Abstract
Background
Carfilzomib (CFZ) is a new proteasome inhibitor used for the treatment of multiple myeloma. Besides heart failure, angina and myocardial ischemia occurred following administration of CFZ, which is not contraindicated in patients with recent myocardial infarction/unstable angina excluded from the safety trials.
Aim of Study
To test the effects of CFZ (10− 9 to 10− 7 mol/L) on vascular tone and reactivity in the isolated rabbit heart and aorta.
Methods and Results
CFZ administered by bolus injection to the isolated heart increased coronary perfusion pressure (CPP) at all tested concentrations and mildly raised left ventricular pressure and heart rate, only at the highest concentration. Addition of CFZ directly into the organ bath increased the basal tone of isolated aortic strips with contraction plateau reached after 10 min. This spasmogenic effect doubled following ablation of the endothelium. Pretreatment with CFZ amplified the vasospastic action exerted by KCl, noradrenaline (NA) and angiotensin II (A) on aortic strips, and impaired vasodilation following administration of nitroglycerin (NTG) and nifedipine (NFP) on the contraction plateau induced by KCl, NA and A. Aortic strips pretreated with CFZ exhibited impaired relaxation, as compared to untreated strips, following administration of acetylcholine (Ach), an endothelium-dependent vasodilating agent, on the plateau of NA contraction (p < 0.05).
Conclusions
CFZ increased CPP, resting vasoconstricting tone and the spasmogenic effect of different agents. Preincubation with CFZ decreased the anti-spasmogenic activity of NTG and NFP, as well as reduced by over 50% the vasodilating effect of Ach, suggesting that CFZ can impair vasodilation via an endothelium dependent mechanism. Further studies are warranted to establish its clinical safety in patients with known CAD and prior history of coronary spasm.
Keywords: Carfilzomib, Proteasome inhibitors, Multiple myeloma, Coronary resistance, Vascular tone
Highlights
-
•
In the isolated aorta, carfilzomib increased basal tone and vasospastic action of KCl, noradrenaline and angiotensin II.
-
•
In the isolated aorta, carfilzomib impaired the anti-spasmogenic activity of nitroglycerin, nifedipine and acetylcholine.
-
•
In the isolated heart, carfilzomib increased coronary perfusion pressure, and mildly left ventricular pressure and heart rate.
Carfilzomib is a new chemotherapeutic agent used for the treatment of multiple myeloma. Our study shows that carfilzomib increases coronary perfusion pressure, resting vasoconstricting tone, and the spasmogenic effect of noradrenaline and angiotensin II, while it curbs the vasodilatory action of nitroglycerine and nifedipine. Our findings are relevant to human health as they warrant caution in the use of carfilzomib in elderly patients with cardiovascular risk factors and, even more importantly, in those with preexisting heart conditions, who are also eligible to receive carfilzomib, even though they were excluded from the safety trials, based on which carfilzomib use was approved.
1. Introduction
Carfilzomib (CFZ) is a novel proteasome inhibitor given by intravenous infusion for the treatment of relapsed and/or refractory Multiple Myeloma (MM) (Neri et al., 2016). Chemically, it is a tetrapeptide epoxyketone derived from epoxomicin, a natural product that has been shown to inhibit the proteasome (Meng et al., 1999). CFZ exerts a potent and irreversible inhibition of the proteasomal chymotrypsin-like activity, by binding selectively the β5 subunit of the 20S proteolytic core particle, while it only has minimal affinity for the β1 and β2 subunits (at doses up to 100 nM) (Demo et al., 2007). The U.S. Food and Drug Administration (FDA) approved CFZ on 20 July 2012 for use in patients with MM, who have received at least two prior therapies [including treatment with bortezomib (BTZ) and an immunomodulatory agent] and who exhibited disease progression within 60 days of completion of the last therapy. More recently, CFZ has also been approved for combined use with lenalidomide and dexamethasone for the treatment of patients with relapsed MM (KYPROLIS (Carfilzomib) [Prescribing Information], 2015).
Proteasomes are protein complexes located in the nucleus and the cytoplasm of all eukaryotic cells. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis (Dahlmann, 2016). Proteins are tagged for degradation with several residues of a small protein called ubiquitin, with final formation of a polyubiquitin chain, which is bound by the proteasome (Yao and Cohen, 2002). The ubiquitin-proteasome pathway (UPP) influences essential cellular functions including cell growth, differentiation, apoptosis, signal transduction, antigen processing and the inflammatory response. Proteasome inhibitors have effective anti-tumor activity. Selective inhibition in cancer cells of proteasome-mediated proteolysis results in a build-up of polyubiquinated proteins, which may cause cell cycle arrest, apoptosis, and inhibition of tumor growth (Grigoreva et al., 2015).
Chronic proteasome inhibition was associated with increased oxidative stress and early occurrence of atherosclerosis in a pig model of coronary artery disease (CAD) (Herrmann et al., 2007). In the same animal model, inhibition of the proteasome resulted in functional and structural alteration of the heart consistent with a hypertrophic-restrictive cardiomyopathy phenotype (Marfella et al., 2008). Sustained proteasome inhibition was also found to promote vascular cell senescence, thereby contributing to plaque progression, in asymptomatic elderly and adult patients undergoing carotid endarterectomy (Herrmann et al., 2013).
Although CFZ has a more favorable safety profile than other proteasome inhibitors, such as BTZ, mostly in regard to the lower incidence of peripheral neuropathy, analysis of the available clinical data seems to endorse the above experimental findings, confirming that treatment with CFZ may be associated with significant cardiovascular complications.
The safety profile of CFZ used as a single agent for the treatment of relapsed and/or refractory MM was analyzed in a total of 526 patients enrolled in the four phase II clinical trials based on which the U.S. FDA approval was granted. The most severe side effects reported include: sudden death (within 24 h from the infusion), pulmonary hypertension, heart failure (HF) (7.2%), myocardial ischemia and infusion reactions. Chest tightness of unknown mechanism has also been described and reported (Siegel et al., 2013).
Likewise, in the phase III, randomized, multicenter studies ASPIRE (Carfilzomib, Lenalidomide, and Dexamethasone versus Lenalidomide and Dexamethasone for the Treatment of Patients with Relapsed Multiple Myeloma) (Stewart et al., 2015) and ENDEAVOR (Phase 3 Study With Carfilzomib and Dexamethasone Versus Bortezomib and Dexamethasone for Relapsed Multiple Myeloma Patients) (Dimopoulos et al., 2015a, Dimopoulos et al., 2015b), the use of CFZ as part of combination regimens in patients with relapsed or refractory MM resulted in significantly higher rates of cardiac and cardiopulmonary adverse events including dyspnea, HF, ischemic heart disease and hypertension.
The goal of our study was to investigate whether CFZ exerts in vitro effects on vascular tone and reactivity in the isolated rabbit heart and thoracic aortic strips. The isolated heart was used to assess the effect of CFZ on myocardial contractility and coronary resistances. The thoracic aortic strips with and without endothelium were used to evaluate the effects of CFZ on vascular smooth muscle tone.
2. Methods
2.1. Rabbit Heart Preparations
All animal experiments were conducted ethically and approved by pertinent ethics committee. New Zealand albino rabbits of both sexes weighing 2–2.5 kg were used. The animals were sacrificed by cervical dislocation. The hearts were removed quickly and placed in an ice-cold Ringer-Locke solution, oxygenated with 100% O2, and containing in millimoles per liter: NaCl, 136.9; KCl, 2.68; MgCl, 0.99; CaCl2, 1.7; NaHPO4, 0.42; NaHCO3, 3.93; and glucose, 5.55 (pH 7.4) (Raddino et al., 1997).
Using a previously described procedure (Broadley, 1979), after removal of the pericardium and surrounding tissues, the hearts were perfused with Ringer-Locke solution according to the nonrecirculating Langendorff technique. The perfusion fluid was continuously gassed with 100% O2, maintained at 37 degrees Celsius, and delivered to the aortic inflow cannula at a constant rate of 22–24 ml/min using a peristaltic pump (Gilson, Miniplus HP2HF). The perfusion pressure was measured by a Statham transducer connected to the sidearm of the perfusion cannula. Since retrograde flow (coronary flow) was kept constant during the experiment, coronary perfusion pressure (CPP) represented a direct measure of the coronary resistance. A fluid-filled balloon connected to a pressure transducer was inserted into the left ventricular cavity through an opening in the left atrium, thus obtaining an isovolumically beating preparation. The balloon was inflated to provide an end-diastolic pressure < 1.0 mm Hg (Ferrari et al., 1996). Both end systolic left ventricular pressure (LVP) and CPP were recorded simultaneously by using a polygraph (OTE Biomedica; C6B). Except for experiments evaluating chronotropic effects, the hearts were electrically paced to exclude LVP variations related to heart rate (HR) oscillations. Rectangular pulses (0.5 V @ 1.0 msec, up to threshold stimulation) were applied to the preparation via two platinum electrodes, one connected to the metal inflow cannula and the other implanted directly in the ventricular apex. The frequency of stimulation was 10% greater than the basal HR. The hearts were left to equilibrate for 30 min prior to drug administration. The maximal effects of the used agents were observed 5–15 min after addition to the perfusion fluid (Ferrari et al., 1996). CFZ was administered in the perfusion buffer or infused at three different concentrations (10− 9, 10− 8 and 10− 7 M) by bolus injection of 1 cc over 5 min, using a collateral arm of the perfusion cannula. The above concentrations were chosen based on prior in vitro work showing that carfilzomib doses ranging from 5 to 80 nmol/L resulted in a significant growth inhibition of mantle cell lymphoma (MCL) cells, harvested from peripheral blood samples or bone marrow aspirates obtained from patients with MCL (Zhang et al., 2013).
2.2. Aortic Preparations
After surgical isolation of the aortic segments, the media was separated from the adventitia and spirally cut into strips, according to Furchgott's technique (Furchgott and Zawadzki, 1980). The thoracic aortic strips (2 cm long and 3 mm wide) were then placed in 10 ml organ baths containing Krebs-Henseleit solution at 37 °C. Contractions were measured by means of an isometric transducer, connected to a pen writing recorder. An initial tension of 2 g was applied for 120 min before the administration of drugs. The effect of three different spasmogenic agents [potassium chloride (KCl, noradrenaline (NA), and angiotensin II (A))] was evaluated on aortic strips either untreated or precontracted for 60 min with CFZ. KCl (10− 1 M), NA (10− 6 M) and A2 (10− 5 M) were administered directly into the organ bath. In a different set of experiments, following achievement of the plateau of contraction (usually 5–10 min after the administration) induced by CFZ, vasodilatory agents, such as nitroglycerin (NTG) and nifedipine (NFP) were added to the organ bath at different concentrations, ranging from 10− 9 to 10− 5 M. Some aortic strips were rubbed to eliminate the endothelial layer, whose absence was pharmacologically tested and confirmed by means of the acetylcholine test (Furchgott and Zawadzki, 1980). Endothelium-dependent vascular reactivity was also assessed by evaluating the antagonism induced by acetylcholine (Ach; 10− 8 to 10− 5 M) during NA-induced specimen contraction (10− 5 M).
2.3. Data Analysis
Results were expressed as mean ± SEM of 6–8 experiments. The inhibitory response on LVP and CPP was calculated as a percentage decrease of the basal value. Likewise, the inhibitory response on coronary spasm was assessed as a percentage of inhibition on the plateau of the CPP increase induced by different spasmogenic compounds. Statistical analysis was performed by analysis of variance and test of simple main effects. Comparison between groups was performed using the Student's t-test for unpaired data. The level of significance was considered as p < 0.05.
2.4. Drugs
All the drugs and reagents were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Carfilzomib was solubilized in dimethyl sulfoxide (DMSO) at 10 mmol/L.
3. Results
3.1. Isolated Heart
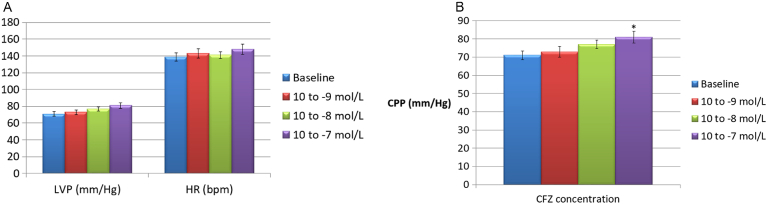
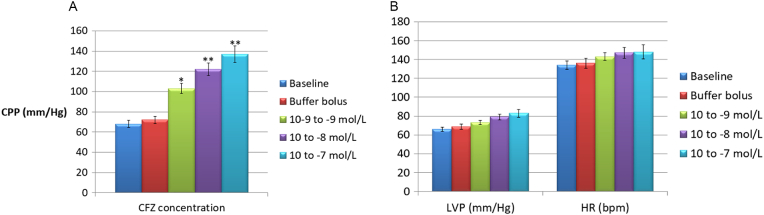
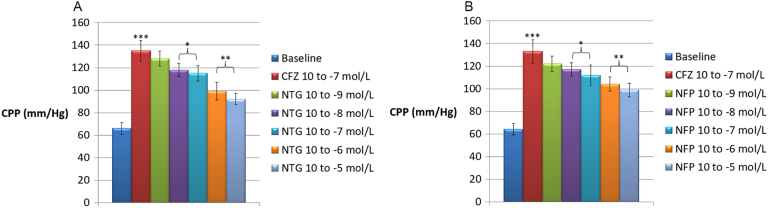
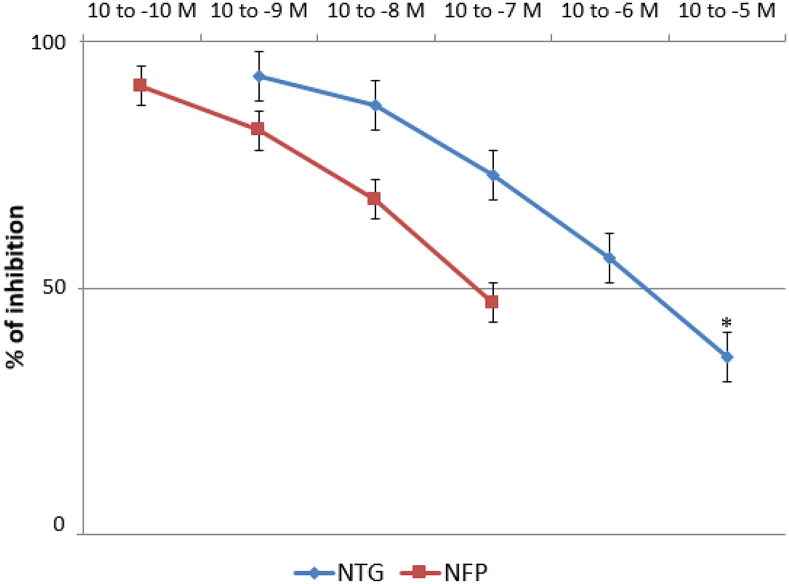
After the stabilization period, the isolated hearts showed a basal LVP of 55.1 ± 5.2 mm Hg and a basal CPP of 62.6 ± 3.9 mm Hg. Basal HR was 135.8 ± 8.5 beats/min (data not shown). Pilot experiments revealed oscillations in basal LVP and CPP < 5% after 120 min; likewise, a spontaneous reduction in HR of 11.7 ± 1.1% was also recorded. CFZ administered in the perfusion fluid at concentrations of 10− 9, 10− 8 and 10− 7 M did not substantially modify LVP and HR values (p > 0.05), whereas CPP was slightly increased from 65.2 ± 4.1 to 78.6 ± 8.3 mm Hg, but only at the highest concentration of 10− 7 mol/L (p < 0.05) (Fig. 1a and b). Administration of CFZ by bolus injection caused a significant increase in coronary resistances at any of the three concentrations used (Fig. 2a), as well as a mild increase in LVP and HR, which was more evident, though still non-statistically significant, at the maximum dosage tested (10− 7 M) (Fig. 2b). As shown in Fig. 3a and b, nitroglycerin (NTG) and nifedipine (NFP) exhibited a significant inhibitory effect on the spasmogenic action exerted by CFZ. At a CFZ concentration of 10− 5 M, the percentage of inhibition for NTG, which peaked at 62.2%, was significantly higher than that for NTG, which peaked at 49.4% (p < 0.05).
Fig. 1.
a) Effects on left ventricular pressure (LVP) and heart rate (HR) of carfilzomib administered in the perfusion buffer (concentrations: 10− 9, 10− 8 and 10− 7 mol/L). b) Effects on coronary perfusion pressure (CPP) of carfilzomib administered in the perfusion buffer (concentrations: 10− 9, 10− 8 and 10− 7 mol/L). *p < 0.05 versus baseline.
Fig. 2.
a) Effects on coronary perfusion pressure (CPP) of carfilzomib administered at three different concentrations (10− 9, 10− 8 and 10− 7 mol/L) by injection of 1 cc boluses over 5 min using a collateral arm of the perfusion cannula. *p < 0.05, **p < 0.01 versus bolus injections of 1 cc buffer over a time frame of 5 min. Baseline value of CPP was recorded during buffer perfusion through the aortic inflow cannula at a constant rate of 22–24 ml/min. b) Effects on left ventricular pressure (LVP) and heart rate (HR) of carfilzomib administered at three different concentrations (10− 9, 10− 8 and 10− 7 mol/L) by injection of 1 cc boluses over 5 min using a collateral arm of the perfusion cannula.
Fig. 3.
a) Effects of nitroglycerin (NTG) on the coronary perfusion pressure (CPP) increase induced by carfilzomib (CFZ) in the isolated rabbit heart. ***p < 0.001 versus baseline; *p < 0.05 and **p < 0.01 versus CPP increase induced by CFZ 10− 7 mol/L. b) Effects of nifedipine (NFP) on the coronary perfusion pressure (CPP) increase induced by carfilzomib (CFZ) in the isolated rabbit heart. ***p < 0.001 versus baseline; *p < 0.05 and **p < 0.01 versus CPP increase induced by CFZ 10− 7 mol/L.
3.2. Aortic Strips
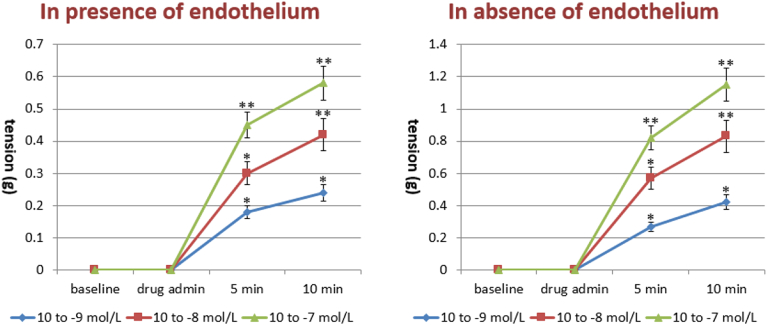
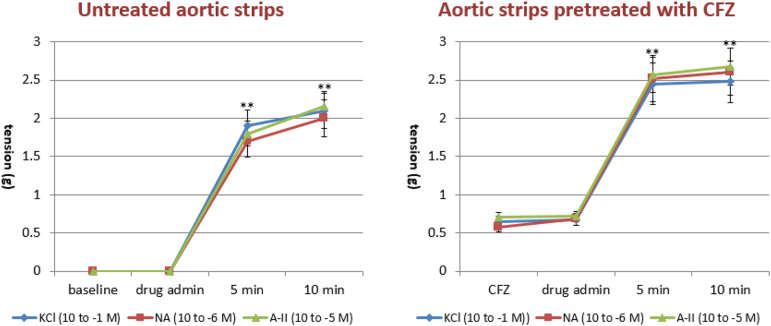
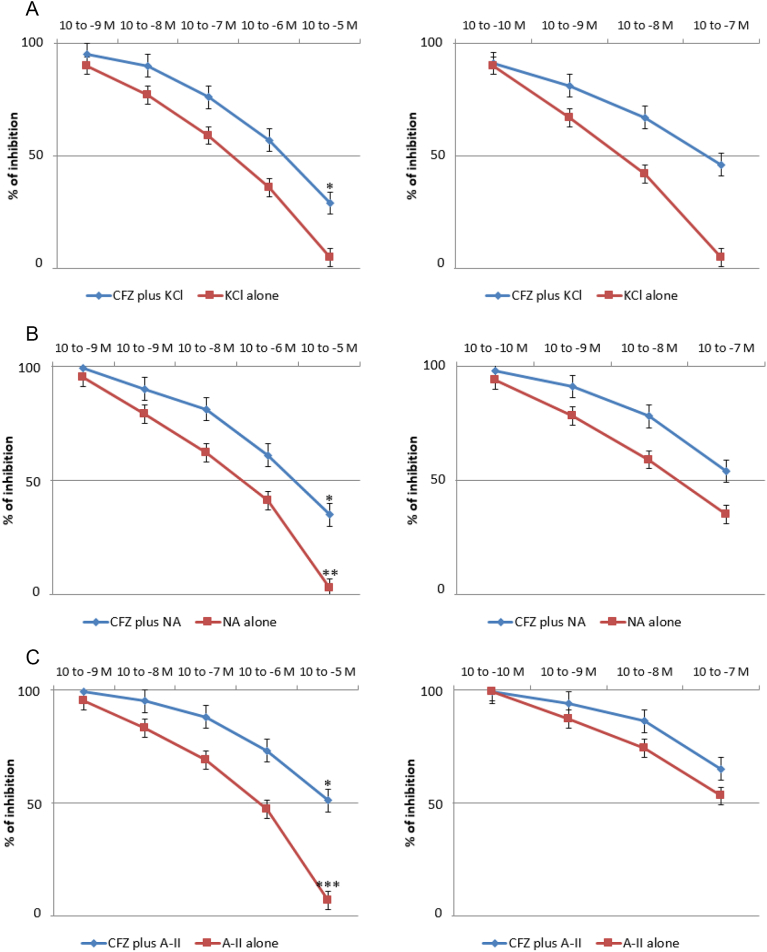
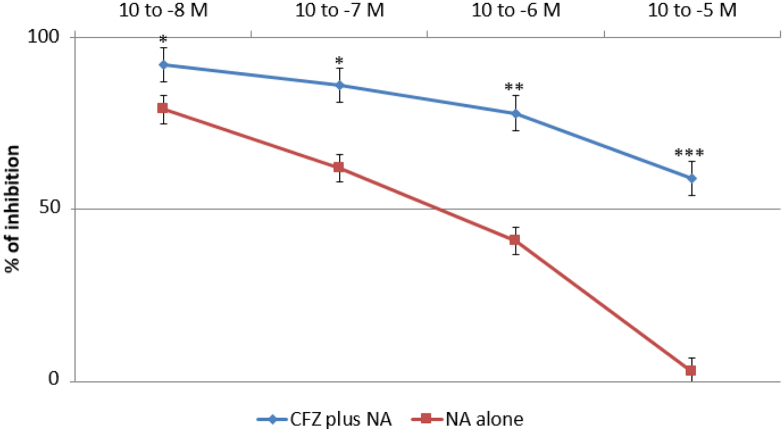
CFZ administered directly into the organ bath (10− 9–10− 7 M) significantly increased the basal tone of the isolated aortic strips with a contraction plateau reached after 10 min incubation (Fig. 4, left panel). The spasmogenic effect was significantly increased following ablation of the endothelium, as shown in Fig. 4 (right panel). Subsequently, we tested the effects of different vasopressors, i.e. KCl (10− 1 M), NA (10− 6 M) and A2 (10− 5 M), on aortic strips either untreated or precontracted with CFZ (10− 7 mol/L) for 15 min. While no difference was observed in the vasoconstrictive action of the three vasopressors, pretreatment with CFZ increased the tension exerted by each of the agents by approximately 30%, as depicted in Fig. 5. The vasodilatory action of NTG and NFP was evaluated on the contraction plateau induced by incubation with CFZ (10− 7 mol/L) for 15 min. Although both NTG and NFP significantly curbed the spasmogenic effects of CFZ, an NTG concentration of 10− 5 M exhibited a more pronounced and statistically significant percentage of inhibition on CFZ-induced vasoconstriction, as compared to an NFP concentration of 10− 7 M (Fig. 6). Of note, neither NTG nor NFP were able to totally reverse the vasoconstrictive effects of CFZ. In another set of experiments, we assessed the vasodilation triggered by NTG and NFP on the contraction plateau of aortic strips incubated with KCl (10− 1 M), NA (10− 6 M) and A-II (10− 5 M) with or without pretreatment with CFZ (15 min at a concentration of 10− 7 mol/L). Pretreatment with CFZ significantly impaired the vasodilatory response to NTG and NFP on the contraction plateau induced by all vasopressors (Fig. 7a, b and c). A comparison between the effects of NTG and NFP used at their highest doses revealed that NTG's ability to cause vasodilation was significantly more pronounced than NFP's both in the presence and absence of CFZ. Endothelium-dependent vascular reactivity was finally assessed by evaluating the antagonism induced by Ach (10− 8 to 10− 5 M) during NA-induced specimen contraction (10− 5 M). As depicted in Fig. 8, aortic strips pretreated with CFZ and contracted with NA exhibited a blunted reactivity to Ach, a well-known endothelium-dependent vasodilating agent (Fig. 8).
Fig. 4.
Effects of carfilzomib (10− 9–10− 7 mol/L) on basal tone of isolated aortic strips in presence and absence of the endothelium. *p < 0.05, **p < 0.01 versus baseline.
Fig. 5.
Spasmogenic effects of potassium chloride (KCl), noradrenaline (NA) and angiontensin II (A2) assessed on aortic strips either untreated or precontracted for 60 min with carfilzomib (CFZ). **p < 0.01 versus baseline.
Fig. 6.
Vasodilatory effect of nitroglycerin (NTG) and nifedipine (NFP) on the plateau of contraction induced by carfilzomib used at a concentration of 10− 7 mol/L. *p < 0.05 in comparing the effects of NTG 10− 5 M versus NFP 10− 7 M.
Fig. 7.
a) Vasodilatory effects of nitroglycerin (NTG) (left panel) and nifedipine (NFP) (right panel) on the plateau of contraction induced by potassium chloride (KCl; 10− 1 M) in presence or absence of carfilzomib (CFZ). *p < 0.05 in the comparison between effects of NTG 10− 5 M and NFP 10−7 M. b) Vasodilatory response to nitroglycerin (NTG) (left panel) and nifedipine (NFP) (right panel) on the plateau of contraction induced by noradrenaline (NA; 10− 6 M) in presence or absence of carfilzomib (CFZ). *p < 0.05 in the comparison between the effects of NTG 10− 5 M and NFP 10−7 M on the plateau of contraction induced by NA in presence of CFZ. **p < 0.01 in the comparison between the effects of NTG 10− 5 M and NFP 10−7 M on the plateau of contraction induced by NA in absence of CFZ. c) Vasodilatory effects of nitroglycerin (NTG) (left panel) and nifedipine (NFP) (right panel) on the plateau of contraction induced by angiotensin II (A-II; 10− 5 M) in presence or absence of carfilzomib (CFZ). *p < 0.05 in the comparison between the effects of NTG 10− 5 M and NFP 10−7 M on the plateau of contraction induced by A-II in presence of CFZ. ***p < 0.001 in the comparison between the effects of NTG 10− 5 M and NFP 10−7 M on the plateau of contraction induced by A-II in absence of CFZ.
Fig. 8.
Vasodilatory effects of acetylcholine (Ach) on noradrenaline (NA)-induced (10− 6 M) contraction of aortic strips either naïve or precontracted with carfilzomib (CFZ). *p < 0.05; **p < 0.01 and ***p < 0.001 in the comparison between the effects of Ach in presence or absence of CFZ.
4. Discussion
Although still incurable, the survival rate of MM, the second most common hematologic malignancy (Siegel et al., 2014), has improved mainly due to the development of new and highly effective agents, such as immunomodulators and proteasome inhibitors (Kumar et al., 2012). CFZ is an irreversible epoxyketone-based proteasome inhibitor, used in the U.S. as a single or combination agent (along with lenalidomide and dexamethasone) in the treatment of relapsed/refractory MM. The elevated response rate and improved progression-free survival (PFS) observed in patients with relapsed or refractory MM receiving CFZ as a single (Siegel et al., 2013) or combination agent (Stewart et al., 2015, Dimopoulos et al., 2015b), as well as its advantageous benefit-risk profile in comparison to BTZ (Dimopoulos et al., 2015b), another proteasome inhibitor, are associated with significantly higher cardiac and cardiopulmonary adverse events. In the ASPIRE trial, for instance, compared to patients receiving lenalidomide and dexamethasone alone, those given CFZ in addition to lenalidomide and dexamethasone reported higher rates of grade 3 dyspnea (2.8% versus 1.8%), HF (grouped term; 3.8% and 1.8%), ischemic heart disease (grouped term; 3.3% and 2.1%), and hypertension (preferred term; 4.3% and 1.8%) (Stewart et al., 2015). Likewise, in the ENDEAVOR study, in comparison to the BTZ-dexamethasone group, the patients assigned to the CFZ-dexamethasone developed increased rates of grade 3 hypertension (8.9% vs. 2.6%), dyspnea (5.4% vs. 2.2%), and HF (4.8% vs. 1.8%) (Dimopoulos et al., 2015b). Recent early-phase clinical trials exploring the safety and efficacy of different CFZ doses in patients with newly diagnosed MM also reported cardiac-related dose-limiting toxicity (DLT), likely related to CFZ administration, with a reported maximum tolerated dose (MTD) of 20–36 mg/m2 (Bringhen et al., 2014, Mikhael et al., 2015). The overall interpretation of the results from the phase I-III clinical trials indicates an increased risk of cardiac-related events following CFZ treatment, with a reported incidence of grade 3 HF ranging from 4 to 6% (Siegel et al., 2013, Stewart et al., 2015, Dimopoulos et al., 2015a). Besides HF, whose etiology and pathophysiology are poorly understood, other common side effects (potentially related to the vasoactive properties of CFZ) include myocardial ischemia, manifesting as angina and myocardial infarction, as well as systemic and pulmonary hypertension. Previous studies also reported the occurrence of chest pain of unclear etiology during CFZ infusion. In line with this finding, in some of our patients receiving CFZ infused at high-doses (≥ 36 mg/m2), we observed cases of chest pain, sometimes associated with ischemic alterations of the electrocardiogram, without release of cardiac biomarkers in the circulation. This group underwent further cardiac evaluation, which failed to unveil inducible myocardial ischemia and/or significant CAD. The current study stemmed from the attempt to explain this inconsistency, by moving backwards from the bedside to the bench. Our data showed for the first time that CFZ exerts powerful in vitro effects on vascular tone and reactivity. CFZ administered by bolus injection heightened coronary resistance and its spasmogenic action was only partly resolved by NTG and NFP. Of note, the percentage inhibition achieved by NTG on CFZ-induced spasm was significantly higher than NFP (62.2% versus 49.4%). This would suggest a more congruent role for nitrates, rather than calcium channel blockers, in the prevention and treatment of the clinical conditions of exaggerated vascular tone, potentially related to CFZ infusion. Furthermore, CFZ increased the resting vasoconstricting tone of aortic strips, an effect which was further amplified by ablation of the endothelium. Moreover, preincubation with CFZ amplified the spasmogenic effect of different vasopressors and minimized the vasodilatory response to NTG and NFP on the contraction plateau caused by several vasopressors. Once again, NTG proved to be more efficacious than NFP in limiting the combined spasm ensuing from CFZ pretreatment and subsequent incubation with any of the vasopressors used. Finally, after preincubation with CFZ, there was a > 50% reduction in the Ach-vasodilating effect. This finding, in conjunction with the stronger antispasmogenic activity consistently exhibited by NTG (as compared to NFP), suggests that CFZ can impair vasodilation via an endothelium dependent mechanism. Since the vasoactive effects of proteasome inhibitors other than CFZ were not investigated, the findings of our study cannot be generalized and assumed to be expression of a drug class effect. This represents a limitation of our study.
The experimental data described above, although in need of proper validation in the clinical setting, should be a cautionary tale in the management of MM patients requiring CFZ. MM typically affects the elderly, a population with greater cardiovascular risk factors and higher incidence of cardiovascular disease. Hence, MM patients whose chemotherapeutic regimen includes CFZ should be evaluated by a cardio-oncologist and undergo comprehensive cardiovascular risk stratification, possibly including an ischemic work-up, prior to receiving CFZ infusion. Remarkably, patients with New York Heart Association Class III–IV HF, myocardial infarction or unstable angina, who were excluded from the phase II studies based on which FDA approval was granted, are also eligible to receive CFZ (KYPROLIS [Prescribing Information], 2016), despite the greater risk of developing cardiovascular complications, as a consequence of the infusion. As CFZ is not contraindicated in this high-risk population and cardiac complications caused by CFZ are serious, it is imperative that cardiovascular risk factors and underlying cardiac conditions (such as HF, cardiac ischemia, systemic and pulmonary hypertension) be thoroughly investigated, diagnosed and treated, prior to proceeding with CFZ infusion.
Author Contributions
Carol Chen-Scarabelli contributed to the manuscript with literature searches, study design, data collection, data analysis, data interpretation and drafting of the paper.
Giovanni Corsetti contributed to the manuscript with literature searches, data collection, data analysis, data interpretation and drafting of some portions of the paper.
Evasio Pasini contributed to the manuscript with supervision of the experimental work, literature searches, study design, data collection, data analysis, data interpretation and drafting of some portions of the paper.
Francesco S. Dioguardi contributed to the manuscript with literature searches, study design, data collection, data analysis, data interpretation and drafting of the paper.
Gagan Sahni contributed to the manuscript with study design and editing of some portions of the paper.
Jagat Narula contributed to the manuscript with data interpretation and editing of some portions of the paper.
Mara Gavazzoni contributed to the manuscript with literature searches, data collection and interpretation.
Hemang Patel contributed to the manuscript with literature searches, data collection and interpretation.
Louis Saravolatz contributed to the manuscript with study design and editing of some portions of the paper.
Richard Knight contributed to the manuscript with data interpretation and editing of the paper.
Riccardo Raddino contributed to the manuscript with study design, data interpretation and drafting of the paper.
Tiziano M. Scarabelli contributed to the manuscript with literature searches, study design, data collection, data analysis, data interpretation, drafting and editing of the paper.
Disclosure
The authors have no conflicts of interest to report.
References
- Bringhen S., Petrucci M.T., Larocca A., Conticello C., Rossi D., Magarotto V., Musto P., Boccadifuoco L., Offidani M., Omedé P., Gentilini F., Ciccone G., Benevolo G., Genuardi M., Montefusco V., Oliva S., Caravita T., Tacchetti P., Boccadoro M., Sonneveld P., Palumbo A. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- Broadley K.J. The Langendorff heart preparation: reappraisal of its role as a research and teaching model for coronary vasoactive drugs. J. Pharmacol. Methods. 1979;2:143–153. [Google Scholar]
- Dahlmann B. Mammalian proteasome subtypes: their diversity in structure and function. Arch. Biochem. Biophys. 2016;591:132–140. doi: 10.1016/j.abb.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Demo S.D., Kirk C.J., Aujay M.A., Buchholz T.J., Dajee M., Ho M.N. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- Dimopoulos M.A., Moreau P., Palumbo A. Carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd) in patients (pts) with relapsed multiple myeloma (RMM): results from the phase III study ENDEAVOR. J. Clin. Oncol. 2015;33(Suppl) (abstr 8509) [Google Scholar]
- Dimopoulos M., Moreau P., Palumbo A. Carfilzomib and dexamethasone improves progression-free survival and response rates vs bortezomib and dexamethasone in patients (PTS) with relapsed multiple myeloma (RMM): The phase 3 study ENDEAVOR. Haematologica. 2015;100(Suppl. 1) (abstr LB2071) [Google Scholar]
- Ferrari R., Cargnoni A., Bernocchi P., Pasini E., Curello S., Ceconi C., Ruigrok T.J. Metabolic adaptation during a sequence of no-flow and low-flow ischemia. A possible trigger for hibernation. Circulation. 1996;94(10):2587–2596. doi: 10.1161/01.cir.94.10.2587. [DOI] [PubMed] [Google Scholar]
- Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Grigoreva T.A., Tribulovich V.G., Garabadzhiu A.V., Melino G., Barlev N.A. The 26S proteasome is a multifaceted target for anti-cancer therapies. Oncotarget. 2015;6(28):24733–24749. doi: 10.18632/oncotarget.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J., Saguner A.M., Versari D., Peterson T.E., Chade A., Olson M., Lerman L.O., Lerman A. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ. Res. 2007;101(9):865–874. doi: 10.1161/CIRCRESAHA.107.152959. (Epub 2007 Sep 6) [DOI] [PubMed] [Google Scholar]
- Herrmann J., Wohlert C., Saguner A.M., Flores A., Nesbitt L.L., Chade A., Lerman L.O., Lerman A. Primary proteasome inhibition results in cardiac dysfunction. Eur. J. Heart Fail. 2013;15(6):614–623. doi: 10.1093/eurjhf/hft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.K., Lee J.H., Lahuerta J.J., Morgan G., Richardson P.G., Crowley J., Haessler J., Feather J., Hoering A., Moreau P., LeLeu X., Hulin C., Klein S.K., Sonneveld P., Siegel D., Bladé J., Goldschmidt H., Jagannath S., Miguel J.S., Orlowski R., Palumbo A., Sezer O., Rajkumar S.V., Durie B.G., International Myeloma Working Group Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyx Pharmaceuticals, Inc., an Amgen Inc. Subsidiary; Thousand Oaks, CA: 2015. KYPROLIS (Carfilzomib) [Prescribing Information] [Google Scholar]
- Onyx Pharmaceuticals, Inc., an Amgen Inc. Subsidiary; Thousand Oaks, CA: 2016. KYPROLIS [Prescribing Information] [Google Scholar]
- Marfella R., Di Filippo C., Laieta M.T., Vestini R., Barbieri M., Sangiulo P., Crescenzi B., Ferraraccio F., Rossi F., D'Amico M., Paolisso G. Effects of ubiquitin-proteasome system deregulation on the vascular senescence and atherosclerosis process in elderly patients. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63(2):200–203. doi: 10.1093/gerona/63.2.200. [DOI] [PubMed] [Google Scholar]
- Meng L., Mohan R., Kwok B.H., Elofsson M., Sin N., Crews C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. U. S. A. 1999;96(18):10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhael J.R., Reeder C.B., Libby E.N., Costa L.J., Bergsagel P.L., Buadi F., Mayo A., Nagi Reddy S.K., Gano K., Dueck A.C., Stewart A.K. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br. J. Haematol. 2015;169(2):219–227. doi: 10.1111/bjh.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri P., Bahlis N.J., Paba-Prada C., Richardson P. Treatment of relapsed/refractory multiple myeloma. Cancer Treat. Res. 2016;169:169–194. doi: 10.1007/978-3-319-40320-5_10. [DOI] [PubMed] [Google Scholar]
- Raddino R., Pelà G., Manca C., Barbagallo M., D'Aloia A., Passeri M., Visioli O. Mechanism of action of human calcitonin gene-related peptide in rabbit heart and in human mammary arteries. J. Cardiovasc. Pharmacol. 1997;29(4):463–470. doi: 10.1097/00005344-199704000-00006. [DOI] [PubMed] [Google Scholar]
- Siegel D., Martin T., Nooka A., Harvey R.D., Vij R., Niesvizky R., Badros A.Z., Jagannath S., McCulloch L., Rajangam K., Lonial S. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98(11):1753–1761. doi: 10.3324/haematol.2013.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Stewart A.K., Rajkumar S.V., Dimopoulos M.A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- Yao T., Cohen R.E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- Zhang L., Pham L.V., Newberry K.J., Ou Z., Liang R., Qian J., Sun L., Blonska M., You Y., Yang J., Lin X., Rollo A., Tamayo A.T., Lee J., Ford R.J., Zhao X., Kwak L.W., Yi Q., Wang M. In vitro and in vivo therapeutic efficacy of carfilzomib in mantle cell lymphoma: targeting the immunoproteasome. Mol. Cancer Ther. 2013;12(11):2494–2504. doi: 10.1158/1535-7163.MCT-13-0156. [DOI] [PubMed] [Google Scholar]