Abstract
HIV-specific broadly neutralizing antibodies (bnAbs) have been isolated from patients with high viremia but also from HIV controllers that repress HIV-1 replication. In these elite controllers (ECs), multiple parameters contribute to viral suppression, including genetic factors and immune responses. Defining the immune correlates associated with the generation of bnAbs may help in designing efficient immunotherapies. In this study, in ECs either positive or negative for the HLA-B*57 protective allele, in treated HIV-infected and HIV-negative individuals, we characterized memory B cell compartments and HIV-specific memory B cells responses using flow cytometry and ELISPOT. ECs preserved their memory B cell compartments and in contrast to treated patients, maintained detectable HIV-specific memory B cell responses. All ECs presented IgG1 + HIV-specific memory B cells but some individuals also preserved IgG2 + or IgG3 + responses. Importantly, we also analyzed the capacity of sera from ECs to neutralize a panel of HIV strains including transmitted/founder virus. 29% and 21% of HLA-B*57 + and HLA-B*57 − ECs, respectively, neutralized at least 40% of the viral strains tested. Remarkably, in HLA-B*57 + ECs the frequency of HIV-Env-specific memory B cells correlated positively with the neutralization breadth suggesting that preservation of HIV-specific memory B cells might contribute to the neutralizing responses in these patients.
Abbreviations: HIV, human immunodeficiency virus; Env, HIV envelope protein; cART, combined antiretroviral therapy; EC, elite controller; IgG, immunoglobulin G; (n)Ab, (neutralizing) antibody; ADCC, antibody-dependent cell-mediated cytotoxicity; CTL, cytotoxic T cell; T/F, transmitted/founder virus; PBMC, peripheral blood mononuclear cells; ASC, antibody secreting cell; AM, activated memory B cells; RM, resting memory B cells; IM, intermediate memory B cells; MZ-like B cells, marginal zone-like B cells; TLM B cells, tissue like memory B cells
Keywords: HIV, Elite controllers, Memory B cells, B cell-ELISPOT, Neutralization, Tier-2 virus, IgG
Highlights
-
•
In contrast to treated HIV-infected patients, elite controllers (ECs) maintain HIV-specific memory B cell responses.
-
•
In HLA-B*57 + ECs, HIV-specific B cell frequency correlates positively with the neutralization breadth of tier-2 HIV strains.
-
•
In HLA-B*57 + and HLA-B*57 − ECs different antibody functions are probably involved in suppressing HIV replication.
A fraction of HIV-1-infected individuals (so-called elite controllers, ECs) naturally control HIV-1 replication maintaining undetectable viral loads. Understanding the mechanisms implicated in natural control of HIV-1 infection will help in developing efficient HIV vaccines. In ECs, we analyzed the influence of B cell antibody responses. We show that in contrast to successfully treated HIV-1-infected patients, ECs preserve memory B cell compartments and maintain HIV-specific B cell responses. In ECs positive for the protective HLA-B*57 allele, HIV-specific memory B cell responses are positively associated with the breadth of HIV neutralization. These findings will help develop novel immunotherapies to fight HIV.
1. Introduction
HIV-1 (HIV) infection alters B cell differentiation resulting in spontaneous immunoglobulin secretion, hypergammaglobulinemia (Lane et al., 1983) and decrease in memory B cell frequencies (Moir et al., 2008, Hu et al., 2015, Buckner et al., 2013). HIV-specific antibodies (Abs), with the capacity to neutralize the autologous virus, appear several months after infection. However, these Abs poorly neutralize heterologous HIV strains (Tomaras et al., 2008, Moog et al., 1997, Wei et al., 2003, Deeks et al., 2006, Richman et al., 2003, Gray et al., 2007). Cross-reactive neutralizing Abs, are produced only 2 to 4 years after seroconversion (Gray et al., 2011, Mikell et al., 2011, Richman et al., 2003) and at low titers in most individuals (Hraber et al., 2014). Only 20% of patients harbor high titers of cross-reactive neutralizing Abs (Doria-Rose et al., 2010). Among them, 1% were identified as elite neutralizers based on the capacity of their plasma to neutralize, across clades, a large panel of HIV strains (Li et al., 2007, Simek et al., 2009). Broadly neutralizing monoclonal Abs (bnAbs) were cloned from HIV-specific memory B cells isolated from these patients (Scheid et al., 2009, Mouquet, 2014, Sok and Burton, 2016). Understanding how these bnAbs are generated in HIV-infected individuals could lead the path to the development of an antibody-based vaccine. In viremic patients, the breadth of neutralization has been associated with higher viral loads (Doria-Rose et al., 2010, Piantadosi et al., 2009, Deeks et al., 2006, Sajadi et al., 2011, Doria-Rose et al., 2009, Sather et al., 2009, Rodriguez et al., 2007), duration of viral exposure and viral diversity (Rusert et al., 2016).
HIV-infected individuals who naturally control HIV infection without combined antiretroviral therapy (cART) (Saez-Cirion and Pancino, 2013), in particular elite controllers (ECs, < 1% of HIV-infected individuals) who maintain very low to undetectable viremia (Lambotte and Delfraissy, 2005, Grabar et al., 2009) represent a unique chance to study immune responses potentially involved in viral suppression (Walker and Yu, 2013). A fraction of ECs exhibit potent cytotoxic CD8 + T cell responses against HIV-infected cells (Sáez-Cirión et al., 2007, Betts et al., 2006, Hersperger et al., 2011), often associated with the expression of the HLA-B*57 allele (Migueles et al., 2000, Lambotte and Delfraissy, 2005, Betts et al., 2006). HIV-specific CD4 + T cells of ECs express high avidity T cell receptors (TCRs) suggesting that T cell helper responses contribute to HIV-control (Benati et al., 2016). In contrast, several studies have shown that ECs present lower cross-neutralizing Ab responses as compared to viremic individuals (Lambotte et al., 2009, Pereyra et al., 2008, Bailey et al., 2006, Sajadi et al., 2011). However, among ECs, there is a marked heterogeneity, some presenting broad cross-neutralizing capacities while others show minimal or no neutralization (Lambotte et al., 2009, Scheid et al., 2009, Pereyra et al., 2008, Bailey et al., 2006, Sajadi et al., 2011). Non-neutralizing Ab responses might also exert significant antiviral activities (Chung et al., 2015). In particular, titers of Abs executing antibody-dependent cell-mediated cytotoxicity (ADCC) have been shown to be higher in ECs (Lambotte et al., 2009) and predominant in HLA-B*57 − ECs as compared to HLA-B57 + ECs (Lambotte et al., 2013). More recently, HIV-control has been linked to the capacity of the sera from ECs to perform multiple effector functions (Ackerman et al., 2016). Indeed, depending on their isotype, Abs exhibit different effector functions such as Fcγ receptors (FcγR) binding, initiation of ADCC and activation of the complement cascades. Although the immunoglobulin G1 (IgG1) subclass dominates HIV-specific responses, the proportion of IgG isotypes might vary depending on individuals, the HLA status and the clinical parameters (Binley et al., 2008, Banerjee et al., 2010, Ackerman et al., 2016, French et al., 2013). Ackerman et al. showed that the sera from ECs exhibiting strong polyfunctional antiviral activities are enriched in Abs of IgG1 and IgG3 subclasses (Ackerman et al., 2016). IgG2 Abs to the HIV Gag protein have been associated with long-term nonprogression (Martinez et al., 2005, Ngo-Giang-Huong et al., 2001) and seem more abundant in HLA-B*57 − ECs compared to HLA-B*57 + ECs (French et al., 2013). Therefore, the quality of Ab responses, defined by the diversity of Ab subclasses should be considered when characterizing Ab responses elicited by HIV infection and vaccine candidates.
A successful vaccine should lead to the generation of long-lived plasma cells and memory B cells that are thought to be essential for sustained humoral immunity. However, during chronic infection, the persistence of viral antigens alters B cell differentiation into memory cells (Colineau et al., 2015). Memory B cells can be divided in 4 subpopulations: activated memory (AM, CD27 + CD21 −), resting memory (RM, CD27 + CD21 +), intermediate memory (IM, CD27 −CD21 +) and tissue-like memory B cells (TLM, CD27 −CD21 −) (Moir et al., 2008). AM and TLM B cells (the latter corresponding to anergic cells) are overrepresented in untreated HIV-infected patients (Moir et al., 2008, Pensieroso et al., 2013) and are associated with higher levels of viremia (Kardava et al., 2014). In contrast, RM cells that contribute to maintaining humoral responses, are decreased upon infection (Good et al., 2009, Moir and Fauci, 2013). Remarkably, HIV-specific B cells are enriched in TLM and AM B cell subsets but decreased in RM cells (Kardava et al., 2014). Compared to the levels in HIV-negative individuals, cART restores TLM and AM B cell proportions and only partially the RM compartment (Moir et al., 2008, Moir et al., 2010, Pensieroso et al., 2013). Although ECs do not clear the infection, studying their memory B cell responses could help understand the maintenance of long lasting humoral immunity in the presence of low to undetectable antigen loads. In ECs, RM and AM B cell proportions are higher compared to treated HIV-infected patients but no differences were observed concerning the percentage of TLM cells (Pensieroso et al., 2013). However, in ECs, the frequency of HIV-specific TLM B cells is reduced compared to treated HIV-infected patients (Buckner et al., 2016). Taken together, these results suggest that ECs preserve their memory B cell compartments but also exhibit features of viremic individuals (increased AM cells). Whether this preservation of the memory B cell subsets, in ECs, is associated with the maintenance and/or a higher frequency of HIV-specific memory B cells expressing various Ab subclasses remains an open question. In addition, potential correlations between HIV-specific memory B cell frequencies and the neutralization breadth in sera have not been investigated so far. Two studies previously analyzed potential correlations between Ab responses and Ab secreting cells (Bussmann et al., 2010, Doria-Rose et al., 2009). Bussman et al. asked whether the frequency of Env/gp120-specific B cells might correlate with the Ab titers to Env/gp120 protein (Bussmann et al., 2010) while Doria-Rose et al. studied the frequency of plasmablasts that spontaneously secret HIV-specific Abs and the breadth of neutralization (Doria-Rose et al., 2009). Both studies failed to observe any correlation between these HIV-specific B cell responses and the Ab profiles.
In the present study, we characterized memory B cell responses in a cohort of ECs either positive or negative for the HLA-B*57 protective allele. We analyzed whether the preservation of B cell compartments might be linked to the capacity of B cells to secrete HIV-specific Abs. We compared B cell responses in HLA-B*57 + and HLA-B*57 − ECs with either that of aviremic patients undergoing successful cART or HIV-negative individuals. We observed a global preservation of memory B cell compartments in ECs with a proportion of TLM comparable to what was observed in cART and HIV-negative individuals. Interestingly, HIV-specific B cells were detected in 82% of ECs. In contrast, only 7% of cART patients presented HIV-specific responses whereas all groups exhibited similar levels of Influenza-specific B cell responses. HIV-specific responses consisted mainly of IgG1 secreting B cells although HIV-specific IgG2 and IgG3 secreting B cells were detected in a third of ECs. Next we analyzed whether these B cell responses might correlate with the capacity of patients' sera to neutralize HIV. For this purpose, we used mostly difficult-to-neutralize tier-2 transmitted/founder (T/F) viruses. 89% of sera from ECs neutralized at least one HIV strain tested and 8% blocked infection of at least 40% of difficult-to-neutralize tier-2 T/F viruses. Remarkably, among HLA-B*57 + ECs, the frequency of Env-specific memory B cells correlated positively with the capacity to neutralize T/F HIV strains, suggesting that these cells might contribute to the neutralizing responses in this group of ECs. Overall, through the analysis of Env-specific memory B cell frequencies, the isotype diversity and the neutralization breadth, our results reveal major differences between HLA-B*57 + and HLA-B*57 − ECs.
2. Materials and Methods
2.1. Patients and Samples
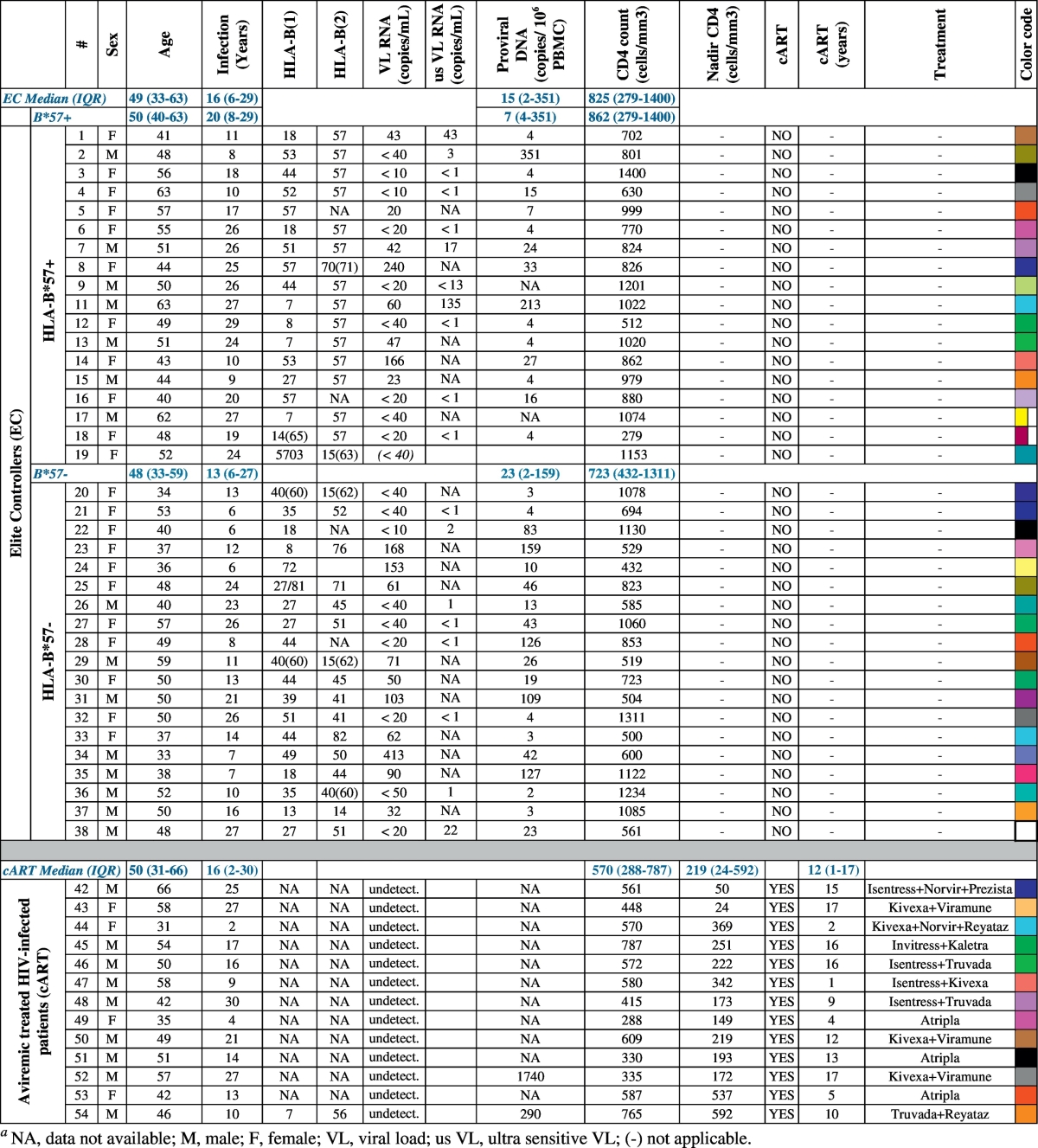
EC (n = 37) were recruited from the CO21 CODEX cohort implemented by the ANRS (Agence nationale de recherches sur le SIDA et les hépatites virales). Whole blood and PBMC were cryopreserved at enrolment. Ten million PBMCs from ECs were available for this study. ECs were defined as HIV-infected individuals maintaining viral loads (VL) under 400 copies of HIV RNA/mL without cART for > 5 years. ECs were divided in 2 groups: HLA-B*57 + (n = 18) or HLA-B*57 − (n = 19). HIV-infected efficiently treated patients (cART) (n = 13) were recruited at Kremlin Bicêtre Hospital. They were treated for at least 1 year (mean of 10 years) and had an undetectable viral load using standard assays. HIV-negative individuals (n = 12) were anonymous blood donors (Établissement Français du sang). A detailed description of the patients is provided in Table 1, including the median and interquartile range for age (at the time of the study), CD4 T cell count and RNA load for each group. HIV-RNA loads were measured on site with different real-time PCR-based assays; depending on the date of enrolment in the cohort and the assay routinely used on each site, the VL detection limit varied from 50 to 10 copies/mL. To better quantify low levels of viral replication, VL were also determined using an ultrasensitive, real-time PCR technique (GENERIC HIV, Biocentric, Bandol, France) with a threshold ranging from 1 to 13 copies/mL, depending on the available plasma volume. Total cells associated HIV-1 DNA levels were quantified using the real time PCR GENERIC HIV-DNA assay (Biocentric, Bandol, France) (Avettand-Fenoel et al., 2009).
Table 1.
Clinical and epidemiological characteristics of the study groups.
2.2. Ethic Statement
All the subjects provided their written informed consent to participate in the study. The CO21 CODEX cohort and this sub-study were funded and sponsored by ANRS and approved by the Ile de France VII Ethics Committee. The study was conducted according to the principles expressed in the Declaration of Helsinki.
2.3. Flow Cytometry
Cell viability was evaluated using LIVE/DEAD® (ThermoFisher Scientific) and the following Abs were used: CD19-APCCy7 (SJ25C1), CD21-APC (B-ly4), CD27-PE (M-T271), IgD-PECF594 (IA6-2), IgG-BV605 (G18-145), CD38-V450 (HB7) (all from BD biosciences), IgM-AF700 (CH2, Exbio) and IgG2-AF488 (HP6002, Southern Biotech). Staining assays were performed using standard procedures in PBS containing 0.5% BSA and 2 mM EDTA (20 min at 4 °C). Samples were processed on a Fortessa cytometer using FACSDiva software (BD Biosciences) and further analyzed using FlowJo2 software (Tree Star).
2.4. Differentiation of B cells into Antibody Secreting Cells
PBMCs were thawed and cultured in Yssel medium supplemented with 1% human AB serum (Institut Jacque Boy), 0.5 μg/mL of TLR7/8 ligand (R848, InVivoGen) and 100 U/mL rhIL-2 (Miltenyi Biotec). Cells were cultured at 1 × 106 cells/mL. After 6 days, cells were harvested, the proportion of B cells evaluated using flow cytometry (using anti-CD19 antibody, not shown) and the frequency of HIV-specific B cells evaluated using B cell-ELISPOT assay (Pinna et al., 2009).
2.5. B Cell-ELISPOT Assay
ELISPOT plates (Millipore MSIPN4550) were pre-wet with 35% ethanol (1 min), washed with PBS and coated overnight at 4 °C with 15 μg/mL anti-IgG antibodies (Mabtech, MT91/145) or viral antigens diluted in PBS. HIV antigens included a trimeric cleavage-deficient recombinant glycoprotein from the YU-2 clade B (tier-2) HIV viral strain (gp140Yu2b, originally described in (Yang et al., 2000)) produced in HEK293T-derived cells by transient transfection as previously described (Lorin and Mouquet, 2015), gp41S30 (Licence N°WO2012101509 A2) and gp160THO (92THO23), oligomeric envelope glycoprotein produced from a hybrid HIV env gp120 from CRF01_AE (92TH023) and subtype B (LAI) gp41, which is deleted in the principal immunodominant domain (PID), expressed by vaccinia virus in BHK21 cells (Thongcharoen et al., 2007). HIV antigens were coated at 10 μg/mL. Influenza antigens (5 μg/mL, 2015 VAXIGRIP vaccine, Sanofi Pasteur Msd) and keyhole limpet hemocyanin (KLH, 10 μg/mL, Sigma-Aldrich) were used as positive and negative controls, respectively. Plates were washed with PBS and saturated with RPMI containing 10% FBS. Six days post activation, 1500 to 3000 or 1.5 × 105 to 3 × 105 B cells/well were plated for total IgG or antigen-specific detections, respectively, and incubated overnight at 37 °C in RPMI + 10% FBS. Plates were then washed with PBS + 0.05% Tween-20 prior incubation with biotinylated anti-IgG (1 μg/mL, MT78/145, Mabtech), anti-IgG1 (1 μg/mL, G17-1, BD), anti-IgG2 (0.2 μg/mL, HP6200, Mabtech) or anti-IgG3 (0.2 μg/mL, HP6050, Southern Biotech) Abs (2 h, RT). Elispots were revealed using alkaline-phosphatase coupled streptavidin (0.5 U/mL, Roche Diagnostics, 1 h RT) and 50 μL BCIP/NBT substrate (15 min, Sigma). The reaction was stopped using water. The number of spots was counted using AID reader (Autoimmun Diagnostika GmbH). For each experimental condition, the Elispot was performed mostly in triplicates and at least in duplicates. Frequency of antigen-specific B cells was calculated taking account the number of CD19 + B cells plated.
2.6. HIV-Specific IgG Ab Detection by Elisa
96-well plates were coated overnight with a sheep anti-human IgG (1 μg/mL in carbonate buffer, Binding Site) for the detection of total IgGs, or with gp140Yu2b or gp41S30 (2 μg/mL in carbonate buffer) for the detection of anti-gp140 or anti-gp41 IgGs, respectively. Plates were washed and saturated with PBS containing 5% BSA and incubated with diluted sera (2 h, at 37 °C). Plates were then washed and a secondary goat anti-human IgG-HRP (HorseRadish Peroxidase) added (1 h at 37 °C, 0.2 μg/mL in PBS, Southern Biotech). Finally, TMB (3,3′, 5,5′ TetraMethylBenzidine) substrate was added. After 30 min, the reaction was stopped (using 25 μL of 1 M H2SO4 per well) and the optical density (OD) read at 450 nm (reference 650 nm). The ratios of HIV-specific IgG were calculated as (OD of anti-gp140 or anti-gp41 IgGs × serum dilution) / (OD of total IgG x serum dilution).
2.7. Viruses and TZM-bl Neutralization Assays
For neutralization, pseudoviruses were produced by cotransfecting 293T cells with HIV-1 env expression plasmid SF162.LS, QH0692.42 and YU2 and the env-deficient HIV-1 backbone plasmid (pSG3ΔEnv). Infectious molecular clones: CH058, CH077, CH106, RHPA, THRO4156.18, REJO 4541.67 and TRJO4551.58 were produced by transfection on 293T cells. These different strains were obtained through the NIH AIDS reagent program.
Sera were tested for their ability to neutralize HIV-1 using TZM-bl neutralization as described previously (Li et al., 2005). As negative controls, HIV-negative sera (purchased at Etablissement Français du Sang) were used and the capacity of EC's sera to neutralize MuLV was assessed. One tier-1 reference strains (SF162.LS), eight tier-2 strains (YU2, QH0692.42, CH058, CH077, CH106, RHPA, THRO4156.18 and REJO4541.67) and one tier-3 strain (TRJO4551.58) were used. CH058, CH077, CH106, RHPA, THRO4156.18 and REJO4541.67 and TRJO4551.58 are T/F viruses. The 50% inhibitory reciprocal dilution (IRD50) was defined as the sample reciprocal dilution that caused a 50% reduction in relative luminescence units (RLU) (Li et al., 2005).
2.8. Statistics
Statistical significances (p-values) were determined using a Kruskal-Wallis test or a Mann-Whitney test (*p < 0.05, **p < 0.01, ***p < 0.001) and associations between continuous variables were evaluated using Spearman rank order correlation test using Prism software (GraphPad) and “Visualization of a Correlation Matrix” R package version 0.77 (https://CRAN.R-project.org/package=corrplot).
3. Results
We characterized memory B cell responses and HIV cross-neutralization potential in a cohort of ECs either positive or negative for the HLA-B*57 protective allele. We compared HLA-B*57 + and HLA-B*57 − ECs with aviremic patients undergoing successful cART or HIV-negative individuals (Table 1).
3.1. Memory B Cell Compartments are Preserved in EC
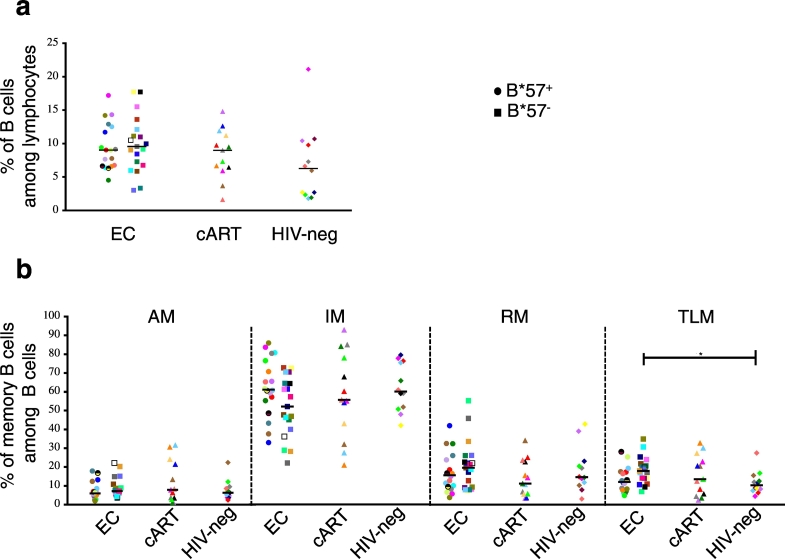
Using flow cytometry, we first analyzed the peripheral memory B cell compartments (as shown in Supplemental Fig. 1). The proportion of CD19 + B cells was slightly higher, although not significantly, in ECs compared to HIV-negative donors (Fig. 1a). ECs, cART and HIV-negative donors presented no significant differences concerning the proportions of AM, IM and RM B cells (Fig. 1b) or other populations studied (total memory B cells, naïve B cells, IgG + and IgG2 + memory B cells, plasmablasts and Marginal Zone-like (MZ-like) B cells (Supplemental Fig. 2). In contrast, a significant increase of TLM B cell proportion was detected in HLA-B*57 − ECs compared to HIV-negative individuals (Fig. 1b, p = 0.0247). Overall, with the exception of the TLM B cells that were slightly expanded in HLA-B57 − ECs, the B cell compartments seemed preserved in ECs.
Fig. 1.
Memory B cell compartments are preserved in ECs.
In PBMC from ECs (n = 37), cART (n = 13) and HIV-negative donors (n = 12): (a) frequency of CD19 + B cells among lymphocytes and (b) frequencies of AM (CD27 + CD21 −), RM (CD27 + CD21 +), IM (CD27 − CD21 +) and TLM (CD27 − CD21 −) B cells among CD19 + B cells. Each individual is represented by a specific dot on each graph (shape and color). Circle: HLA-B*57 + EC; Square: HLA-B*57 − ECs. The statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn's test (*p < 0.05). Bars indicate median values.
3.2. EC Maintain HIV-Specific Memory B Cell Responses with Stronger Proliferative Capacity
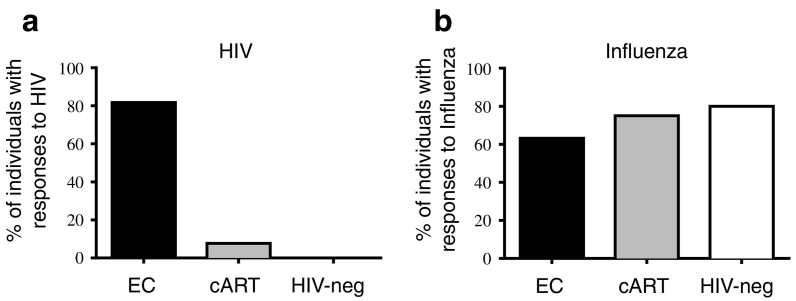
We then evaluated the frequency of HIV-specific memory B cells using ELISPOT (Fig. 2 and Supplemental Fig. 3). The frequencies of B cells secreting total IgG, IgG1 or IgG3 were not significantly different between the 3 groups (Supplemental Fig. 3). In contrast, we noticed a reduced frequency of IgG2 secreting B cells in ECs compared to HIV-negative donors (p = 0.0131, Supplemental Fig. 3c). To detect antigen-specific B cell responses, we used different HIV envelopes (HIV Env): gp140Yu2b, gp41S30, gp160THO and Influenza antigens or KLH as positive and negative controls, respectively. The gp140Yu2b trimers can capture the B-cells and bind to non-neutralizing and cross-neutralizing Abs (Scheid et al., 2009, Mouquet et al., 2011) of all specificities and more importantly, of all types of bNAbs and clonal variants (Scheid et al., 2011, Mouquet et al., 2012), except PG16-like Abs. Gp41S30 is the target of Abs to gp41 such as bNAbs against the MPER (e.g. 2F5, 4E10 and 10E8) or non-NAbs against the PID (e.g. 7B2). Gp160THO was used in the RV144 vaccine trial that showed an estimated 31% vaccine efficacy (Rerks-Ngarm et al., 2009). It allows the capture of Abs directed to gp120 and to gp41 except Abs directed to the PID and PG16-like Abs. As an additional control, the frequency of HIV-specific B cells was evaluated in healthy HIV-negative donors (Fig. 2a). Remarkably, 82% of ECs (27/33 ECs tested) but only 7% of cART patients (1/13) exhibited a positive response against HIV Envs (Fig. 2a). This very low frequency of responders among cART patients was consistent with other studies describing that upon initiation of cART the frequency of HIV-specific B cells drops to low or undetectable levels (Morris et al., 1998, Bussmann et al., 2010, Buckner et al., 2016). This difference is strictly specific to HIV antigens since both groups reacted to Influenza antigens (63% and 75% reacting ECs and cART-patients, respectively) (Fig. 2b). Therefore, although both ECs and cART patients have undetectable viral loads, HIV-specific memory B cells were detected mainly in ECs.
Fig. 2.
ECs maintain HIV-specific memory B cell responses.
Percentage of ECs (n = 33), cART (n = 13) and HIV-negative (n = 6) donors presenting memory B cell responses against (a) at least one HIV antigen (gp140Yu2b, gp41S30 or gp160THO) and (b) Influenza vaccine antigens (2015 VAXIGRIP vaccine). B cell memory responses were evaluated by B cell ELISPOT.
3.3. HIV-Specific Memory B Cell Responses are Mainly of the IgG1 Isotype
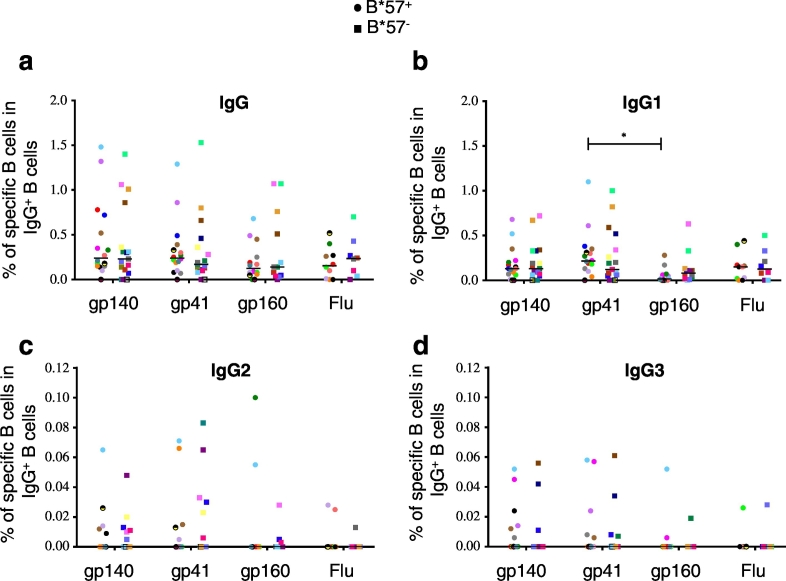
We next analyzed the specificity and isotype of HIV-specific secreted Abs in ECs (Fig. 3). Patients with detectable antibody responses to gp140 also reacted against gp41 and gp160 (Fig. 3A). In ECs, IgG + B cells specific for gp140, gp41 and gp160 represented respectively 0.24, 0.20 and 0.13 mean % of IgG + B cells (Fig. 3A). In ECs, the frequencies of IgG + Influenza-specific and HIV Env-specific B cells were in the same order of magnitude (Fig. 3a). Note that in ECs, cART and HIV-negative donors, we did not observe a significant difference in the frequency of Influenza-specific B cells (Supplemental Fig. 4).
Fig. 3.
In ECs, HIV-specific memory B cell responses are mainly of the IgG1 isotype. Percentage of antibody secreting B cells specific for HIV-Env antigens (gp140Yu2b, gp41S30 or gp160THO) and Influenza vaccine antigen (Flu, 2015 VAXIGRIP vaccine) presented according to the Ab isotype: (a) total IgG +, (b) IgG1 +, (c) IgG2 + and (d) IgG3 + antigen-specific ASC. Each individual is represented by a specific dot on each graph (shape and color). Statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn's test (*p < 0.05). Bars indicate median values.
HIV Env- and Flu-specific B cell responses in ECs were mainly mediated by IgG1 Abs, which represented nearly 50% of responses (Fig. 3b). In addition, all patients reacting to HIV Env antigens exhibited an HIV-specific IgG1 response. In contrast, only 35% and 26% of ECs showed gp140-specific IgG2 + or IgG3 + B cell responses, respectively (Fig. 3c–d). Note that IgG2 + and IgG3 + responses were not necessarily detected in the same patients (Fig. 3c–d).
Finally, whatever the targeted antigens, the proportion of ECs presenting IgG, IgG1, IgG2 and IgG3 Env-specific memory B cell responses and the magnitude of these responses were similar in HLA-B*57 − and HLA-B*57 + ECs (Fig. 3).
3.4. In HLA-B*57 + ECs, the Frequency of Env-Specific Memory B Cells Correlates with the Neutralization Breadth
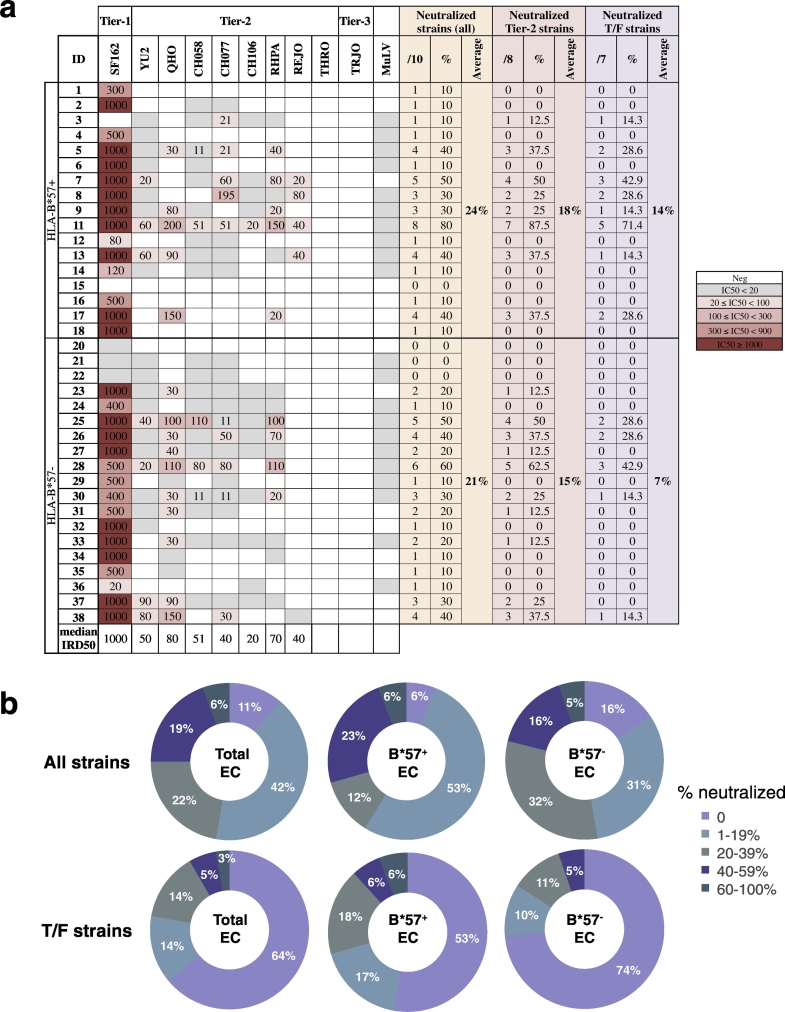
We then asked whether HIV-specific B cell memory responses might be associated with the capacity of ECs to neutralize HIV-1 infection. To this end, we tested the ability of the sera from ECs to neutralize a reference (tier-1) strain, 8 difficult-to-neutralize tier-2 strains (including 7 transmitted founder (T/F) strains) and one highly difficult-to-neutralize tier-3 T/F strain (Fig. 4). Sera from most ECs (87%) neutralized the neutralization-sensitive HIV-1SF162 strain. 25% of sera neutralized at least 4 HIV strains out of the 10 tested (Fig. 4). 48% blocked infection of at least one tier-2 virus. 36% blocked at least one tier-2 T/F virus (Fig. 4b) and 8% blocked at least 40% of tier-2 T/F strains (Fig. 4b). This demonstrates that some ECs effectively neutralize difficult-to-neutralize tier-2 T/F. Interestingly, when examining the neutralizing potential, the sera from HLA-B*57 + ECs had a twofold higher capacity to neutralize T/F strains than the sera from HLA-B*57 - ECs (Fig. 4a, average of 14% T/F neutralized versus 7% for HLA-B*57 + and HLA-B*57 − ECs, respectively), although this difference was not statistically significant (p = 0.19). In addition, 30% of the HLA-B*57 + versus 16% of the HLA-B*57 − ECs' sera neutralized > 20% of the T/F viruses tested (Fig. 4b). This difference was almost exclusively due to the capacity of HLA-B*57 + ECs' sera to neutralize the T/F virus REJO.
Fig. 4.
Capacity of ECs to neutralize tier-2 T/F HIV strains in the TZM-bl assay.
Sera from ECs (n = 36) were tested against one lab and 8 difficult-to-neutralize tier-2 HIV strains, including 7 T/F strains. The capacity to neutralize MuLV was used as a negative control. (A) Neutralization data are shown as the reciprocal serum dilution that neutralized 50% of the infection (IRD50) tested. A color code (right) indicates the potency of neutralization based on IRD50. The gray color indicates a decreased infection (at 1/20 dilution) that did not reach consistently 50% inhibition. An absence of color means that no neutralization was found at the lowest dilution tested (1/20). (B) Neutralization breadth expressed as percentage of neutralized strains for all strains (top panel) and T/F strains (lower panel) in all ECs (Total ECs, left rings), HLA-B*57 + (B*57 + ECs, middle rings) and HLA-B*57 − ECs (B*57 − ECs, right rings). The numbers indicate the % of patients for each fraction.
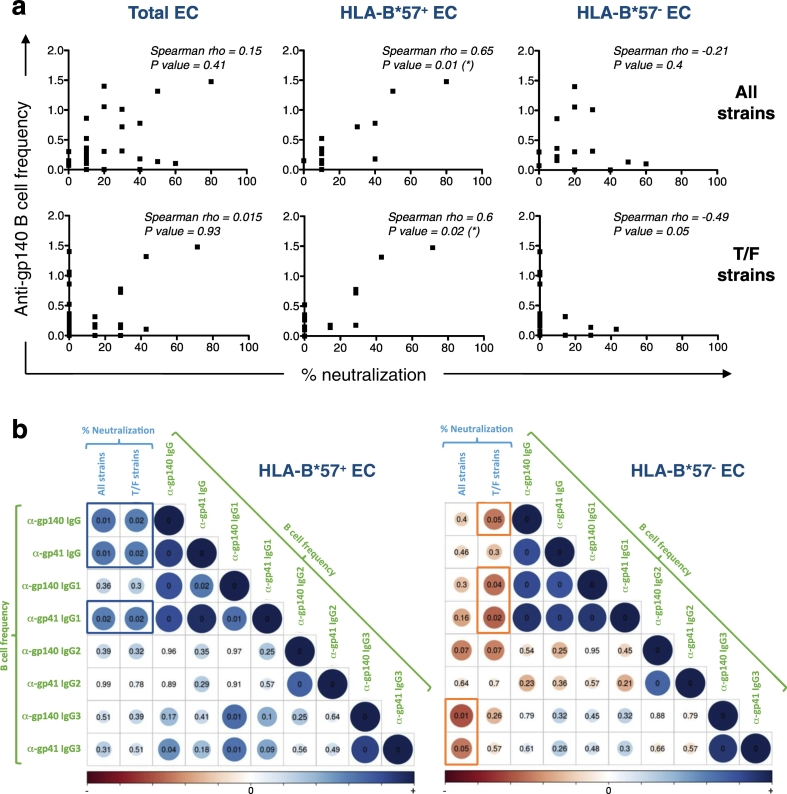
To further analyze Ab responses in ECs, we examined potential correlations between the frequency of Env-specific B cells, evaluated in ELISPOT, and the percentage of neutralized strains for each individual (Fig. 5). In ECs, the capacity to neutralize all HIV strains or T/F viruses was neither associated with the frequency of anti-gp140 nor anti-gp41 secreting B cells (among IgG + B cells), for all IgG subclasses analyzed (Fig. 5a and not shown). However, by separating ECs into HLA-B*57 + and HLA-B*57 − donors, we showed that, among HLA-B*57 + ECs the frequency of gp140- and gp41-specific B cells correlated with the capacity to neutralize all HIV strains (p = 0.01) including T/F viruses (p = 0.02) (Fig. 5a–b, blue square). Dissecting Env-specific isotypic responses in HLA-B*57 + ECs, we observed that gp41-specific IgG1 + B cell frequencies also correlated with the neutralization of all HIV strains (p = 0.02) and T/F strains (p = 0.02) (Fig. 5b, blue rectangle). In addition in these ECs, gp140-specific B cell frequencies correlated with the IRD50 for 6 out of 8 tier-2 T/F neutralized (Fig. 4a and not shown). Interestingly in HLA-B*57 + ECs, Env-specific IgG3 + memory B cell frequencies correlated positively with both total IgG and IgG1 Env-specific responses without being directly associated to the neutralization potential (Fig. 5b).
Fig. 5.
In HLA-B*57 + ECs, the frequency of HIV-specific B cells correlates with the neutralization of tier-2 T/F virus.
(a) Association of HIV gp140-specific B cell frequency (among IgG + B cells) and percent of neutralization for all HIV strains tested (top panel) or T/F HIV strains (bottom panel) for all ECs (Total EC), HLA-B*57 + and HLA-B*57 − ECs. (*) p < 0.05. (b) Spearman correlation matrices between the frequency of gp140- and gp41-specific B cell of various IgG isotypes and the percentage of neutralized strains (All strains) or T/F strains and the frequency of Env-specific B cell of various IgG subtypes. Left panel HLA-B*57 + and right panel HLA-B*57 − ECs. Strength and significance are represented as size and color intensity: blue for positive correlation and red for negative correlation. The numbers are p values.
In contrast, in HLA-B*57 − ECs, the frequency of HIV-specific B cells did not correlate positively with any of the neutralizing parameters analyzed. In fact in HLA-B*57 − ECs, an inverse correlation was observed between the frequency of gp140-specific IgG B cells, or of gp140- and gp41-specific IgG1 B cells and the number of T/F viruses neutralized (Fig. 5b, red rectangles, p < 0.05). A significant inverse correlation was also observed between the frequencies of gp140- and gp41-specific IgG3 + B cells and the number of total viruses neutralized (Fig. 5b, red rectangle, p < 0.05). Note that these opposite correlations observed in HLA-B*57 + and HLA-B*57 − ECs, between HIV-specific memory B cell frequencies and the neutralization potentials, did not mirror the quantity of HIV-specific IgGs or IgG subclasses present in their sera (Supplemental Fig. 6 and not shown). Indeed, using Elisa, we detected similar levels of HIV-specific IgGs in the sera of both groups (Supplemental Fig. 6a). In addition, no correlation was found between the frequency of HIV-specific memory B cells and the quantity of HIV-specific IgGs present in the sera (Supplemental Fig. 6b).
Altogether, these results show that HLA-B*57 + and HLA-B*57 − ECs have similar sustained frequency of HIV-specific B cells responses, similar HIV-specific Abs responses and non-statistically different neutralizing capacities. Despite these findings, the strong correlation observed in HLA-B*57 + ECs between the frequency of Env-specific memory B cells and the neutralizing parameters suggests that the quality of the Env-specific Abs induced differ with that of HLA-B*57 − ECs. In HLA-B*57 + ECs, the maintenance of HIV-specific memory B cells may sustain the production of functionally relevant neutralizing Ab responses.
4. Discussion
In this study, we show that, in contrast to treated patients, ECs naturally preserve their memory B cell compartments and maintain HIV-specific memory B cell responses despite low to undetectable viral loads. HIV-specific B cells mainly express the IgG1 + Ab isotype and some ECs also express anti-HIV IgG2 and IgG3 Abs. The sera from a fraction of ECs exhibit a broad cross-neutralization capacity against difficult-to-neutralize tier-2 T/F viruses. Remarkably, the frequency of Env-specific B cells, in HLA-B*57 + ECs, correlates with a broader cross-neutralizing capacity. Our results suggest that, in HLA-B*57 + ECs, memory B cell responses might contribute to the maintenance of broad neutralization capacities and perhaps to the natural control of HIV infection.
Ex vivo analysis of B cell compartments in ECs, cART and HIV-negative donors showed a global preservation of the B cell subsets. This is in contrast to other studies that have observed a slight decrease of RM B cells in cART patients (Pensieroso et al., 2013, Buckner et al., 2016) compared to HIV-negative donors and an increase of AM B cells in ECs (Pensieroso et al., 2013). However, we noticed a clear increase of TLM B cell proportion in HLA-B*57 − compared to HLA-B*57 + ECs and to HIV-negative donors. This expansion of TLM B cells cannot be explained by a higher residual replication in HLA-B*57 − ECs compared to HLA-B*57 + individuals, as neither the levels of plasma RNA nor cell associated viral DNA were significantly different between the two groups. Unfortunately, owing to the limited amount of cells available for this study, we could not directly analyze the phenotype of HIV-specific B cells using for instance gp140-fluorescent probes and flow cytometry. We analyzed the frequency of HIV Env-specific memory B cell responses using B-cell ELISPOT, that requires fewer cells but whose results correlate with the frequency obtained using fluorescent antigens and flow cytometry (Buckner et al., 2016).
Remarkably, despite very low to undetectable viral loads, the majority of ECs presented Env-specific memory B cell responses. We obtained similar results using HIV Gag p24 as antigens (not shown). In ECs, the magnitude of gp140-specific IgG + memory B cell responses were 0.24%, a result slightly higher than reported by Bussman et al. (0.1% of memory B cell responses specific to gp120) using a cohort of 10 controllers with a median viral load above 400 RNA copy/ml (Bussmann et al., 2010). In contrast, we showed that memory B cells from cART patients, under successful virological control, rarely reacted to HIV antigens. This observation is consistent with other studies describing that upon initiation of cART the frequency of HIV-specific Ab secreting cells is strongly reduced to low or undetectable levels (Morris et al., 1998, Bussmann et al., 2010, Buckner et al., 2016, Fondere et al., 2004). Interestingly, using six ECs, Buckner et al. recently showed that the initiation of cART led to a decrease of HIV-specific memory B frequencies (Buckner et al., 2016). Therefore, although in our study, the frequency of HIV-specific memory B cells in ECs did not correlate with any virological parameters (Table 1), the maintenance of HIV-specific memory B cell responses might be due to a persistent low viral replication in the blood and/or tissues (Hatano et al., 2009, Pereyra et al., 2009).
Several studies have highlighted that ECs present heterogeneous cross-neutralizing Ab responses, with some ECs exhibiting broad cross-neutralizing capacities while others show minimal or no neutralization (Deeks et al., 2006, Scheid et al., 2009, Pereyra et al., 2008, Bailey et al., 2006, Sajadi et al., 2011, Doria-Rose et al., 2009, Braibant et al., 2008). In this work, we also observed some heterogeneity between ECs and we identified 8% of ECs with broader cross-neutralizing activities. This value is slightly lower than the 12% of ECs with broad cross-neutralization responses reported in previous studies (Sajadi et al., 2011, Scheid et al., 2009). As previously observed in ECs (Palmer et al., 2016, Ranasinghe et al., 2015), we did not find a correlation between residual viral loads and cross-neutralization potentials. In two studies on ECs (defined as controllers with viral loads below 2000 RNA copy/ml), the breadth of cross-neutralization was linked to an expansion of terminally differentiated CD57 + CD8 + T cells (Palmer et al., 2016) and an enhancement of HIV-specific CD4 + T cell responses (Ranasinghe et al., 2015). Recently, Martin-Gayo et al. observed in controllers a correlation between the enrichment of CXCR5 + CXCR3 + PD-1low CD4 + Tfh-like cells and the neutralization breadth (Martin-Gayo et al., 2017) while Dugast et al. identified a unique inflammatory profile that might be linked to the evolution of the neutralization breadth (Dugast et al., 2017).
Remarkably, we show here that in HLA-B*57 + ECs, the frequency of Env-specific B cells, correlated positively with the breadth of viruses neutralized. Previous studies analyzed potential correlations between antibody secreting cells and Ab responses (Bussmann et al., 2010, Doria-Rose et al., 2009). We confirmed the findings from Bussman et al. that Env-specific B cell frequencies do not correlate with the Ab titers to Env in the sera of ECs (Bussmann et al., 2010). Doria-Rose et al. did not observe an association between the frequency of plasmablasts spontaneously secreting HIV-specific Abs and the breadth of neutralization (Doria-Rose et al., 2009). In our study, we focused on long-lived memory B cells that provide not only an archive of contemporaneous HIV-specific Ab secreting cells but also of historic of Ab responses to the infection. In HLA-B*57 + ECs, we found a positive correlation between Env-specific memory B cell responses and the neutralizing breadth. However, it is important to note that this association was lost or correlated inversely when considering the global ECs population or HLA-B*57 − ECs, respectively.
Taking this study together with previously published work, we are proposing that in HLA-B*57 + ECs, an early and spontaneous control of viral replication (Goujard et al., 2009), probably mediated by CTL (Sáez-Cirión et al., 2007) and innate components of immunity (Barblu et al., 2012), might favor an early preservation of CD4 + Tfh cells and the establishment of efficient memory B cell responses sustaining the generation of potent antiviral responses including broad cross-neutralization. It is tempting to speculate that as recently observed in a unique HLA-B*57 + individual (Freund et al., 2017), broad neutralization in HLA-B*57 + ECs may also contribute to the control of HIV infection.
Abs can also mediate antiviral functions independently of their ability to neutralize viruses, for instance through the binding of FcR and the initiation of ADCC. Interestingly, when comparing 9 nonneutralizing and 5 broadly neutralizing monoclonal Abs, a recent publication reported that bnAbs, due to their enhanced capacity to recognize HIV-infected cells, mediate more potent ADCC than non-neutralizing Abs (Bruel et al., 2017). However, in sera from patients, neutralization-independent Ab activities have been previously associated with long-term control of HIV infection (Lambotte et al., 2009, Lambotte et al., 2013, Baum et al., 1996). In particular, using HIV-infected cells, Lambotte et al. observed that HLA-B*57 − ECs present significantly higher ADCC Ab titers than HLA-B*57 +. They suggested that ADCC plays a role in the immune control of HIV, especially in HLA-B*57 − ECs (Lambotte et al., 2013). In the present study, in HLA*B57 − ECs, we reveal an inverse correlation between Env-specific memory B cell frequencies and the neutralization breadth. Therefore in both group of ECs, whether HIV-specific memory B cell frequency might be associated with the potency of non-neutralizing antiviral Ab functions, such as ADCC of infected cells, needs to be further addressed. In a study, using the sera of 9 controllers and 11 progressors, Smalls-Mantey et al. analyzed potential correlations between ADCC of infected cells and various parameters including neutralization (Smalls-Mantey et al., 2012). They observed similar ADCC Ab titers in both groups and did not find a correlation between ADCC and neutralization activities (Smalls-Mantey et al., 2012). However, the controler cohort was limited in size and they did not compare the ADCC and neutralization profiles based on the HLA-B genotype (Smalls-Mantey et al., 2012).
Ackerman et al. proposed that the capacity to exert multiple antiviral functions might be linked to HIV-control (Ackerman et al., 2016). Indeed, depending on their isotype, Abs exert different antiviral functions. For instance, in contrast to IgG2 and IgG4, IgG1 and IgG3 bind strongly to FcR on phagocytic cells inducing efficient effector activity. Ackerman et al. showed that the sera from ECs exhibiting strong polyfunctional antiviral activities are enriched in Abs of IgG1 and IgG3 subtypes (Ackerman et al., 2016). In contrast, in viremic controllers, anti-HIV IgG2 production has been previously associated with HIV-control and slow progression (Martinez et al., 2005, Ngo-Giang-Huong et al., 2001). In this work, we show that in ECs, Env-specific memory B cell responses were mainly composed of IgG1 Abs. Only few ECs presented IgG2 + or IgG3 + responses. Interestingly in HLA-B*57 + ECs, Env-specific IgG3 + memory B cell frequencies correlated positively with both total IgG and IgG1 Env-specific responses. In contrast, in HLA-B*57 − ECs, Env-specific IgG3 + memory B cell frequencies were negatively associated with the neutralization breadth of HIV. These observations suggest that depending on the HLA genotype (e.g. HLA-B*57 +/−) different Ab isotypes and/or functions might be involved in immune control of HIV infection.
To summarize, our work highlights the facts that ECs maintain HIV-specific memory B cell responses associated to effective antiviral humoral activities and that Env-specific memory B cell responses are positively associated with the neutralization breadth in HLA-B*57 + ECs. We propose that promoting HIV-specific B cell polyfunctional responses by therapeutic vaccination might be highly beneficial in cART treated patients.
The following are the supplementary data related to this article.
Phenotypic analysis of circulating B cell populations using flow cytometry. B cells were identified using CD19, and further subdivided in naïve (CD27–IgD+), total memory B cells (CD27+ IgD–), activated memory (AM, CD27+ CD21 −), resting memory (RM, CD27+ CD21+), intermediate memory (IM, CD27− CD21+), tissue-like memory (TLM, CD27 − CD21 −), plasmablasts (CD27+ IgD− CD38hig) and marginal zone-like B cells (MZ-B cells, CD27+ IgD− IgM+ CD38− CD21+). IgG+ and IgG2+ memory B cells were also identified.
Proportions of memory B cell populations in HIV+ patients and HIV − donors. (a) Frequency of total memory B cells (CD27+ IgD −) among CD19+ B cells. (b) Frequency of naïve B cells (CD27 − IgD+) among CD19+ B cells. (c) Frequency of IgG + cells among memory B cells. (d) Frequency of IgG2+ cells among memory B cells. (e) Frequency of CD38hi plasmablasts among memory B cells. (f) Frequency of IgM+ CD38 − CD21+ MZ-like B cells among memory B cells. Each individual is represented by a specific dot on each graph (shape and color). Statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn’s test. Bars indicate median values; HIV-neg: HIV-negative donors.
Distribution and frequency of B cells according to their Ab isotype in HIV+ and HIV − donors. Frequencies of B cells secreting IgG (a), IgG1 (b), IgG2 (c) or IgG3 (d) among total B cells in EC (circle: HLA-B*57+ or square: HLA-B*57 −), cART and HIV-negative donors. Each individual is represented by a specific dot on each graph (shape and color). Statistical significance were calculated using a Kruskal-Wallis test followed by a Dunn’s test (*P < 0.05). Bars indicate median values.
Memory B cell responses against Influenza vaccine antigens. Frequency of Flu-specific IgG+ B cells in EC, cART and HIV-negative donors. Each individual is represented by a specific dot on each graph (shape and color). Circle: HLA-B*57+ EC; square: HLA-B*57 − EC. Statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn’s test. Bars indicate median values.
Proportion of HLA-B57+ and HLA-B57 − EC responding to viral antigens. HLA-B57+ EC (n = 16) are represented in green and HLA-B*57 − (n = 17) in orange. Proportions of patient presenting (a) IgG +, (b) IgG1 +, (c) IgG2 + and (d) IgG3 + B cell responses against HIV-Env antigens (gp140Yu2b, gp41S30 or gp160THO) and Influenza vaccine antigens (Flu, VAXIGRIP vaccine).
The quantity of HIV-specific IgG in patient’s sera does not correlate with HIV-specific B cell frequency. (a) Quantity of HIV-specific IgG normalized to total IgG, evaluated using gp41 and gp140 HIV antigens, in sera from HLA-B*57 + (yellow circles) and HLA-B*57 − (pink squares) EC. Bars indicate median values. (b) Spearman correlation between the anti-gp140 B cell frequency (among IgG + B cells) and the ratio of anti-gp140 IgG Abs to total IgG Abs for all EC (left panel), HLAB*57 + EC (middle panel) and HLA-B*57 − EC (right panel).
Funding Sources
This work was granted by the ANRS (Agence nationale de recherches sur le SIDA et les hépatites virales). We thank the Dormeur Foundation, Vaduz, for providing AID ELISPOT Reader. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization: CR OL BA CM AM.
Funding acquisition: CR OL BA CM AM.
Investigation: AR JK BS SE CR AS GL VAF FB CR OL BA SGD CM AM.
Methodology: AR JK BS SE CR AS GL VAF FB LH HM CR OL BA SGD CM AM.
Project administration: FB CR OL BA SGD CM AM.
Resources: FB LH HM CR OL BA CM AM.
Software: AR JK NP CM AM.
Supervision: CM SGD AM.
Validation: CM AM.
Writing – original draft: AR AM.
Making of figures: AR JK CM AM.
Writing – review & editing: AR JK BS CR AS VAF FB LH HM CR OL BA SGF CM AM.
Acknowledgments
We thank Camille Lécuroux and Francoise Churaqui for help and assistance. We thank all participants of the ANRS CODEX cohort. We thank Mia Rozenbaum for English editing.
Contributor Information
Christiane Moog, Email: c.moog@unistra.fr.
Arnaud Moris, Email: arnaud.moris@upmc.fr.
References
- Ackerman M.E., Mikhailova A., Brown E.P., Dowell K.G., Walker B.D., Bailey-Kellogg C., Suscovich T.J., Alter G. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avettand-Fenoel V., Chaix M.L., Blanche S., Burgard M., Floch C., Toure K., Allemon M.C., Warszawski J., Rouzioux C., French Pediatric Cohort Study, A.-C. O. G LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01) J. Med. Virol. 2009;81:217–223. doi: 10.1002/jmv.21390. [DOI] [PubMed] [Google Scholar]
- Bailey J.R., Lassen K.G., Yang H.C., Quinn T.C., Ray S.C., Blankson J.N., Siliciano R.F. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee K., Klasse P.J., Sanders R.W., Pereyra F., Michael E., Lu M., Walker B.D., Moore J.P. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res. Hum. Retrovir. 2010;26:445–458. doi: 10.1089/aid.2009.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barblu L., Machmach K., Gras C., Delfraissy J.F., Boufassa F., LEAL M., Ruiz-Mateos E., Lambotte O., Herbeuval J.P., Group, A. E. H. C. S Plasmacytoid dendritic cells (pDCs) from HIV controllers produce interferon-alpha and differentiate into functional killer pDCs under HIV activation. J. Infect. Dis. 2012;206:790–801. doi: 10.1093/infdis/jis384. [DOI] [PubMed] [Google Scholar]
- Baum L.L., Cassutt K.J., Knigge K., Khattri R., Margolick J., Rinaldo C., Kleeberger C.A., Nishanian P., Henrard D.R., Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J. Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- Benati D., Galperin M., Lambotte O., Gras S., Lim A., Mukhopadhyay M., Nouel A., Campbell K.A., Lemercier B., Claireaux M., Hendou S., Lechat P., de Truchis P., Boufassa F., Rossjohn J., Delfraissy J.F., Arenzana-SEISDEDOS F., Chakrabarti L.A. Public T cell receptors confer high-avidity CD4 responses to HIV controllers. J. Clin. Invest. 2016;126:2093–2108. doi: 10.1172/JCI83792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts M.R., Nason M.C., West S.M., de Rosa S.C., Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., Roederer M., Koup R.A. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8 + T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binley J.M., Lybarger E.A., Crooks E.T., Seaman M.S., Gray E., Davis K.L., Decker J.M., Wycuff D., Harris L., Hawkins N., Wood B., Nathe C., richman D., Tomaras G.D., Bibollet-Ruche F., Robinson J.E., Morris L., Shaw G.M., Montefiori D.C., Mascola J.R. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braibant M., Agut H., Rouzioux C., Costagliola D., Autran B., Barin F. Characteristics of the env genes of HIV type 1 quasispecies in long-term nonprogressors with broadly neutralizing antibodies. J. Acquir. Immune Defic. Syndr. (1999) 2008;47:274–284. doi: 10.1097/QAI.0b013e318162cac2. [DOI] [PubMed] [Google Scholar]
- Bruel T., Guivel-Benhassine F., Lorin V., Lortat-Jacob H., Baleux F., Bourdic K., Noel N., Lambotte O., Mouquet H., Schwartz O. Lack of ADCC breadth of human nonneutralizing anti-HIV-1 antibodies. J. Virol. 2017;91 doi: 10.1128/JVI.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner C.M., Kardava L., Zhang X., Gittens K., Justement J.S., Kovacs C., Mcdermott A.B., Li Y., Sajadi M.M., Chun T.-W., Fauci A.S., Moir S. Maintenance of HIV-specific memory B-cell responses in elite controllers despite low viral burden. J. Infect. Dis. 2016;214(3):390–398. doi: 10.1093/infdis/jiw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner C.M., Moir S., Ho J., Wang W., Posada J.G., Kardava L., Funk E.K., Nelson A.K., Li Y., Chun T.-W., Fauci A.S. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J. Virol. 2013;87:5800–5811. doi: 10.1128/JVI.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann B.M., Reiche S., Bieniek B., Krznaric I., Ackermann F., Jassoy C. Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of the humoral immune response against HIV. Virology. 2010;397:7–13. doi: 10.1016/j.virol.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Chung A.W., Kumar M.P., Arnold K.B., Yu W.H., Schoen M.K., Dunphy L.J., Suscovich T.J., Frahm N., Linde C., Mahan A.E., Hoffner M., Streeck H., Ackerman M.E., Mcelrath M.J., Schuitemaker H., Pau M.G., Baden L.R., Kim J.H., Michael N.L., Barouch D.H., Lauffenburger D.A., Alter G. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163:988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colineau L., Rouers A., Yamamoto T., Xu Y., Urrutia A., Pham H.P., Cardinaud S., Samri A., Dorgham K., Coulon P.G., Cheynier R., Hosmalin A., Oksenhendler E., Six A., Kelleher A.D., Zaunders J., Koup R.A., Autran B., Moris A., Graff-Dubois S. HIV-infected spleens present altered follicular helper T cell (Tfh) subsets and skewed B cell maturation. PLoS One. 2015;10:e0140978. doi: 10.1371/journal.pone.0140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S.G., Schweighardt B., Wrin T., Galovich J., Hoh R., Sinclair E., Hunt P., Mccune J.M., Martin J.N., Petropoulos C.J., Hecht F.M. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 2006;80:6155–6164. doi: 10.1128/JVI.00093-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N.A., Klein R.M., Daniels M.G., O'Dell S., Nason M., Lapedes A., Bhattacharya T., Migueles S.A., Wyatt R.T., Korber B.T., Mascola J.R., Connors M. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose N.A., Klein R.M., Manion M.M., O'Dell S., Phogat A., Chakrabarti B., Hallahan C.W., Migueles S.A., Wrammert J., Ahmed R., Nason M., Wyatt R.T., Mascola J.R., Connors M. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 2009;83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugast A.S., Arnold K., Lofano G., Moore S., Hoffner M., Simek M., Poignard P., Seaman M., Suscovich T.J., Pereyra F., Walker B.D., Lauffenburger D., Kwon D.S., Keele B.F., Alter G. Virus-driven inflammation is associated with the development of bNAbs in spontaneous controllers of HIV. Clin. Infect. Dis. 2017;64:1098–1104. doi: 10.1093/cid/cix057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondere J.-M., Huguet M.-F., Macura-Biegun A., Baillat V., Ohayon V., Reynes J., Vendrell J.-P. Detection and enumeration of circulating HIV-1-specific memory B cells in HIV-1-infected patients. J. Acquir. Immune Defic. Syndr. (1999) 2004;35:114–119. doi: 10.1097/00126334-200402010-00002. [DOI] [PubMed] [Google Scholar]
- French M.A., Center R.J., Wilson K.M., Fleyfel I., Fernandez S., Schorcht A., Stratov I., Kramski M., Kent S.J., Kelleher A.D. Isotype-switched immunoglobulin G antibodies to HIV gag proteins may provide alternative or additional immune responses to 'protective' human leukocyte antigen-B alleles in HIV controllers. AIDS (London, England) 2013;27:519–528. doi: 10.1097/QAD.0b013e32835cb720. [DOI] [PubMed] [Google Scholar]
- Freund N.T., Wang H., Scharf L., Nogueira L., Horwitz J.A., Bar-On Y., Golijanin J., Sievers S.A., Sok D., Cai H., Cesar Lorenzi J.C., Halper-Stromberg A., Toth I., Piechocka-Trocha A., Gristick H.B., van Gils M.J., Sanders R.W., Wang L.X., Seaman M.S., Burton D.R., Gazumyan A., Walker B.D., West A.P., Jr., Bjorkman P.J., Nussenzweig M.C. Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller. Sci. Transl. Med. 2017:9. doi: 10.1126/scitranslmed.aal2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K.L., Avery D.T., Tangye S.G. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J. Immunol. 2009;182:890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- Goujard C., Chaix M.L., Lambotte O., Deveau C., Sinet M., Guergnon J., Courgnaud V., Rouzioux C., Delfraissy J.F., Venet A., Meyer L., Agence Nationale de Recherche Sur Le Sida, P. S. G Spontaneous control of viral replication during primary HIV infection: when is “HIV controller” status established? Clin. Infect. Dis. 2009;49:982–986. doi: 10.1086/605504. [DOI] [PubMed] [Google Scholar]
- Grabar S., Selinger-Leneman H., Abgrall S., Pialoux G., Weiss L., Costagliola D. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS. 2009;23:1163–1169. doi: 10.1097/QAD.0b013e32832b44c8. [DOI] [PubMed] [Google Scholar]
- Gray E.S., Madiga M.C., Hermanus T., Moore P.L., Wibmer C.K., Tumba N.L., Werner L., Mlisana K., Sibeko S., Williamson C., Abdool Karim S.S., Morris L. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4 + T cell decline and high viral load during acute infection. J. Virol. 2011;85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E.S., Moore P.L., Choge I.A., Decker J.M., Bibollet-Ruche F., LI H., Leseka N., Treurnicht F., Mlisana K., Shaw G.M., Karim S.S.A., Williamson C., Morris L. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 2007;81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano H., Delwart E.L., Norris P.J., Lee T.-H., Dunn-Williams J., Hunt P.W., Hoh R., Stramer S.L., Linnen J.M., Mccune J.M., Martin J.N., Busch M.P., Deeks S.G. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J. Virol. 2009;83:329–335. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger A.R., Migueles S.A., Betts M.R., Connors M. Qualitative features of the HIV-specific CD8 + T-cell response associated with immunologic control. Curr. Opin. HIV AIDS. 2011;6:169–173. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraber P., Seaman M.S., Bailer R.T., Mascola J.R., Montefiori D.C., Korber B.T. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Luo Z., Wan Z., Wu H., Li W., Zhang T., Jiang W. HIV-associated memory B cell perturbations. Vaccine. 2015;33:2524–2529. doi: 10.1016/j.vaccine.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardava L., Moir S., Shah N., Wang W., Wilson R., Buckner C.M., Santich B.H., Kim L.J.Y., Spurlin E.E., Nelson A.K., Wheatley A.K., Harvey C.J., Mcdermott A.B., Wucherpfennig K.W., Chun T.-W., Tsang J.S., Li Y., Fauci A.S. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J. Clin. Invest. 2014;124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotte O., Delfraissy J.-F. HIV controllers: a homogeneous group of HIV-1 infected patients with a spontaneous control of viral replication. Clin. Infect. Dis. 2005;41:1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- Lambotte O., Ferrari G., Moog C., Yates N.L., Liao H.-X., Parks R.J., Hicks C.B., Owzar K., Tomaras G.D., Montefiori D.C., Haynes B.F., Delfraissy J.-F. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS (London, England) 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambotte O., Pollara J., Boufassa F., Moog C., Venet A., Haynes B.F., Delfraissy J.F., Saez-Cirion A., Ferrari G. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One. 2013;8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H., Masur H., Edgar L., Whalen G., Rook A., Fauci A. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1983;309:453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Li M., Gao F., Mascola J.R., Stamatatos L., Polonis V.R., Koutsoukos M., Voss G., Goepfert P., Gilbert P., Greene K.M., Bilska M., Kothe D.L., Salazar-Gonzalez J.F., Wei X., Decker J.M., Hahn B.H., Montefiori D.C. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Migueles S.A., Welcher B., Svehla K., Phogat A., Louder M.K., Wu X., Shaw G.M., Connors M., Wyatt R.T., Mascola J.R. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 2007;13:1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorin V., Mouquet H. Efficient generation of human IgA monoclonal antibodies. J. Immunol. Methods. 2015;422:102–110. doi: 10.1016/j.jim.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Martin-Gayo E., Cronin J., Hickman T., Ouyang Z., Lindqvist M., Kolb K.E., Schulze Zur Wiesch J., Cubas R., Porichis F., Shalek A.K., van Lunzen J., Haddad E.K., Walker B.D., Kaufmann D.E., Lichterfeld M., Yu X.G. Circulating CXCR5 + CXCR3 + PD-1lo Tfh-like cells in HIV-1 controllers with neutralizing antibody breadth. JCI Insight. 2017;e89574:2. doi: 10.1172/jci.insight.89574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V., Costagliola D., Bonduelle O., N'Go N., Schnuriger A., Théodorou I., Clauvel J.P., Sicard D., Agut H., Debré P., Rouzioux C., Autran B. Combination of HIV-1- specific CD4 Th1 cell responses and IgG2 antibodies is the best predictor for persistence of long-term non progression. J. Infect. Dis. 2005;191(191):2053–2063. doi: 10.1086/430320. [DOI] [PubMed] [Google Scholar]
- Migueles S.A., Sabbaghian M.S., Shupert W.L., Bettinotti M.P., Marincola F.M., Martino L., Hallahan C.W., Selig S.M., Schwartz D., Sullivan J., Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikell I., Sather D.N., Kalams S.A., Altfeld M., Alter G., Stamatatos L. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 2011;7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S., Buckner C.M., Ho J., Wang W., Chen J., Waldner A.J., Posada J.G., Kardava L., Shea M.A.O., Kottilil S., Chun T.-W., Proschan M.A., Fauci A.S. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116:5571–5579. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S., Fauci A.S. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol. Rev. 2013;254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
- Moir S., Ho J., Malaspina A., Wang W., Dipoto A.C., O'Shea M.A., Roby G., Kottilil S., Arthos J., Proschan M.A., Chun T.-W., Fauci A.S. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog C., Fleury H.J.A., Pellegrin I., Kirn A., Aubertin A.M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L., Binley J.M., Clas B.A., Bonhoeffer S., Astill T.P., Kost R., Hurley A., Cao Y., Markowitz M., Ho D.D., Moore J.P. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H. Antibody B cell responses in HIV-1 infection. Trends Immunol. 2014;35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Mouquet H., Klein F., Scheid J.F., Warncke M., Pietzsch J., Oliveira T.Y., Velinzon K., Seaman M.S., Nussenzweig M.C. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One. 2011;6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet H., Warncke M., Scheid J.F., Seaman M.S., Nussenzweig M.C. Enhanced HIV-1 neutralization by antibody heteroligation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:875–880. doi: 10.1073/pnas.1120059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Giang-Huong N., Candotti D., Goubar A., Autran B., Maynart M., SICARD D., Clauvel J.P., Agut H., Costagliola D., Rouzioux C. HIV type 1-specific IgG2 antibodies: markers of helper T cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res. Hum. Retrovir. 2001;17:1435–1446. doi: 10.1089/088922201753197105. [DOI] [PubMed] [Google Scholar]
- Palmer C.D., Romero-Tejeda M., Scully E.P., Lockhart A., Seaman M.S., Goldenthal A., Piechocka-Trocha A., Walker B.D., Chibnik L.B., Jost S., Porichis F. Increased frequencies of CD8 + CD57 + T cells are associated with antibody neutralization breadth against HIV in viraemic controllers. J. Int. AIDS Soc. 2016;19 doi: 10.7448/IAS.19.1.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensieroso S., Galli L., Nozza S., Ruffin N., Castagna A., Tambussi G., Hejdeman B., Misciagna D., Riva A., Malnati M., Chiodi F., Scarlatti G. B-cell subset alterations and correlated factors in HIV-1 infection. AIDS (London, England) 2013;27:1209–1217. doi: 10.1097/QAD.0b013e32835edc47. [DOI] [PubMed] [Google Scholar]
- Pereyra F., Addo M.M., Kaufmann D.E., Liu Y., Miura T., Rathod A., Baker B., Trocha A., Rosenberg R., Mackey E., Ueda P., Lu Z., Cohen D., Wrin T., Petropoulos C.J., Rosenberg E.S., Walker B.D. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- Pereyra F., Palmer S., Miura T., Block B.L., Wiegand A., Rothchild A.C., Baker B., Rosenberg R., Cutrell E., Seaman M.S., Coffin J.M., Walker B.D. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J. Infect. Dis. 2009;200:984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi A., Panteleeff D., Blish C.A., Baeten J.M., Jaoko W., Mcclelland R.S., Overbaugh J. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J. Virol. 2009;83:10269–10274. doi: 10.1128/JVI.01149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna D., Corti D., Jarrossay D., Sallusto F., Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur. J. Immunol. 2009;39:1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe S., Soghoian D.Z., Lindqvist M., Ghebremichael M., Donaghey F., Carrington M., Seaman M.S., Kaufmann D.E., Walker B.D., Porichis F. HIV-1 antibody neutralization breadth is associated with enhanced HIV-specific CD4 + T cell responses. J. Virol. 2015;90:2208–2220. doi: 10.1128/JVI.02278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., PARIS R., Premsri N., Namwat C., de Souza M., Adams E., Benenson M., Gurunathan S., Tartaglia J., Mcneil J.G., Francis D.P., Stablein D., Birx D.L., Chunsuttiwat S., Khamboonruang C., Thongcharoen P., Robb M.L., Michael N.L., Kunasol P., Kim J.H., Investigators M.-T. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Richman D.D., Wrin T., Little S.J., Petropoulos C.J. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S.K., Sarr A.D., Macneil A., Thakore-Meloni S., Gueye-Ndiaye A., Traore I., Dia M.C., Mboup S., Kanki P.J. Comparison of heterologous neutralizing antibody responses of human immunodeficiency virus type 1 (HIV-1)- and HIV-2-infected Senegalese patients: distinct patterns of breadth and magnitude distinguish HIV-1 and HIV-2 infections. J. Virol. 2007;81:5331–5338. doi: 10.1128/JVI.02789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusert P., Kouyos R.D., Kadelka C., Ebner H., Schanz M., huber M., Braun D.L., Hoze N., Scherrer A., Magnus C., Weber J., Uhr T., Cippa V., Thorball C.W., Kuster H., Cavassini M., Bernasconi E., Hoffmann M., Calmy A., Battegay M., Rauch A., Yerly S., Aubert V., Klimkait T., Boni J., Fellay J., Regoes R.R., Gunthard H.F., Trkola A., Swiss H.I.V.C.S. Determinants of HIV-1 broadly neutralizing antibody induction. Nat. Med. 2016;22:1260–1267. doi: 10.1038/nm.4187. [DOI] [PubMed] [Google Scholar]
- Sáez-Cirión A., Lacabaratz C., Lambotte O., Versmisse P., Urrutia A., Boufassa F., Barré-Sinoussi F., Delfraissy J.-F., Sinet M., Pancino G., Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A., Pancino G. HIV controllers: a genetically determined or inducible phenotype? Immunol. Rev. 2013;254:281–294. doi: 10.1111/imr.12076. [DOI] [PubMed] [Google Scholar]
- Sajadi M.M., Guan Y., Devico A.L., Seaman M.S., Hossain M., Lewis G.K., Redfield R.R. Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J. Acquir. Immune Defic. Syndr. 2011;57:9–15. doi: 10.1097/QAI.0b013e3182100c1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather D.N., Armann J., Ching L.K., Mavrantoni A., Sellhorn G., Caldwell Z., Yu X., Wood B., Self S., Kalams S., Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid J.F., Mouquet H., Feldhahn N., Seaman M.S., Velinzon K., Pietzsch J., Ott R.G., Anthony R.M., Zebroski H., Hurley A., Phogat A., Chakrabarti B., Li Y., Connors M., Pereyra F., Walker B.D., Wardemann H., Ho D., Wyatt R.T., Mascola J.R., Ravetch J.V., Nussenzweig M.C. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Scheid J.F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T.Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K., Hurley A., Myung S., Boulad F., Poignard P., Burton D.R., Pereyra F., Ho D.D., Walker B.D., Seaman M.S., Bjorkman P.J., Chait B.T., Nussenzweig M.C. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek M.D., Rida W., Priddy F.H., Pung P., Carrow E., Laufer D.S., Lehrman J.K., Boaz M., Tarragona-Fiol T., Miiro G., Birungi J., Pozniak A., Mcphee D.A., Manigart O., Karita E., Inwoley A., Jaoko W., Dehovitz J., Bekker L.G., Pitisuttithum P., Paris R., Walker L.M., Poignard P., Wrin T., Fast P.E., Burton D.R., Koff W.C. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalls-Mantey A., Doria-Rose N., Klein R., Patamawenu A., Migueles S.A., Ko S.Y., Hallahan C.W., Wong H., Liu B., You L., Scheid J., Kappes J.C., Ochsenbauer C., Nabel G.J., Mascola J.R., Connors M. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4 + T cells is directly associated with the magnitude of surface IgG binding. J. Virol. 2012;86:8672–8680. doi: 10.1128/JVI.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D., Burton D.R. HIV broadly neutralizing antibodies: taking good care of the 98. Immunity. 2016;45:958–960. doi: 10.1016/j.immuni.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongcharoen P., Suriyanon V., Paris R.M., Khamboonruang C., de Souza M.S., Ratto-Kim S., Karnasuta C., Polonis V.R., Baglyos L., Habib R.E., Gurunathan S., Barnett S., Brown A.E., Birx D.L., Mcneil J.G., Kim J.H., Thai A.V.E.G. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J. Acquir. Immune Defic. Syndr. 2007;46:48–55. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- Tomaras G.D., Yates N.L., Liu P., Qin L., Fouda G.G., Chavez L.L., Decamp A.C., Parks R.J., Ashley V.C., Lucas J.T., Cohen M., Eron J., Hicks C.B., Liao H.-X., Self S.G., Landucci G., Forthal D.N., Weinhold K.J., Keele B.F., Hahn B.H., Greenberg M.L., Morris L., Karim S.S.A., Blattner W.A., Montefiori D.C., Shaw G.M., Perelson A.S., Haynes B.F. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B.D., Yu X.G. Unravelling the mechanisms of durable control of HIV-1. Nat. Rev. Immunol. 2013;13:487–498. doi: 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- Wei X., Decker J.M., Wang S., Hui H., Kappes J.C., Wu X., Salazar-Gonzalez J.F., Salazar M.G., Kilby J.M., Saag M.S., Komarova N.L., Nowak M.A., Hahn B.H., Kwong P.D., Shaw G.M. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Yang X., Farzan M., Wyatt R., Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 2000;74:5716–5725. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypic analysis of circulating B cell populations using flow cytometry. B cells were identified using CD19, and further subdivided in naïve (CD27–IgD+), total memory B cells (CD27+ IgD–), activated memory (AM, CD27+ CD21 −), resting memory (RM, CD27+ CD21+), intermediate memory (IM, CD27− CD21+), tissue-like memory (TLM, CD27 − CD21 −), plasmablasts (CD27+ IgD− CD38hig) and marginal zone-like B cells (MZ-B cells, CD27+ IgD− IgM+ CD38− CD21+). IgG+ and IgG2+ memory B cells were also identified.
Proportions of memory B cell populations in HIV+ patients and HIV − donors. (a) Frequency of total memory B cells (CD27+ IgD −) among CD19+ B cells. (b) Frequency of naïve B cells (CD27 − IgD+) among CD19+ B cells. (c) Frequency of IgG + cells among memory B cells. (d) Frequency of IgG2+ cells among memory B cells. (e) Frequency of CD38hi plasmablasts among memory B cells. (f) Frequency of IgM+ CD38 − CD21+ MZ-like B cells among memory B cells. Each individual is represented by a specific dot on each graph (shape and color). Statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn’s test. Bars indicate median values; HIV-neg: HIV-negative donors.
Distribution and frequency of B cells according to their Ab isotype in HIV+ and HIV − donors. Frequencies of B cells secreting IgG (a), IgG1 (b), IgG2 (c) or IgG3 (d) among total B cells in EC (circle: HLA-B*57+ or square: HLA-B*57 −), cART and HIV-negative donors. Each individual is represented by a specific dot on each graph (shape and color). Statistical significance were calculated using a Kruskal-Wallis test followed by a Dunn’s test (*P < 0.05). Bars indicate median values.
Memory B cell responses against Influenza vaccine antigens. Frequency of Flu-specific IgG+ B cells in EC, cART and HIV-negative donors. Each individual is represented by a specific dot on each graph (shape and color). Circle: HLA-B*57+ EC; square: HLA-B*57 − EC. Statistical significance was calculated using a Kruskal-Wallis test followed by a Dunn’s test. Bars indicate median values.
Proportion of HLA-B57+ and HLA-B57 − EC responding to viral antigens. HLA-B57+ EC (n = 16) are represented in green and HLA-B*57 − (n = 17) in orange. Proportions of patient presenting (a) IgG +, (b) IgG1 +, (c) IgG2 + and (d) IgG3 + B cell responses against HIV-Env antigens (gp140Yu2b, gp41S30 or gp160THO) and Influenza vaccine antigens (Flu, VAXIGRIP vaccine).
The quantity of HIV-specific IgG in patient’s sera does not correlate with HIV-specific B cell frequency. (a) Quantity of HIV-specific IgG normalized to total IgG, evaluated using gp41 and gp140 HIV antigens, in sera from HLA-B*57 + (yellow circles) and HLA-B*57 − (pink squares) EC. Bars indicate median values. (b) Spearman correlation between the anti-gp140 B cell frequency (among IgG + B cells) and the ratio of anti-gp140 IgG Abs to total IgG Abs for all EC (left panel), HLAB*57 + EC (middle panel) and HLA-B*57 − EC (right panel).