Abstract
Introduction
Platelet markers (soluble CD40 ligand [sCD40L] and soluble p selectin [sPselectin]) are associated with platelet activation and cardiovascular events. We sought to investigate the reproducibility of these markers over time and the effect of low-dose aspirin on sCD40L and sPselectin in plasma and serum.
Methods
Following an overnight fast, 40 healthy volunteers had weekly phlebotomy and were administered aspirin 81 mg/day between weeks 3 and 4. Reproducibility over time was assessed by coefficient of variation (CV) and Inter-class correlation coeffient (ICC). Correlation between markers was assessed using Pearson r statistic. Difference between levels pre- and post-aspirin was measured with Wilcoxon Signed- Rank test. Data are presented as median [interquartile range].
Results
sCD40L and sPselectin measurements were reproducible over time in plasma and serum (CV<10%). Measurement of sCD40L and sPselectin in plasma correlated with levels in serum before aspirin and after aspirin. There was no significant correlation between sCD40L and sPselectin. After 1-week of aspirin 81mg/day, there was a reduction in sCD40L and sPselectin in serum and plasma, respectively.
Conclusion
Soluble CD40L and sPselectin are independent markers that are reproducible over time in both plasma and sera and are reduced by 1-week of low-dose aspirin.
Keywords: platelets, CD40-Ligand, P-Selectin, Biomarker, Aspirin
Introduction
Increased levels of soluble CD40 ligand (sCD40L) and soluble P-selectin (sPselectin ) are considered biomarkers of platelet activation that have been associated with future cardiovascular events.1–3 P-selectin, a cell surface glycoprotein stored within the alpha granules of platelets and the Weibel-Palade bodies of endothelial cells, is mobilized to the cell surface within minutes of platelet and/or endothelial cell activation and is proteolytically cleaved generating sPselectin which mediates several early processes in inflammatory cell adhesion and induces a procoagulant state.4 CD40 ligand is a trimeric transmembrane protein in the tumor necrosis factor family, found in a variety of cell types, including endothelial cells, smooth muscle cells, monocytes and macrophages, however, it is predominantly derived from platelets. Following platelet activation, the membrane bound form is mobilized to the cell surface and undergoes cleavage generating sCD40L.3 Both membrane bound CD40L and sCD40L interact with CD40 on vascular cells resulting in inflammatory and prothrombotic responses in vascular cells and increased expression of adhesion molecules and secretion of inflammatory cytokines in endothelial cells.5
Both sPselectin and sCD40L have been proposed to identify healthy individuals at increased risk for cardiovascular events suggesting that each biomarker has direct prothrombotic properties that may mediate early atherogenesis, plaque rupture, and thrombosis.6,7 However, the data correlating these biomarkers with future cardiovascular events is inconsistent.2 Before incorporation of these biomarkers into routine clinical or research practices, one needs to better understand the optimal method of measurement. To better assess the potential role of these markers in identifying and evaluating cardiovascular risk, we sought to investigate the reproducibility of these markers, use of plasma or sera for measurement, and the effect of low dose aspirin on both sCD40L and sPselectin. Since both sPselectin and sCD40L have been proposed as potential biomarkers, we investigate the relationship between them.
Materials and methods
Study Population
Healthy adults >18 years of age were recruited to participate via flyer announcement. Exclusion criteria for this study included age < 18 years, medications known to affect platelet function, including non-steroidal anti-inflammatory drugs (including aspirin), antihistamines, and selective serotonin reuptake inhibitors during the 5 days prior to baseline phlebotomy; platelet count <100,000 or > 450,000, renal failure (creatinine clearance <30ml/min or on dialysis), history of coronary artery disease, diabetes, presence of co-existing inflammatory disease, coexisting cancer, or any known hemorrhagic diathesis. This study was approved by the New York University School of Medicine Institutional Review Board, and informed consent was obtained from each volunteer.
Study Design
Baseline characteristics and medical history were obtained via direct interview, questionnaire and physical exam. Volunteers had blood collection every week for 4 consecutive weeks and took aspirin 81mg daily for 7 days between weeks 3 and 4. Volunteers fasted overnight and refrained from intensive exercise and tobacco use for 4 hours prior to an early-morning phlebotomy to avoid any circadian changes in platelet activity.
Phlebotomy
After informed consent, volunteers rested comfortably for 10 minutes prior to phlebotomy. Blood was collected from a clean, problem-free venipuncture, using a 19 gauge needle after a 2cc discard (a tourniquet may have been used to obtain access - however, it was removed before blood collection). Blood was collected into 3.2% (0.105 moles/L) sodium citrate tubes (Becton Dickinson, Franklin Lakes, NJ, USA) for plasma and serum separator tubes (SST; Becton Dickinson) for serum. After collection, each tube was gently inverted 3 times. After phlebotomy, blood was immediately transferred to the laboratory for processing. Complete blood count including MPV was performed using a Sysmex (Mundelein, Illinois, USA) XE-2100 hematology analyzer, and was performed within 30 minutes of phlebotomy.
Processing
Sodium citrate anti-coagulated blood was centrifuged within 15 minutes of phlebotomy at 2500 rcf for 10 minutes yielding platelet free plasma. Blood in SST tubes were allowed to clot for 30 minutes at room temperature and then centrifuged at 2500 rcf for 10 minutes yielding serum. Plasma and serum were aliquoted no more than five minutes after completion of centrifugation and stored at −80 C until time of assay.
Concentrations of sPselectin and sCD40L were determined in plasma and serum by commercially available quantitative enzyme-linked immunosorbent assay (Bender MedSystems: BMS219 & BMS293, respectively) according to manufacturer’s instructions. In brief, plasma and serum for sPslectin measurements were diluted 1:10 with the sample diluent provided by kit. Plasma for sCD40L measurement was diluted 1:2 while serum was diluted 1:5. All samples were assayed in duplicate. After addition of sample or standard, in either sPselectin or sCD40L plates, 50 uL of horseradish peroxidase conjugated antibody was added to all wells and plates were incubated at room temperature for two hours on a microplate shaker at 100 rpm. After incubation, wells were washed 3 times with approximately 400 uL of wash buffer and after all liquid was removed, 100 uL of 3,3′,5,5′-Tetramethylbenzidine solution was added to each well for color development. Plates were incubated in the dark at room temperature for 30 minutes. When color development was complete, 100 uL of stop solution was added to each well and the plate was read within 15 minutes on a spectro-photometer using 450nm as the primary wavelength. Mean optical densities were converted to concentrations using the standard curve run for each individual plate and multiplied by their corresponding dilution factors.
Statistical Analyses
Reproducibility over time of levels of sPselectin and sCD40L was assessed by coefficient of variation (CV) and intraclass correlation coefficient (ICC) using a two way mixed effect model. The difference between levels pre- and post-aspirin was measured by Wilcoxin Signed- Rank test. Correlation of sPselectin and sCD40L in plasma versus serum was assessed using Pearson r statistic. Correlation of sPselectin and sCD40L with mean platelet volume (MPV) and platelet count was performed using the Pearson r statistic. Statistical analyses were performed using GraphPad Prism, Version 4 (La Jolla, USA). Statistical significance was defined as a two-sided P value < 0.05. Data is expressed as median [interquartile range]
Results
Baseline characteristics of our study population are described in Table 1.
Table 1.
Baseline Characteristics
| N=40 | |
|---|---|
| Age, median [IQR] | 30.2 [21–61] |
| Female | 20 |
| Race | |
| White | 21 |
| Asian | 15 |
| Black | 1 |
| Other | 3 |
| Ethnicity | |
| Hispanic | 3 |
| Non-Hispanic | 37 |
| BMI, kg/m2, median [IQR] | 24.5 [22.5–25.9] |
| Current Smoker | 1 |
| Past Smoker | 8 |
| Family History of CVD | 6 |
IQR, interquartile ratio; BMI, body mass index
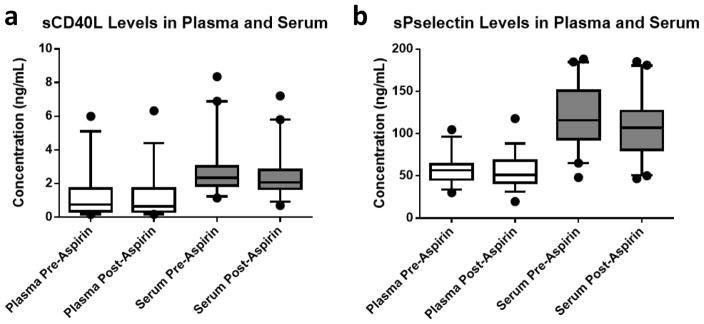
Soluble CD40L measurements were reproducible over time in plasma (Week 1: 0.72 ng/mL [0.35–1.63], Week 2: 0.73 ng/mL [0.36–1.69], Week 3: 0.67 ng/mL [0.34– 1.7]). The CV of sCD40L in plasma was 9.6% and the ICC was 0.96. When measured in serum, levels of sCD40L were higher than in plasma, yet, levels remained reproducible overtime (Week 1: 2.43 ng/mL [2.11– 2.99], Week 2: 2.27 ng/mL [1.8– 2.98], Week 3: 2.44 ng/mL [1.75– 3.04]). The CV in serum was 8% and the ICC was 0.99. One week of low-dose aspirin reduced levels of sCD40L in both plasma and serum (Figure 1a). Measurement of sCD40L in plasma correlated with levels in serum before aspirin (Week 1: r=.877 and p<0.0001, Week 2: r=.852 and p<0.0001, Week 3: r=0.812 and p<0.0001) and after aspirin (Week 4: r=0.87, p< 0.0001). The correlation in plasma and serum measurements before and after aspirin suggests that aspirin does not act differentially on sCD40L in plasma versus sera. No difference in baseline demographics (age, sex, race/ethnicity) was found among quartiles of sCD40L in plasma or serum (data not shown). Soluble CD40L did not correlate with platelet count in plasma before aspirin (Weeks 1–3: r=0.195, p=0.248) or after aspirin (Week 4: r= 0.189, p=0.261), nor did it correlate in sera before aspirin (Weeks 1–3: r= 0.179, p=0.268) or after aspirin (r=0.159, p=0.329). Since mean platelet volume is associated with platelet activity and incident cardiovascular events,8,9 we investigated the association between MPV and sCD40L. There was no significant association between sCD40L and MPV in plasma before aspirin (Weeks 1–3: r=−0.174, p=0.304) or after aspirin (Week 4: r=−0.211, p=0.210), nor in serum before aspirin (Weeks 1–3: r=−0.169, p=0.295) or after aspirin (Week 4: r= −0.1698, p=0.295).
Figure 1. Levels of a) soluble CD40 Ligand and b) soluble P-selectin before and after 1-week of low-dose aspirin in plasma and sera, respectively.
sCD40L and sPselectin were measured in individuals at baseline (before ingestion of aspirin) and then again after 1-week of aspirin 81mg per day.
- After 1-week of aspirin 81mg/day there was a reduction in sCD40L in plasma (0.76 ng/ml [0.35 – 1.72] vs. 0.64 [0.34 – 1.70], P=0.11) and serum (2.35 ng/ml [1.87 – 3.03] vs. 2.07 [1.69 – 2.80], P<0.001).
- After 1-week of aspirin 81mg/day there was a reduction in sP-selectin in plasma (56.5 ng/ml [45.8 – 63.9] vs. 51.1 [41.9 – 68.2], P=0.02) and serum (115.8 ng/ml [93.3 – 151] vs. 107.1 [80.8 – 126.7], P<0.001).
Soluble P-selectin measurements were reproducible over time in plasma (Week 1: 58.9 ng/mL [46.2–70.8], Week 2: 55.6 ng/mL [45.4–68.6], Week 3: 52.6 ng/mL [43.8–62.8]). The CV of sPselectin in plasma was 9.4% and ICC was 0.92. When measured in serum, levels of sPselectin were higher than in plasma, yet, levels remained reproducible over time (Week 1: 116 ng/mL [94.6–145.5], Week 2: 113.6 ng/mL [90.7–149.6], Week 3: 111.9 ng/mL [94.1–148.8]). The CV in serum was 6% and ICC was 0.98. One week of low dose Asprin reduced levels of sPselectin in both plasma and serum (Figure 1b). Levels of sPselectin in plasma correlate with levels in serum before aspirin (Week 1: r=0.660 and p< 0.0001, Week 2: r=0.583 and p= 0.0001, Week 3: r=0.7258 and p<0.0001) and after aspirin (r=0.54, p= 0.0004). The correlation in measurements before and after aspirin suggests that aspirin does not act differentially on sCD40L in plasma versus sera. No difference in baseline demographics (age, sex, race/ethnicity) was found among quartiles of sPselectin in plasma or serum (data not shown). Soluble p-selectin did not correlate with platelet count in plasma before aspirin (Weeks 1–3: r=0.054, p=0.743) or after aspirin (Week 4: r=0.089, p=0.591), nor did it correlate in sera before aspirin (Weeks 1–3: r=0.143, p=0.376) or after aspirin (r=0.192, p=0.230). Soluble P-selectin levels also did not correlate with MPV in plasma before aspirin (Weeks 1–3: r= 0.025, p=0.879) or after aspirin (Week 4: r=0.061, p=0.710), nor in serum before aspirin (Weeks 1–3: r=0.124, p=0.447) or after aspirin (Week 4: r=0.169, p=0.296).
Since sCD40L and sPselectin are both considered biomarkers of platelet activity, we investigated the relationship between them. There was no significant correlation between sCD40L and sPselectin before (r=−0.06, p=0.704; r= 0.03, p=0.853) or after aspirin (r=−0.096, p=0.573; r=0.11, p=0.482) in plasma or in sera, respectively. This data suggests that that circulating levels of sCD40L and sPselectin may have different biological relevance.
Discussion
Our results demonstrate that, with a standardized protocol, sPselectin and sCD40L levels are reproducible over time in both plasma and sera, respectively (Supplementary Figure). Importantly, both sPselectin and sCD40L are decreased by 1-week of low aspirin 81mg per day and thus careful attention to antiplatelet therapy is crucial for proper interpretation. This study suggests that with a standardized phlebotomy, strict laboratory protocol, and with careful attention to use of concomitant medications, there is reproducibility to the assessment of both sPselectin and sCD40L. Importantly, this study is unable to comment on the clinical usefulness of these assays.
In routine clinical laboratory practice, different protocols are used for blood collection and processing. Variation exists in phlebotomy techniques, type of sample collection (anticoagulation used, plasma/sera, etc.), time of day for the collection, time between collection and centrifugation, centrifugation speed, time to freezing, freezing temperatures, thawing/freezing cycles, and assay performance. Previous studies have reported that many of these pre-analytical conditions affect the results of platelet activity and other markers of coagulation.10–13 As noted previously,10,14,15 the plasma values were markedly lower than serum values for both markers. This is the first demonstration that both sCD40L and sPselectin from plasma and serum are reproducible over time. Consistent with prior publications,16,17 aspirin decreases levels of these markers. Our study reinforces that even 1-week of low-dose aspirin is effective at decreasing both sCD40L and sPselectin in both plasma and sera, respectively. The present study suggests that with a standardized protocol, measurement of sPselectin and sCD40L in both plasma and sera is reproducible over time.
There did not appear to be any meaningful correlation between sPselectin and sCd40L, suggesting unique characteristics of each marker, such as cell origin and differences in proteolytic mechanisms.18,19 Understanding the association between each of these markers and other markers of platelet activity and/or other markers of cardiovascular risk are needed. Future studies should address whether sPselectin and/or sCD40L are associated with future cardiovascular events in carefully performed studies.
There are several important limitations that need to be kept in mind when interpreting the results of our study. This study was not designed to address long-term follow-up or associations with clinical endpoints but does provide insight into future study designs utilizing both sCD40L and sPselectin. While this study evaluated two platelet markers in plasma and sera, there are other methods of measuring platelet activity that were not chosen for this study. The effect of a longer duration of aspirin or other more potent anti-platelet medications was not evaluated.
In conclusion, the current data suggests that both sCD40L and sPselectin is reproducible over time in both plasma and sera with a standardized protocol. The significant effect of a short-term course of low-dose aspirin on both of these markers underscores the importance of antiplatelet therapy when interpreting the results of these biomarkers. For the proper interpretation of results in clinical and research laboratories, one must be aware of the limitations and methodology of these biomarkers.
Supplementary Material
Acknowledgments
This study was funded, in part, by a grant from the Doris Duke Charitable Foundation (2010055). Dr Berger was partially funded by the National Heart and Lung Blood Institute of the National Institutes of Health (RO1HL114978), American Heart Association Clinical Research Program (13CRP14410042) and Doris Duke Charitable Foundation (2010055).
Footnotes
Disclosures
None
References
- 1.Blann AD, Lip GY. Hypothesis: is soluble P-selectin a new marker of platelet activation? Atherosclerosis. 1997;128:135–8. doi: 10.1016/s0021-9150(96)05980-1. [DOI] [PubMed] [Google Scholar]
- 2.Sharma G, Berger JS. Platelet activity and cardiovascular risk in apparently healthy individuals: a review of the data. Journal of thrombosis and thrombolysis. 2011;32:201–8. doi: 10.1007/s11239-011-0590-9. [DOI] [PubMed] [Google Scholar]
- 3.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. Journal of the American College of Cardiology. 2009;54:669–77. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 4.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–6. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 5.Freedman JE. CD40-CD40L and platelet function: beyond hemostasis. Circulation research. 2003;92:944–6. doi: 10.1161/01.RES.0000074030.98009.FF. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491–5. doi: 10.1161/01.cir.103.4.491. [DOI] [PubMed] [Google Scholar]
- 7.Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104:2266–8. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- 8.Shah B, Valdes V, Nardi MA, Hu L, Schrem E, Berger JS. Mean platelet volume reproducibility and association with platelet activity and anti-platelet therapy. Platelets. 2014;25:188–92. doi: 10.3109/09537104.2013.793794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. Journal of thrombosis and haemostasis : JTH. 2010;8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thom J, Gilmore G, Yi Q, Hankey GJ, Eikelboom JW. Measurement of soluble P-selectin and soluble CD40 ligand in serum and plasma. Journal of thrombosis and haemostasis : JTH. 2004;2:2067–9. doi: 10.1111/j.1538-7836.2004.00962.x. [DOI] [PubMed] [Google Scholar]
- 11.Mason PJ, Chakrabarti S, Albers AA, et al. Plasma, serum, and platelet expression of CD40 ligand in adults with cardiovascular disease. The American journal of cardiology. 2005;96:1365–9. doi: 10.1016/j.amjcard.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Iversen LH. Pre-analytical variation in the measurements of sensitive markers of coagulation and fibrinolysis: the influence of venipuncture and mixing of blood. Haemostasis. 1997;27:119–24. doi: 10.1159/000217443. [DOI] [PubMed] [Google Scholar]
- 13.Lee RD, Barcel DA, Williams JC, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thrombosis research. 2012;129:80–5. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn ER, Lander G, Jy W, et al. Differences of soluble CD40L in sera and plasma: implications on CD40L assay as a marker of thrombotic risk. Thrombosis research. 2004;114:143–8. doi: 10.1016/j.thromres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Varo N, Nuzzo R, Natal C, Libby P, Schonbeck U. Influence of pre-analytical and analytical factors on soluble CD40L measurements. Clinical science. 2006;111:341–7. doi: 10.1042/CS20060047. [DOI] [PubMed] [Google Scholar]
- 16.Riondino S, Martini F, La Farina F, Spila A, Guadagni F, Ferroni P. Increased plasma levels of soluble CD40 ligand correlate with platelet activation markers and underline the need for standardized pre-analytical conditions. Clinical biochemistry. 2010;43:666–70. doi: 10.1016/j.clinbiochem.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Ferroni P, Martini F, Riondino S, et al. Soluble P-selectin as a marker of in vivo platelet activation. Clinica chimica acta; international journal of clinical chemistry. 2009;399:88–91. doi: 10.1016/j.cca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Archivum immunologiae et therapiae experimentalis. 2006;54:75–84. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- 19.Santilli F, Basili S, Ferroni P, Davi G. CD40/CD40L system and vascular disease. Internal and emergency medicine. 2007;2:256–68. doi: 10.1007/s11739-007-0076-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.