Abstract
Background
The most recent European Association for the Study of the Liver (EASL) 2016 Guidelines on treatment of hepatitis C (HCV), allowed for shortening the course of treatment for some subsets of patients with sofosbuvir/ledipasvir and with grazoprevir/elbasvir based on cutoff baseline HCV RNA values. We hypothesized that it would be prudent to also consider an objectively assuring very rapid, on-treatment, virologic response to therapy at week 2 (vRVR) before taking the decision of shortening the treatment duration. So we planned this study to test whether a dual sofosbuvir/daclatasvir (SOF/DCV) treatment duration tailored according to achieving vRVR to 8 or 12 weeks is non-inferior to the recommended fixed 12 weeks course in non-cirrhotic Egyptian chronic HCV genotype-4 patients.
Methods
The study was conducted in an outpatient setting according to a prospective, randomized, open-label, comparative, non-inferiority study design. A hundred twenty eligible, non-cirrhotic, chronic HCV patients were randomly assigned (1:1) to receive daily doses in the form of one Gratisovir 400 mg table (generic sofosbuvir produced by Pharco Pharmaceuticals, Alexandria, Egypt) plus one Daktavira 60 mg tablet (generic daclatasvir produced by Dawood Pharm, Egypt) for either a fixed 12 weeks duration (reference group) or a response tailored duration (test group). In the test group the treatment duration was tailored according to the virus load tested by real time PCR into 8 weeks for patients who had undetectable HCV RNA level in their serum by the end of the second week of treatment (vRVR)), or 12 weeks for those who did not show vRVR. The primary outcome of the trial was the proportions of patients achieving SVR12 (HCV RNA below lower level of quantification at week 12 after end of treatment). The comparison between groups was based on testing the null hypothesis of inferiority of the response-tailored group with a pre-specified margin of non-inferiority (NI-m) of 0.1 (10%).
The protocol was registered with a WHO Clinical Trial Registration ID: ACTRN12617000263392. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372041
Findings
Starting from Jun, 5 2016, a hundred twenty eligible patients from 4 outpatient clinics in Alexandria, Egypt were randomized to either a fixed duration group (reference group: n = 60 patients) or a response tailored duration group (test group: n = 60 patients). During the whole period of the study, only 1 patient dropped-out from each group. Both were lost to follow-up after the 4th week's visit. Baseline characteristics in both groups were almost matching. Fifty eight out of the total 60 intention-to-treat (ITT) patients in the reference group achieved SVR12 (96.67% (95% confidence interval (CI): 88.64–99%). Whereas, 59 out of the total 60 (ITT) patients in the test group achieved SVR12 (98.33% (CI: 91.14–99.71%). The per-protocol (PP) analysis, excluding patients who dropped-out before collecting their final result, showed that 58/59 (98.31% (CI: 91–99.7%)) of patients in the reference group and 59/59 (100% (CI: 93.89–100%) of the test group achieved SVR12. Non-inferiority was declared since the upper bound of the two-sided 95% CI for the difference in proportions of SVR12 between groups (P(reference) − P(test)) did not exceed the specified non-inferiority margin of + 0.1 (10%), both in ITT population (− 1.67%, CI: − 9.8%–+5.9%), and in the PP population (− 1.69%, CI: − 9%–+ 4.58%). No fatalities or serious adverse events were reported during the period of the study. Similar rates of non-serious adverse events were reported in both groups with a trend of higher incidence rate in the fixed 12 weeks group; all were mild in severity.
Interpretation
Shortening the duration of therapy based on observed vRVR could provide a prudent basis to avoid unnecessary long treatment courses. This could not only reduce the drug exposure and the risk of adverse drug reactions, but also cut the cost of full treatment course with such expensive medications by one third. This could economize the treatment budget at the individual out-of-pocket level as well as the public health services and insurance levels and allow for better utilization of public health resources.
Keywords: Chronic hepatitis C infection, Sofosbuvir, Daclatasvir, Response tailored therapy, Very rapid virologic response, SVR12
Highlights
-
•
It would be prudent to consider vRVR to therapy at week 2 before shortening HCV treatment duration with SOF/DCV to 8 weeks.
-
•
This will consider the variability of response as a factor at individualized level not just a point of estimate at a population level.
-
•
Response-tailored duration of 8 or 12 weeks based on achieving vRVR was non-inferior to the fixed 12 weeks course.
The decision of shortening the duration of therapy of non-cirrhotic chronic hepatitis C genotype-4 patients with dual sofosbuvir plus daclatasvir to 8 weeks instead of the recommended 12 weeks, if based on achieving viral negativity in serum at week 2 as an on-treatment qualifier, could provide a prudent basis to avoid unnecessary long treatment courses. This could not only reduce the drug exposure and the risk of adverse drug reactions, but also cut the cost of full treatment course with such expensive medications by one third.
1. Introduction
The most recent European Association for the Study of the Liver (2016) Guidelines on treatment of hepatitis C, allowed for shortening the course of treatment with Sofosbuvir/Ledipasvir and with Grazoprevir/Elbasvir for subsets of patients with lower virus load based on cutoff values of baseline serum HCV RNA level (EASL, 2017; Kowdley et al., 2014, Curry et al., 2016).
Although low baseline virus load is considered one of the predictive factors for SVR12, many other host, virus and drug related factors are also important and can sometimes affect the virus response to treatment regardless of baseline virus load. The significant existence of Resistance Associated Substitutions (RASs) is an example of an unpredictable virus related factor that can affect the response rate and it is still not routinely tested for on a wide scale (Di Maio et al., 2017).
We had suggested in a previous study report (Yakoot et al., 2016), that the speed of virus response during the first 2 weeks of therapy could be regarded as an efficacy marker with a high positive predictability for sustained virologic response. It combines the measured displacement (reduction) of the virus load divided by the time which is another independent factor for response (Pineda et al., 2017, Sulkowski et al., 2016); (Speed (v) = Displacement of virus load (d)/Time(t)).
The very rapid virologic response (vRVR), defined as undetectable serum HCV RNA level at week 2 of therapy, was found, in our study to be a good positive predictor for SVR12 to dual therapy with sofosbuvir and ribavirin in patients with chronic HCV genotype-4. It had a high positive predictive value (PPV) of 100% (95% CI, 90.8–100%) and a high sensitivity of 82.6% (CI, 68.6–92.2%) but with low negative predictive value (Yakoot et al., 2016).
We suggested that achieving a vRVR could be used as a prudent objective qualifier to shorten duration of therapy. It objectively demonstrates that the treatment has been working against at least insignificant drug resistance and maintained for a period of at least 6 weeks after reaching viral negativity in the serum. This, in our assumption, might be enough time for the complete eradication of the virus from all liver cells and other hidden potential reservoirs such as platelets and mononuclear cells in blood or RES (ex: spleen) that could probably be the potential initiators for relapse.
Our new suggestion of tailoring the duration of therapy according to achieving a vRVR can be regarded as a positive response guided therapy. This is totally different from the previously implemented negative response guided therapy, in the pre- directly acting antiviral drugs (DAAs) era. Because now with the lower failure rates (> 90% success rates), achieved with DAAs, the early viral response kinetics (i.e. time to viral negativity) became strong positive predictors for treatment success but weak negative predictors for treatment failure (Maasoumy et al., 2016).
Although sofosbuvir with daclatasvir (SOF/DCV) dual therapy is the most widely used treatment protocol for chronic hepatitis C in Egypt, the country with highest prevalence of infection and the greatest number of treated patients, published data about this combination is still scanty.
All non-cirrhotic patients are being treated with a fixed 12 weeks protocol according to the Egyptian and EASL guidelines.
We have observed during our real life practice that the success rate is very high reaching above 95% and the majority of patients respond very early during the first 2 weeks of treatment (vRVR) and remain with undetectable HCV RNA in serum till the end of the 12 weeks post-treatment follow up period (SVR12). Also, we have noticed in many incidents that non-cirrhotic patients, who for any reason, interrupted treatment at as early as 8 weeks did not relapse.
This observation has lead us to further study our above mentioned suggestion that the vRVR might be used as a prudent qualifier to shorten the duration of therapy in this setting to 8 weeks as it allows a period of at least 6 weeks on observably working treatment after reaching undetectable HCV RNA level in serum. We planned this research to study whether the proportion of SVR12 in the response tailored duration (test group) is non-inferior to that in the recommended fixed 12 weeks duration (reference group). So we tested the null hypothesis of inferiority of a protocol tailored according to vRVR of 8/12 weeks versus the recommended fixed 12 weeks course of dual SOF/DCV treatment in non-cirrhotic Egyptian chronic HCV genotype-4 patients.
2. Methods
2.1. Study Design and Participants
The study was conducted in an outpatient setting according to a prospective, randomized, open-label, comparative, non-inferiority study design.
Male or female patients, between 18 and 70 years old who had a diagnosis of chronic hepatitis C and serum HCV RNA level above 10,000 IU/ml were screened for inclusion. Key exclusion criteria were pregnancy or lactation; concomitant other causes of hepatitis; concurrent HIV virus infection; active schistosomiasis; Child-Pugh score > 6; alanine or aspartate aminotransferase higher than 7 times the upper limit of normal, albumin < 2.8 g/dL; international normalized ratio > 2.3; transient elastography (by FibroScan) result of > 12.5 kPa at screening and/or an aspartate aminotransferase to platelet ratio index (APRI) of > 2; platelet count < 50 × 109/L; severe anemia (hemoglobin grade 3 or higher (< 8 g/dL)); any malignancy; Alfa-fetoprotein (AFP) level above 200 ng/ml; critically ill or more than slight limitation of activity; unwilling to participate or to sign the informed consent.
The study protocol was reviewed and approved by Green Clinic and Research ethical committee according to the Declaration of Helsinki (IRB00008268). All subjects gave written informed consent before any treatment interventions were performed. The study has been registered with a WHO Clinical Trial Registration ID: ACTRN12617000263392.
2.2. Randomisation and Masking
Eligible patients were randomly assigned (1:1) to receive daily doses in the form of one Gratisovir 400 mg table (generic sofosbuvir produced by Pharco Pharmaceuticals, Alexandria, Egypt) plus one Daktavira 60 mg tablet (generic daclatasvir produced by Dawood Pharm, Egypt) for either a fixed 12 weeks duration (reference group) or a response tailored duration (test group). In the test group the treatment duration was tailored according to the virus load tested by real time PCR into 8 weeks for patients who had undetectable HCV RNA level in their serum by the end of the second week of treatment (very rapid virologic response (vRVR)), or 12 weeks for those who did not show vRVR.
A research support statistician, who was not involved with the conduct of the study or analysis of data prepared the random allocation sequence table by a software generated block randomisation technique stratified by site, with a block size of four, and kept the allocation sequence of each study site concealed in sealed opaque envelopes that were consecutively numbered on the outside and stored in a locked cabinet in each study site, to be opened by investigators just at the entry of each eligible patient according to his/her consecutive randomization number.
The study was unmasked and open label because practically investigators had to determine the response tailored duration of either 8 or 12 weeks on the basis of the result of serum virus load (HCV RNA levels) at week 2; while in addition, the primary endpoint (HCV RNA levels) is very robust objective outcome measure that is hard to be affected by the patient knowledge of taking an active treatment for whichever duration.
2.3. Procedures
Starting from 5/6/2016, all patients presenting, with chronic hepatitis C infection, to 4 outpatient clinics in Alexandria, Egypt were subjected to full screening for eligibility to be included in this study. The first 120 of those who fulfilled all eligibility criteria were randomized into two balanced groups (reference and test) by a computer based block randomization technique stratified by site.
The reference group (n = 60) was assigned to a fixed 12 weeks duration of a dual combination of Gratisovir (generic sofosbuvir) one 400 mg table daily and Daktavira (generic daclatasvir) one 60 mg tablet daily. The test group (n = 60) was assigned to the same drugs and doses for a duration tailored according to the achievement of vRVR. Those who achieved vRVR were given the treatment for a shorter duration of 8 weeks, while those who did not show vRVR were asked to complete 12 weeks course.
At the randomization visit (day 0), patients were given the treatment kit sufficient for 4 weeks, and were asked to visit the treatment site at the end of week 1, 2, 4, 6, 8, 12, 16, 20, 24 and to call by phone or visit at any unscheduled time for reporting any adverse event or query.
During the screening visits and all other study visits all patients were subjected to full physical examination and laboratory investigations including the complete blood count (CBC), serum bilirubin, serum albumin, Alanine aminotransferase (ALT) & Aspartate aminotransferase (AST), prothrombin time (PT), serum creatinine and ultrasonographic abdominal scan.
Serum HCV-RNA level was tested using the Polymerase Chain Reaction (PCR) quantitative measurements by COBAS Amplicor 2.0, Roche Molecular Diagnostics, Pleasanton, CA, USA (lower limit of detection of 10 IU/mL) during the screening visit and all the study visits.
While the screening tests to exclude pregnancy, active schistosomiasis, hepatitis B, HIV and autoimmune hepatitis as well as the test for genotype “if not known” using GEN-C 2.0 Reverse Hybridization Strip Assay (Nuclear Laser Medicine, Settala, Italy) were done only at the screening visits.
2.4. Outcomes
The primary outcome of the trial was the proportions of patients achieving SVR12 (HCV RNA below lower level of quantification at week 12 after end of treatment).
We tested whether the proportion of SVR12 in the response tailored duration (test group) is non-inferior to that in the recommended fixed 12 weeks duration (reference group).
The secondary outcome measures were to compare both groups for the efficacy endpoints (proportions of SVR12) and the safety endpoints (proportions of serious and above grade 1 severity adverse events) using the suitable superiority tests.
We defined on-treatment virologic failure as the failure to reach a virus load (VL) less than the lower limit of quantification (LLOQ) by the end of treatment, or a confirmed rise of VL above the LLOQ after being below the limit. We defined post-treatment failure (relapse) as two consecutive post-treatment HCV RNA measurements at LLOQ or higher within 12 weeks after the end of treatment with HCV RNA concentration below LLOQ.
Any adverse events reported by patients or observed by investigators during the study visits or any deviation from a baseline normal laboratory test, occurring after administration of the first dose of study drugs until 30 days after the last dose was considered a treatment-emergent adverse event (TEAE). These were further evaluated by investigators for causality using the Uppsala Monitoring Center (UMC) causality categorization. Adverse events deemed to have certain, probable or possible causality category were considered in analysis and graded according to seriousness (serious/non-serious) and severity using the Common Terminology Criteria for Adverse Events v3.0 (CTCAE) into grade 1 (mild), 2 (moderate), 3 (severe), 4 (life threatening) or 5 (death related). Adverse events were categorized by system organ class and coded by both lowest level terms and preferred terms using the Medical dictionary for regulatory activity (MedDRA) version 19.
2.5. Statistical Analysis
Sample sizes of 60 in each group achieve 83% power to detect a non-inferiority margin difference between the group proportions of 0.10 (10%). The reference (fixed 12 weeks) group proportion is 0.95. The test (response tailored) group proportion is assumed to be 0.85 or lower under the null hypothesis of inferiority. The power was computed for the case when the actual treatment group proportion is 0.95 (zero difference). The test statistic used is the one-sided Z test (unpooled). The significance level of the test was targeted at 0.05 (PASS 2008, version 08.0.2; NCSS).
The comparison between groups was based on testing the null hypothesis of inferiority of the test group with a pre-specified margin of non-inferiority (NI-m) of 0.1.
H0 (Null hypothesis): (P(reference group) − P(test group)) ≥ 0.1 (NI-m);
H1 (alternative hypothesis): (P(reference group) − P(test group)) < 0.1 (NI-m).
Non-inferiority was declared if the upper bound of the two-sided 95% confidence interval (CI) for the absolute difference between the proportions of SVR12 in both groups (P(reference) − P(test)) did not exceed a non-inferiority margin of + 0.1, equivalent to one-sided z test with an alpha value of 0.025. The reference group proportion was assumed to be 0.95 according to an average estimate of data from published and unpublished clinical studies on the treatment of genotype-4 with SOF/DCV for 12 weeks duration in Egypt and abroad (Alavian and Rezaee-Zavareh, 2016, Llaneras et al., 2017, Rockstroh et al., 2016). The non-inferiority margin was predefined as 0.1 (10%) based on clinical acceptability and with regards to the reduction by one third in the total cost and duration of therapy as well as the reduction of the risk of drug exposure.
We calculated the percentage of patients achieving the efficacy outcome measure (SVR12) or the safety outcome measures (serious and above grade 1 severity of treatment emergent adverse events) in each treatment group. We computed both the two-sided 95% CI using the Wilson score method and the z test for proportions to compare the efficacy outcomes between groups. Secondary outcomes were compared using standard two-sided superiority tests (z test for proportions or Exact test.
3. Results
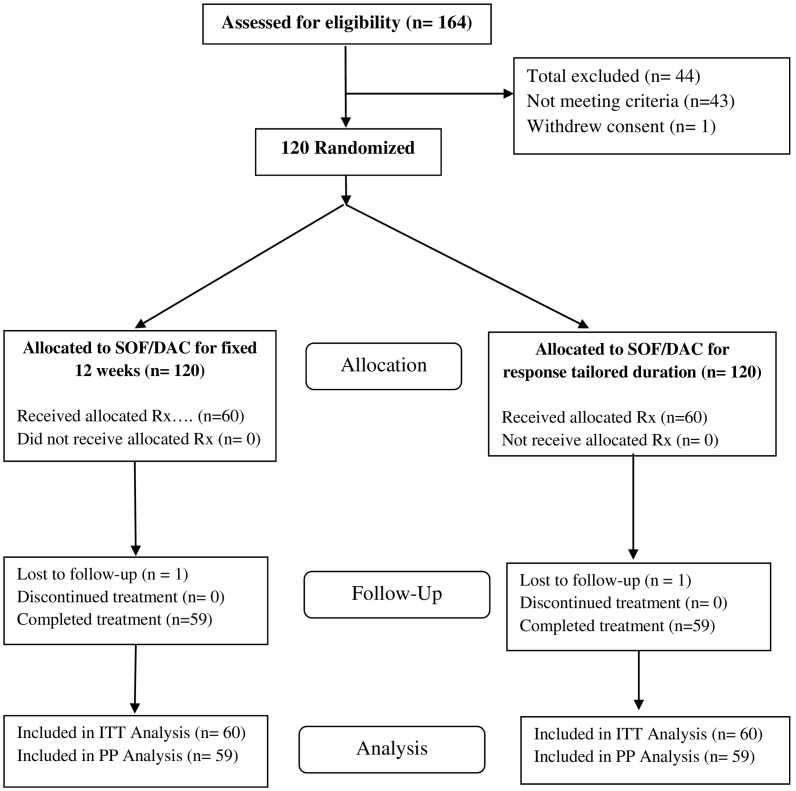
A hundred twenty eligible patients were included to be treated with dual SOF/DCV and randomized for either a fixed 12 weeks duration group (reference group: n = 60 patients) or a response tailored 8/12 weeks durations group (test group: n = 60 patients). During the whole period of the study, only 1 patient dropped-out from each group. Both were lost to follow-up after the 4th week's visit. One of them achieved vRVR at week 2 and the other became negative at week 4 of treatment. Their results have been included in the intention to treat analysis (ITT) as not achieving SVR12. We also conducted a per-protocol efficacy analysis in which we excluded these 2 patients from analysis and included only those who completed the full protocol (Flowchart: Fig. 1).
Fig. 1.
Patient flowchart.
The baseline characteristics of both groups were almost comparable (Table 1.).
Table 1.
Patients' demographics and baseline characteristics of the 2 groups.
| Characteristics | Fixed 12 weeks reference group (n = 60) | Response tailored test group (n = 60) |
|---|---|---|
| Age-yrs.: (mean ± SD) | 43.6 ± 8.15 | 45.4 ± 7.86 |
| Sex count: M/F | 36/24 | 32/28 |
| Body mass index Kg/m2: (mean ± SD) | 29.74 ± 4.7 | 28.26 ± 4.4 |
| Baseline HCV RNA (log10 IU/mL): (mean ± SD) | 5.97 ± 0.632 | 6.12 ± 0.678 |
| Baseline APRI (mean ± SD) | 0.617 ± 0.616 | 0.577 ± 0.432 |
| Interferon treatment history: (naive/relapser/non-responder) | 48/5/7 | 43/8/9 |
The vRVR rates and the SVR12 rates calculated per each intention-to-treat (ITT) population and for those who completed the full protocol (PP) in each group are presented in (Table 2).
Table 2.
The virologic responses in both groups.
| Response | Fixed 12 weeks (Ref.) | Response-tailored (test) | Proportion difference (Ref-test) | Z | P (2-tail) |
|---|---|---|---|---|---|
| vRVR (ITT) | 49/60 (81.67%) (CI: 70.08–89.44%) | 48/60 (80%) (CI: 68.22–88.17%) | 1.67%) (CI: − 12.51–+15.78%) | 0.232 | 0.817 |
| SVR12 (ITT) | 58/60 (96.67%) (CI: 88.64–99%) | 59/60 (98.33%) (CI: 91.14–99.71%) | (− 1.67%) (CI: − 9.8%–+5.9%) | 0.6 | 0.569 |
| SVR12 (PP) | 58/59 (98.31%) (CI: 91–99.7%) | 59/59 (100%) (CI: 93.89–100%) | (− 1.69%) (CI: − 9%–+4.58%) | 1 | 0.315 |
| SVR12/8w (ITT) | 47/48 (97.92%) (CI: 89.1–99.6%) | ||||
| SVR12/8w (PP) | 47/47 (100%) (CI: 92.4–100%) |
vRVR (very rapid virologic response at week 2).
SVR12 (sustained virologic response at 12 weeks post-treatment).
SVR12/8w (sustained virologic response at 12 weeks after the end of 8 weeks-treatment course).
Data are n/N (%) (95% Confidence Interval (CI:) by Wilson score method).
ITT (Intention-to-treat population); PP (Per Protocol analysis for those who completed the full protocol).
The vRVR rates showed no statistically significant difference between groups (49/60 (81.67% (95% confidence interval (CI): 70.08–89.44%) in the fixed 12 weeks group, versus 48/60 (80% (CI: 68.22–88.17%) in the response-tailored group; p = 0.817)).
Fifty eight out of the total 60 (ITT) patients in the reference group (fixed 12 weeks) achieved SVR12 (96.67% (CI: 88.64–99%)). Whereas, 59 out of the total 60 (ITT) patients in the test group (tailored duration) achieved SVR12 (98.33% (CI: 91.14–99.71%)).
The per-protocol (PP) analysis, excluding patients who dropped-out before collecting their final result, showed that 58 out of 59 (98.31% (CI: 91–99.7%)) of patients who completed the full protocol in the reference group and 59 out of 59 (100% (CI: 93.89–100%) of the test group achieved SVR12.
One out of the 48 patients who achieved vRVR in the test group (tailored duration) dropped-out during the study; all the remaining 47 patients (100% (CI: 92.4–100%)) who completed their full 8 weeks treatment protocol (PP analysis) finally achieved sustained virologic response at 12 weeks post-treatment (SVR12/8w). Therefore, the SVR12/8w rate in the total subset eligible for 8 weeks (ITT) analysis was 97.92% (CI: 89.1–99.6%).
The differences between the 2 groups in the vRVR rates and in SVR12 rates (both ITT and PP) were not statistically significant by confidence interval and the z test for proportion (Table 2).
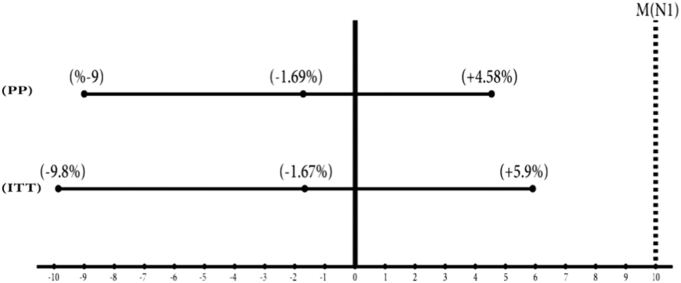
Non-inferiority was confirmed since the upper bound of the two-sided 95% confidence interval (CI) for the absolute difference in proportions of SVR12 between groups (P(reference) − P(test)) did not exceed the specified non-inferiority margin of + 0.1 (10%), both in the intention to treat (ITT) population (− 1.67%, CI: − 9.8%–+5.9%), and in the per-protocol (PP) population (− 1.69%, CI:− 9%–+ 4.58%). (Fig. 2).
Fig. 2.
The 95% Confidence Intervals for absolute difference in proportions of SVR12.
All treatment-emergent-adverse-events with causality category possible or above were included in the intention-to-treat safety analysis. No fatalities or serious adverse events were reported during the period of the study. Similar rates of non-serious, adverse events were reported in both groups. There was no statistically significant difference by z test for proportions but a non-significant trend of higher total adverse events rate in the fixed 12 weeks group; all were mild in severity (Table 3).
Table 3.
Treatment-emergent-adverse-events.
| System organ class (SOC) | Preferred term | Fixed 12 weeks group (n = 60) | Response-tailored group (n = 60) | Z | P (2-tail) |
|---|---|---|---|---|---|
| Frequency/% | Frequency/% | ||||
| Gastrointestinal disorders | Abdominal pain | 6 (10%) | 5 (8.33%) | 0.316 | 0.752 |
| Nausea | 7 (11.67%) | 5 (8.33%) | 0.609 | 0.543 | |
| General disorders and administration site conditions | Fatigue | 10 (16.67%) | 8 (13.33%) | 0.511 | 0.609 |
| Musculoskeletal and connective tissue disorders | Joint pain | 0 | 2 (3.33%) | 1.4 | 0.154 |
| Nervous system disorders | Headache | 12 (20%) | 10 (16.67%) | 0.472 | 0.637 |
| Skin and subcutaneous tissue disorders | Pruritus | 9 (15%) | 6 (10%) | 0.828 | 0.408 |
| Total adverse events | 44 (73.3%) | 36 (60%) | 1.549 | 0.121 | |
| Total Serious event/death | 0 | 0 | |||
| Total Severe (≥ grade 3) | 0 | 0 |
Data are n (%) in ITT patients.
4. Discussion
To our knowledge, this is the first study to address the evaluation of a shortened duration of 8 weeks for a dual sofosbuvir plus daclatasvir therapy based on an on-treatment qualifier.
The SVR12 rate in the group of patients that were randomly assigned to treatment duration tailored according to achievement of vRVR was non-inferior to that of the fixed 12 weeks duration which is the duration recommended in guidelines so far.
The European Association for the Study of the Liver (EASL) latest 2016 Guidelines on treatment of hepatitis C, allowed for shortening the course of treatment for genotype 1 infected patients with Sofosbuvir/Ledipasvir combination to 8 weeks in treatment-naive patients without cirrhosis if their baseline HCV RNA level is below 6 million IU/ml (EASL, 2017).
This recommendation was based on a post-hoc analysis of ION-3 clinical study results which demonstrated that 8 weeks of treatment yielded an SVR12 rate (sustained virologic response rate measured at 12 weeks after end of treatment) of 97% (119/123) in treatment-naïve patients without cirrhosis when baseline serum virus load was < 6 million IU/ml (Kowdley et al., 2014, Curry et al., 2016).
In this study, 100% of those who had achieved vRVR and completed the full protocol finally achieved SVR12. Because all investigators belong to the same medical school that values the role of psychological and spiritual support as factors affecting the immune system; all patients in both groups were treated the same with full psychological and spiritual support throughout the study visits with assurance, explanation and positive suggestions. In addition to this, perceiving the result of treatment with negative or very marked drop in the virus load as early as only 2 weeks of therapy, in our experience, may have a positive impact on our patients' spirits, immune responses, compliance and final results.
Our results agree with the strategy of shortening the duration of dual NS5B/NS5A combined therapy to 8 weeks, but based on an on-treatment response predictor rather than a baseline variable. This supports the findings of many other studies for the validity of early on-treatment response kinetics as a powerful positive predictor for the sustained virologic response to different combinations of DAAs therapy (Sulkowski et al., 2016, Maasoumy et al., 2016, D'Offizi et al., 2017).
To our mind this patient-centered approach is more prudent as it objectively consider an assuring response to therapy before taking the decision.
Shortening the duration of therapy based on witnessed very rapid virologic response could provide a prudent basis to avoid unnecessary long treatment courses. This could not only reduce the drug exposure and the risk of adverse drug reactions, but also cut the cost of full treatment course with such expensive medications by one third.
We acknowledge the limitation of a small sample size based on rather a large non-inferiority margin of 0.1. With this margin we tolerated 10% lower SVR12 rate to gain almost over 30% reductions in the cost and the exposure to the drugs in addition to economize our overall budget of the study which is an important research concern in our community.
We encourage investigators to replicate this study model with larger sample size and smaller non-inferiority margins in a way for establishing more personalized therapy that consider the variability of response at a patient level rather than the mean or other points of estimate at a population level.
Role of the Funding Source
Abbass Helmy Charity and Pharco shared in funding the study, they contributed to study design, participated in the collection, analysis, and interpretation of data, and in preparation and approval of this report. All authors had access to all relevant study data, reviewed and approved the final report, and take full responsibility for the accuracy of the data and statistical analysis. The corresponding author had full access to all relevant study data and had final responsibility for the decision to submit for publication.
Contributors
Yakoot M, Abdo AM, Abdel-Rehim S & Helmy S conceived and designed the study. Yakoot M, Abdel-Rehim S and Abdo AM recruited the participants and collected the clinical data. Yakoot M prepared the first draft of the paper. All authors reviewed the paper, and approved the submitted version.
Declaration of Interests
AA has been a clinical investigator for Pharco; SH works and holds stock in Pharco; SA has nothing to declare; MY has been a clinical investigator for Pharco.
Acknowledgments
We thank Abbass Helmy Charity establishment and Pharco for their sincere help and support for the study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.05.011.
Appendix A. Supplementary data
Primer information, supporting materials, and mass spectormetry data.
References
- Alavian S.M., Rezaee-Zavareh M.S. Daclatasvir-based treatment regimens for hepatitis C virus infection: a systematic review and meta-analysis. Hepat. Mon. 2016;16(9):e41077. doi: 10.5812/hepatmon.41077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry M., Modi A.A., Pungpapong S., Leise M., Aqel B., Llewellyn J. Realworld effectiveness of ledipasvir/sofosbuvir (LDV/SOF) in treatment-experienced cirrhotic genotype 1 patients with chronic hepatitis C: a comparative analysis of Gilead sponsored trials with 4 real-world cohorts. J. Hepatol. 2016;64:S797. [Google Scholar]
- Di Maio V.C., Cento V., Lenci I. HCV Italian resistance network study group. Multiclass HCV resistance to direct-acting antiviral failure in real-life patients advocates for tailored second-line therapies. Liver Int. 2017 doi: 10.1111/liv.13327. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- D'Offizi G., Cammà C., Taibi C. Clinical and virological predictors of sustained response with an interferon-based simeprevir regimen for patients with chronic genotype 1 hepatitis C virus infection. New Microbiol. 2017;40(1):19–26. [PubMed] [Google Scholar]
- European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2016. J. Hepatol. 2017;66(1):153–194. doi: 10.1016/j.jhep.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Kowdley K.V., Gordon S.C., Reddy K.R., Rossaro L., Bernstein D.E., Lawitz E. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- Llaneras J., Riveiro-Barciela M., Buti M., Esteban R. Hepatitis C virus genotype 4: genotype 1's little brother. J. Viral Hepat. 2017;24(1):4–12. doi: 10.1111/jvh.12620. [DOI] [PubMed] [Google Scholar]
- Maasoumy B., Vermehren J., Welker M.W. Clinical value of on-treatment HCV RNA levels during different approved sofosbuvir-based antiviral regimens. J. Hepatol. 2016;65(3):473–482. doi: 10.1016/j.jhep.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Pineda J.A., Morano-Amado L.E., Granados R. Grupo de Estudio de Hepatitis Vírica.; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica: GEHEP-SEIMC and Grupo de Estudio de Hepatitis Vírica.; Sociedad Andaluza de Enfermedades Infecciosas y Microbiología Clínica: HEPAVIR/Red de Investigación en SIDA (RIS-HEP07): Week 4-response predicts sustained virologic response to all-oral direct-acting antiviral-based therapy in cirrhotic patients with hepatitis C virus genotype 3 infection. Clin. Microbiol. Infect. 2017:30005–30008. pii: S1198-743X (17) [Google Scholar]
- Rockstroh J.K., Ingiliz P., Petersen J. Daclatasvir plus sofosbuvir, with or without ribavirin, in real-world patients with HIV-HCV coinfection and advanced liver disease. Antivir. Ther. 2016 doi: 10.3851/IMP3108. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Sulkowski M.S., Flamm S., Kayali Z. Short-duration treatment for chronic hepatitis C virus with daclatasvir, asunaprevir, beclabuvir, and sofosbuvir (FOURward study) Liver Int. 2016 doi: 10.1111/liv.13335. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Yakoot M., Abdo A.M., Yousry A., Helmy S. Very rapid virologic response and early HCV- response kinetics, as quick measures to compare efficacy and guide a personalized response-guided therapy. Drug Des. Dev. Ther. 2016;10:2659–2667. doi: 10.2147/DDDT.S111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer information, supporting materials, and mass spectormetry data.