirate the supernatant.
- Add 10 ml PBS, centrifuge at 400 × g for 10 min, and aspirate the supernatant Based on study goals, use preparation as is, or conduct additional purifications (e.g., density-gradient separation).
- Specifically, layer every 4 ml of perfusate over 4 ml of density-gradient separation mass in a 50 cc polypropylene centrifuge tube. Spin the tubes at 754 × g, RT, for 30 min with the break off.
- Obtain the mononuclear layers at the density-gradient separation-supernatant interface by manual pipetting, wash in PBS, and insert into a 5 ml tube.
Representative Results
In F344 rats we compared the cytotoxicity of MH-NK cells (collected from the liver sinusoids by forced liver perfusion) to cytotoxicity of the entire liver cell population following mechanical grinding of the liver tissue, and to the cytotoxicity of circulating leukocytes. All cell preparations were washed at least 3 times, as routine in immunological assays, and as target cell lines we used the allogenic YAC-1 or the syngeneic MADB106 target cell lines. As indicated in Figures 1–4, whereas MH-NK cells exhibited profound cytotoxicity against the MADB106 and the YAC-1 target cell lines (~80% and up to 100% respectively), both the entire liver cell population (Figure 1) and circulating leukocytes (Figures 2 and 3) displayed profoundly lower levels of cytotoxicity, and almost no killing against the syngeneic MADB106 target cell line. The same relative lower levels of cytotoxicity were evident when mechanically processed livers also underwent washing and density gradient separation (Figure 4), levels that are typical in the literature. Thus, MH-NK cells exhibit uniquely high levels of cytotoxicity in F344 rats. FACS analysis was conducted and revealed profound differences in the composition of following cell subpopulations: monocytes (CD161dim), NK cells (CD161 bright), T cells (CD3), B cells (CD45), and NKT (CD161 and CD3) cells. Major differences in maturation profile (CD11b/CD27) were also evident between the different compartments (Figure 5).
Figure 1. A Comparison of the Cytotoxicity of MH-NK Cells, to Cytotoxicity of the Entire Liver Cell Population (YAC-1 Target Cell Line).
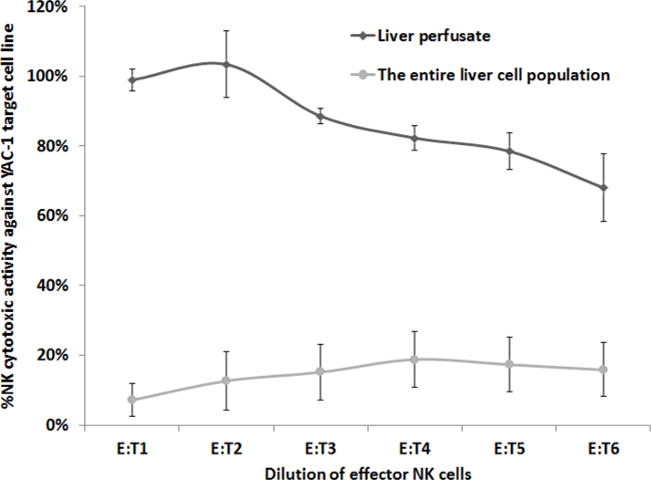
MH-NK cells (harvested from the MH-compartment through the herein described approach) exhibited profound cytotoxic activity against the YAC-1 target cell line, while the entire liver cell population, harvested through mechanical processing of the liver, displayed minimal levels (p <0.05). X axis represents effector-to-target cells ratio. Data is presented as mean ± SEM.
Figure 4. A Comparison of the Cytotoxicity of MH-NK Cells, to Cytotoxicity of the Entire Liver Lymphocytes which was Washed and Enriched Through Density-Gradient Separation (YAC-1 target cell line).
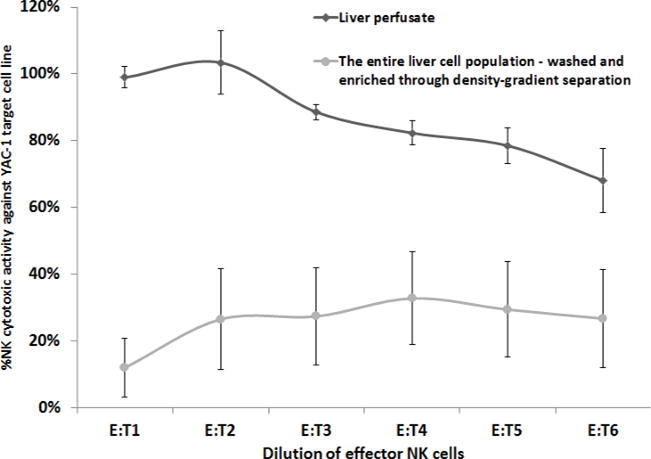
MH-NK cells (harvested from the MH-compartment through the herein described approach) exhibited profound cytotoxicity against the YAC-1 target cell line, while the entire liver lymphocytes (following mechanical processing, which was washed and enriched through density-gradient separation), displayed significantly lower levels of cytotoxicity (up to 30%) (p <0.05) that are typical in the literature. X axis represents effector-to-target cells ratio. Data is presented as mean ± SEM.
Figure 2. A Comparison of the Cytotoxicity of MH-NK Cells, to Cytotoxicity of Circulating Leukocytes (YAC-1 Target Cell Line).
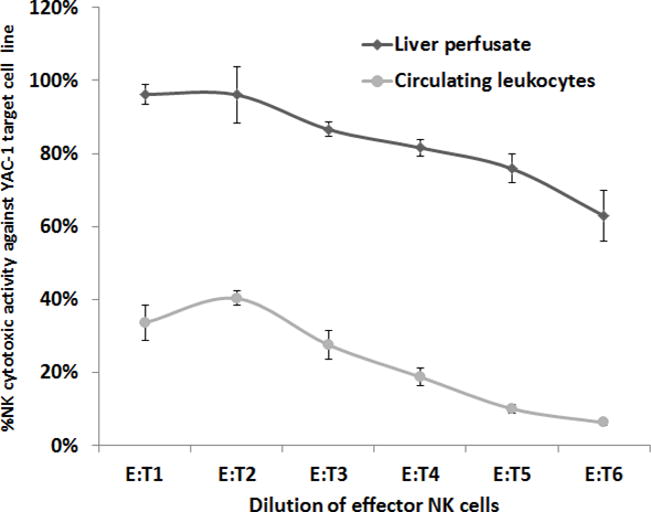
MH-NK cells (harvested from the MH-compartment through the herein described approach) exhibited profound cytotoxicity against the YAC-1 target cell line, while circulating leukocytes displayed minimal levels of cytotoxicity (p <0.05). X axis represents effector-to-target cells ratio. Data is presented as mean ± SEM.
Figure 3. A Comparison of the Cytotoxicity of MH-NK cells, to Cytotoxicity of Circulating Leukocytes (MADB106 Target Cell Line).
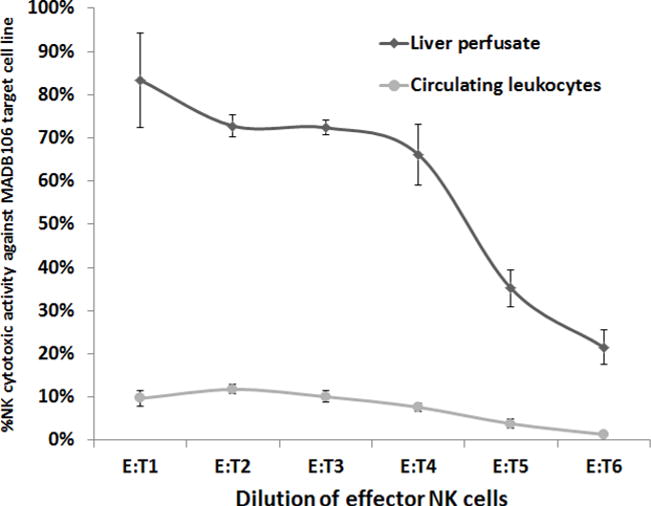
MH-NK cells (harvested from the MH-compartment through the herein described approach) exhibited profound cytotoxicity against the syngeneic MADB106 target cell line, while circulating leukocytes displayed minimal levels of cytotoxicity (p <0.05). X axis represents effector-to-target cells ratio. Data is presented as mean ± SEM.
Figure 5. A Comparison of Mononuclear Cell Subsets and NK Cell Subsets From the Circulating Mononuclear, the MH-Mononuclear, and the Entire Liver Mononuclear Cells.
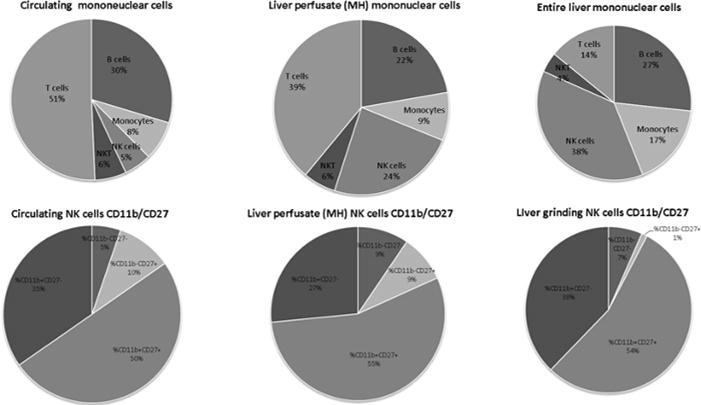
FACS analysis shows clear differences in the composition of following cell subpopulations: monocytes (CD161dim), NK cells (CD161 bright), T cells (CD3), B cells (CD45), and NKT (CD161 and CD3) cells as well as differences in the maturation profile (CD11b/CD27) between different compartments.
Discussion
The liver perfusion method presented herein enables a selective harvesting and studying of the unique population of marginating hepatic leukocytes. Hepatic NK cells, also called pit cells3, constitute a distinctive NK cell population that reside in the hepatic sinusoids. They are found in rats, mice17 and in humans18,19. Compared to isolated peripheral NK cells, pit cells demonstrated higher cytotoxicity against YAC-1 and CC531s target cell lines20, were shown to have a higher number of smaller cytoplasmic granules20, and a higher expression of surface antigens, such as LFA-112. Additionally, unlike isolated blood NK cells, pit cells can lyse NK-resistant P815 cells. Similar to rat pit cells, human hepatic NK cells have a higher cytotoxicity against NK-sensitive K562 target cells compared to circulating NK cells18,19, and were shown to lyse NK-resistant target cells18. Overall, as the liver is specifically and uniquely involved in various diseases, immunity should be studied within this organ, and cells of the MH compartment should be distinguished from parenchymal, Kupffer, and stellate cells.
As elaborated in the Introduction, our perfusion approach is advantageous over the common mechanical grinding/biological degradation procedures in selectively harvesting MH leukocytes, and in better preserving the integrity, the activity, and the physiological milieu of MH leukocytes. Indeed, assessing NK cytotoxicity, MH leukocytes harvested through our approach exhibited a markedly higher NK cytotoxicity than leukocytes harvested through the mechanical grinding procedure, despite similar number of NK cells.
The hepatic perfusion technique is only operable in animal studies. However, specific insights obtained from this approach regarding marked differences between immune compartments may question the biological and clinical significance of observations based solely on circulating leukocytes. Animal studies that measure immune indices in different immune compartments may suggest the degree to which each immunological index in the circulation reflects or correlates with its levels in the MH compartment. An interesting observation refers to employing this technique in mice, where the perfused MH-NK cells will only demonstrate significant cytotoxic activity against target cells following an a-priori immune stimulation of the animal21.
Few critical aspects of the technique should be indicated. In both rats and mice, the chest cavity should be opened widely immediately following complete cessation of respiration, and with minimal bleeding. In rats, it is recommended that maximal amount of cardiac blood is withdrawn before the perfusion. In mice, it is not recommended to withdrawn blood from the heart, as it reduces the blood pressure in the portal vein and makes it harder to insert the needle through it to continue the process. This needle insertion should be made adjacent but rostral to the splenic vein, as one wishes to ensure that the distance from the insertion to the entrance of the portal vein into the liver is sufficient for the needle to be in a stable position and allows all the lobes to get perfused. The needle should be inserted into the portal vein while parallel to its surface, so the risk of rupturing the vessel wall is minimized. In rats, while inserting a needle to the caudal vena cava for the collection of the perfusate, it is crucial to maintain the entrance point as narrow as possible to avoid a potential leakage of perfusate. In mice, it is important to precociously cut the vena cava inside the chest cavity without rupturing other blood vessels or the lungs, in order to avoid unnecessary blood contamination. The major advantage of using a peristaltic pump is a stable control over the perfusion rate, within and between animals. It is important to maintain a low flow rate during the entire procedure in order to avoid rupture of the portal vein. As the first milliliters of liver perfusate are contaminated with blood, they should be discarded as shown. To achieve standardization between animals, the criterion to start collecting the MH perfusate is based on color transition from dark brown to light brown (rather than collection of a specific volume).
Along conducting the procedure, several malfunctions may occur, but most are reversible. If the needle through which the PBS is transfused into the liver slips out, it is recommended to stop the peristaltic pump, soak the surrounding area of the gut (to achieve better visibility), re-insert the needle in a slightly more rostral position, and re-start the pump.
The liver perfusion technique described herein enables a selective harvesting of the MH leukocyte population in a fairly simple process. Given that this population displays distinctive features compared to leukocytes from other immune compartments, and may have biological and clinical significance to the organism, we recommend the use of this method when feasible, as leukocytes from other compartments may not reflect the profile of the MH population. Additionally, as this technique is not doable in humans, we further suggest employing it in animals to clarify specific circulating biomarkers that correlate with the MH characteristics, particularly when studying hepatic-related processes and diseases.
Acknowledgments
The authors and this work were supported by NIH/NCI grant # R01CA172138 (to SBE).
Footnotes
Video Link
The video component of this article can be found at http://www.jove.com/video/53918/
Disclosures
The authors have nothing to disclose.
References
- 1.Melamed R, et al. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33:16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benish M, Melamed R, Rosenne E, Neeman E, Sorski L, Levi B, Shaashua L, Matzner M, Ben-Eliyahu S. The marginating-pulmonary immune compartment in mice exhibits increased NK cytotoxicity and unique cellular characteristics. Immunologic Research. 2014;58:28–39. doi: 10.1007/s12026-013-8435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisse E, van’t Noordende JM, van der Meulen J, Daems WT. The pit cell: description of a new type of cell occurring in rat liver sinusoids and peripheral blood. Cell Tissue Res. 1976;173:423–435. doi: 10.1007/BF00224305. [DOI] [PubMed] [Google Scholar]
- 4.Vermijlen D, et al. Hepatic natural killer cells exclusively kill splenic/blood natural killer-resistant tumor cells by the perforin/granzyme pathway. J Leukoc Biol. 2002;72:668–676. [PubMed] [Google Scholar]
- 5.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneda K. Liver-associated large granular lymphocytes: morphological and functional aspects. Arch Histol Cytol. 1989;52:447–459. doi: 10.1679/aohc.52.447. [DOI] [PubMed] [Google Scholar]
- 7.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 8.Kamada MM, et al. Identification of carboxypeptidase and tryptic esterase activities that are complexed to proteoglycans in the secretory granules of human cloned natural killer cells. J Immunol. 1989;142:609–615. [PubMed] [Google Scholar]
- 9.Wisse E, et al. On the function of pit cells, the liver-specific natural killer cells. Semin Liver Dis. 1997;17:265–286. doi: 10.1055/s-2007-1007204. [DOI] [PubMed] [Google Scholar]
- 10.Bouwens L, Remels L, Baekeland M, Van Bossuyt H, Wisse E. Large granular lymphocytes or “pit cells” from rat liver: isolation, ultrastructural characterization and natural killer activity. Eur J Immunol. 1987;17:37–42. doi: 10.1002/eji.1830170107. [DOI] [PubMed] [Google Scholar]
- 11.Vanderkerken K, et al. Origin and differentiation of hepatic natural killer cells (pit cells) Hepatology. 1993;18:919–925. doi: 10.1002/hep.1840180425. [DOI] [PubMed] [Google Scholar]
- 12.Luo D, et al. The role of adhesion molecules in the recruitment of hepatic natural killer cells (pit cells) in rat liver. Hepatology. 1996;24:1475–1480. doi: 10.1002/hep.510240629. [DOI] [PubMed] [Google Scholar]
- 13.Shresta S, MacIvor DM, Heusel JW, Russell JH, Ley TJ. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci U S A. 1995;92:5679–5683. doi: 10.1073/pnas.92.12.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo D, et al. Involvement of LFA-1 in hepatic NK cell (pit cell)-mediated cytolysis and apoptosis of colon carcinoma cells. J Hepatol. 1999;31:110–116. doi: 10.1016/s0168-8278(99)80170-6. [DOI] [PubMed] [Google Scholar]
- 15.Wiltrout RH, et al. Augmentation of mouse liver-associated natural killer activity by biologic response modifiers occurs largely via rapid recruitment of large granular lymphocytes from the bone marrow. J Immunol. 1989;143:372–378. [PubMed] [Google Scholar]
- 16.Schluter K, et al. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am J Pathol. 2006;169:1064–1073. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiltrout RH, et al. Augmentation of organ-associated natural killer activity by biological response modifiers. Isolation and characterization of large granular lymphocytes from the liver. J Exp Med. 1984;160:1431–1449. doi: 10.1084/jem.160.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winnock M, et al. Functional characterization of liver-associated lymphocytes in patients with liver metastasis. Gastroenterology. 1993;105:1152–1158. doi: 10.1016/0016-5085(93)90961-b. [DOI] [PubMed] [Google Scholar]
- 19.Hata K, et al. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol. 1990;56:401–419. doi: 10.1016/0090-1229(90)90160-r. [DOI] [PubMed] [Google Scholar]
- 20.Vanderkerken K, Bouwens L, Wisse E. Characterization of a phenotypically and functionally distinct subset of large granular lymphocytes (pit cells) in rat liver sinusoids. Hepatology. 1990;12:70–75. doi: 10.1002/hep.1840120112. [DOI] [PubMed] [Google Scholar]
- 21.Sorski L, Melamed R, Lavon H, Matzner P, Rosenne E, Ben-Eliyahu S. PNIRS Annual Scientific meeting; Seattle. 2015. [Google Scholar]


