Abstract
The mismatch negativity (MMN) is an event-related potential that is consistently attenuated in people with schizophrenia. Within the predictive coding model of psychosis, MMN impairment is thought to reflect the same prediction failures that are also thought to underlie the development and crystallization of delusions and hallucinations. However, the true relationship between symptom severity and MMN impairment across studies has not yet been established. The present meta-analysis used meta-regressions to examine the relationship between MMN impairment (quantified as Hedges' g) and PANSS positive and negative symptom totals across 62 and 68 samples, respectively. Furthermore, we examined the relationship between MMN impairment and group differences in educational achievement (n = 47 samples), cognitive ability (n = 36 samples), and age (n = 86 samples). Overall, we found no significant associations between MMN impairment and symptom severity (p's > 0.50); however, we did observe a trend-level association between MMN impairment and lower education (p = 0.07) and a significant association with older age (p < 0.01) in the schizophrenia patient group. Taken together, these results challenge a simple predictive coding model of psychosis, and suggest that MMN impairment may be more closely associated with premorbid functioning than with the expression of psychotic symptoms.
1. Introduction
Although hallucinations and delusions represent core features of schizophrenia, a viable explanatory model that accounts for these symptoms has not been forthcoming. Recently, there has been considerable interest in the predictive coding (PC) framework to conceptualize the emergence and maintenance of positive symptoms (Fletcher and Frith, 2009, Adams et al., 2013). PC posits that one's beliefs about the world (posterior beliefs) result from an integration of prior beliefs with incoming sensory information. A mismatch between what is expected (priors) and what is perceived generates a prediction error (PE) which then updates beliefs about future events. In the healthy brain, PEs serve to orient attention to events not accounted for by prior beliefs and motivate an update of those beliefs. In the context of psychotic symptoms, PC failures have been used to explain the formation and crystallization of delusions (Adams et al., 2013), as well as hallucinations (Horga et al., 2014). For example, inappropriate PE signaling coincident with common events may imbue those events with added salience, spurring delusion formation (Kapur, 2002, Heinz, 2002, Corlett et al., 2007).
Despite the promising conceptual link between the PC framework and phenomenology, evidence supporting an association between experimentally-elicited PEs and symptom severity has not been consistently observed. For instance, there is considerable disagreement regarding the relationship between positive symptoms and mismatch negativity (MMN), an electrophysiological index of auditory processing that is commonly described as a prototypical PC phenomenon (e.g. Wacongne et al., 2012) and is robustly impaired in schizophrenia (Erickson et al., 2015). The MMN is an event-related potential elicited when a sequence of regular auditory stimulation is infrequently interrupted by a tone that deviates from the standard stimulus along one or more dimensions (e.g., pitch or duration). Within the context of the PC framework, the MMN can be conceptualized as a PE that is generated when the prior belief—expectation of a standard stimulus—is violated by the presentation of a deviant tone. MMN production also appears to rely on the same biological processes that are thought to be involved in prediction formation and PE signaling. Glutamate disruption via administration of NMDA antagonists such as ketamine attenuates the MMN in humans (e.g. Umbricht et al., 2000; see also Rosburg and Kreitschmann-Andermahr, 2016 for a meta-analysis) and animals (Tikhonravov et al., 2008). PC theory posits that prior expectations are signaled top-down most commonly via NMDA receptors and PEs most commonly via AMPA receptors. The MMN may therefore be an important biomarker that links neurochemical disruption in schizophrenia with a cognitive model that accounts for the development of psychosis.
One proposed mechanism by which this relationship emerges is through weak development of priors following NMDA disruption. Insufficient priors may yield abnormally weak PEs that manifest as MMN impairment, as well as aberrant belief formation and sensory experiences. Accordingly, reports have emerged suggesting that MMN amplitude is indeed associated with hallucinations (Fisher et al., 2011), and positive symptoms in general (Kärgel et al., 2014, Fisher et al., 2014). However, a number of other reports failed to find a strong relationship (e.g. Baldeweg et al., 2002, Devrim-Üçok et al., 2008, Jahshan et al., 2012, Kim et al., 2014, Perez et al., 2014, Salisbury et al., 2002), including one study with over 800 participants in which the correlation between positive symptoms and MMN amplitude was only 0.08 (Light et al., 2015). Similarly, it has been reported that MMN amplitude is associated with negative symptom severity (Catts et al., 1995), although this observation has also been challenged by a number of failures to replicate these findings (e.g. Baldeweg and Hirsch, 2015, Devrim-Üçok et al., 2008, Dulude et al., 2010, Grzella et al., 2001, Horton et al., 2011, Perez et al., 2014). As a result, the relationship between MMN impairment and symptom severity is not well-understood.
In the present analysis we evaluate the PC theory of psychosis by taking a meta-analytic approach to measure the relationship between MMN and symptom severity. We would interpret a significant association between MMN effect size and symptom severity as strong support for the PC model of psychosis. In the event that MMN impairment is not associated with psychosis, a secondary aim of this work was to identify sample characteristics that are consistently associated with reduced MMN in schizophrenia. For instance, it has been suggested that MMN impairment may be more strongly associated with low premorbid IQ than with the emergence of psychosis (Salisbury et al., 2017). This suggestion is consistent with other reports of significant associations between cognitive ability and MMN amplitude (Hermens et al., 2010, Higuchi et al., 2013); however, the results from this literature are mixed, and a meta-analytic approach is necessary to determine the robustness of this association across studies.
We examined the relationship between MMN impairment and positive and negative symptoms across 68 studies, as well as the relationship between MMN impairment and educational achievement and cognitive ability across 47 and 36 samples, respectively. We found that MMN impairment was not significantly associated with either positive or negative symptoms, but did appear to be meaningfully associated with lower educational achievement and older age in the patient group compared to healthy participants. Finally, we discuss this pattern of results and its implications for how best to consider the MMN within the PC framework.
2. Methods
2.1. Literature search and study selection
The present meta-analysis is an extension of a recent meta-analysis examining MMN effect sizes by group, illness duration, and stimulus type (Erickson et al., 2015). As such, the search parameters were identical for the present study, inclusive of all peer-reviewed research published through December 31, 2016. Briefly, the literature search was conducted through Web of Science (Thompson Reuters Corporation, New York, New York) and PubMed (National Center for Biotechnology Information, National Institutes of Health, Bethesda, Maryland) using the following search terms: schizophrenia, schizoaffective, psychosis, prodromal, bipolar disorder, mismatch negativity, and MMN (years 1987 to 2016). Although prodromal and bipolar samples were investigated in the previous study, they were not included in the present meta-analysis. Only peer-reviewed manuscripts written in English were considered. This initial search strategy identified 237 articles.
The following inclusion/exclusion criteria were then applied: (1) the MMN amplitude must be reported as a difference wave (deviant-minus-standard); (2) group differences in MMN must be reported either in terms of mean and standard deviation, t-test, F-test, effect size, or as a precise p-value; (3) the study must include at least one psychiatrically healthy control group and one comparison group of participants who have been diagnosed with a schizophrenia-spectrum disorder according to contemporary diagnostic standard (e.g., DSM-III or later, ICD-9 or later); (4) for consistency, only electroencephalogram (not magnetoencephalogram) studies of MMN were included in the present analysis; (5) only studies that presented original data were included; (6) only studies that reported symptom severity as a total or summary score from the PANSS or SAPS/SANS were included; and (7) only studies that reported educational achievement (in years) and/or cognitive test performance by group were included. Cognitive assessment tools such as the National Adult Reading Test (Nelson and Willison, 1991), subtests from the Wechsler Adult Intelligence Scale (Wechsler, 1997), and subtests from the MATRICS Consensus Cognitive Battery (Nuechterlein et al., 2008) were among the most commonly used assessments.
Finally, there were no additional inclusion/exclusion criteria for the healthy comparison sample, with the exception that they must not have a diagnosis of schizophrenia or bipolar disorder. A list of inclusion/exclusion criteria used by individual studies can be found in Supplementary Table 1.
Table 1.
Demographic, clinical, and cognitive variables.
| Healthy controls | SZ-All | SZ-F | SZ-C | |
|---|---|---|---|---|
| Total N | 3526 | 3485 | 419 | 214 |
| Age | 32.07 (7.25) | 35.14 (7.31) | 23.40 (3.42) | 34.88 (6.32) |
| Percent male | 57.87% (15.54%) | 68.46% (16.57%) | 62.07% (16.03%) | 75.96% (15.32%) |
| Education | 14.56 (1.39) | 12.63 (0.82) | 11.11 (3.87) | 12.72 (1.20) |
| Cognition (z-score) | – | − 0.89 (0.55) | − 1.55 (1.98) | − 1.42 (2.28) |
| PANSS positive | – | 16.23 (3.22) | 20.59 (2.70) | 17.19 (1.15) |
| PANSS negative | – | 18.61 (3.88) | 22.12 (3.57) | 18.79 (3.61) |
| Hedges' g | – | 0.94 | 0.41 | 0.82 |
Using these criteria, 84 unique articles were included in the meta-analysis (Supplementary Table 2). These 84 articles included 90 samples of patients with a schizophrenia-spectrum disorder, 16 of which were first episode (SZ-F), and 9 of which were chronic samples (SZ-C). The remaining samples were comprised of patients with mixed illness duration (SZ-All). Ten of the 90 samples (4 SZ-F; 6 SZ-All) included patients with other psychotic disorders, such as Psychosis NOS and Delusional disorder (see Supplementary Table 3 for a list of diagnoses). 153 articles were rejected from the meta-analysis (see Supplementary Table 4 for the list of studies and reasons for exclusion).
2.2. Effect size and meta-regression calculations
All effect size and meta-regression calculations were conducted using Comprehensive Meta-Analysis Software (Biostat, Inc., Englewood, New Jersey). Hedges' g (Hedges and Olkin, 1985) was used to estimate effect size, which is calculated as (M1 − M2)/(SDpooled). For all included studies, Hedges' g was estimated on the basis of (1) group means and standard deviations; (2) t-tests or F-tests of the group effect; (3) p-value and sample size, or (4) Cohen's d and sample size. Many studies examined MMN amplitude across multiple deviant types, probabilities, and magnitudes; the effects of these stimulus parameters were explored in previous work (Erickson et al., 2015). For the present analysis, only the group effect across all levels of deviant type, magnitude, and probability was examined.
To examine the relationship between effect size and symptom severity, positive and negative symptom severity scores were regressed on Hedges' g in two separate meta-regressions. Symptom severity was quantified using PANSS positive and negative symptom subscales for majority of samples (n = 42). The SAPS and SANS was used for 20 and 26 samples, respectively; for these studies, PANSS positive and negative symptom equivalent scores were calculated using the following equations (developed by van Erp et al., 2014):
To examine the impact of cognitive ability on MMN effect size, two meta-regressions were conducted in which (1) difference in education (in years) was regressed on Hedges' g, and (2) the standardized difference in mean cognitive performance was regressed on Hedges' g. Given the variety of cognitive tests employed by different research groups, we were unable to estimate ability for individual cognitive domains; rather, a coarse index of cognition was calculated by averaging the patients' z-transformed cognitive performance across all neuropsychological tests in a given study. Given that most neuropsychological measures are substantially intercorrelated, we expect that this coarse metric provides an approximate measure of general cognitive ability. Information about educational achievement was available for 47 samples, and cognitive performance was reported for 36 samples. Of the 36 samples in which cognitive performance was measured, 13 completed a battery of neuropsychological assessments that assessed at least three cognitive domains. An additional, separate meta-regression was conducted on this subset of samples with a more thorough neuropsychological evaluation to further explore the relationship between cognitive impairment and MMN attenuation.
3. Results
3.1. Sample characteristics and effect size by group
The combined demographic, cognitive, and clinical information from all included studies is presented in Table 1. The average age for healthy, SZ-All, and SZ-C groups was 32 to 35 years, whereas the average age for SZ-F participants was 23 years. A larger percentage of participants was male in the psychosis groups (62%–76%) compared to the healthy control group (58%). On average, healthy control participants had two more years of education (14.56 years) compared to the psychosis groups (11.11–12.72 years). SZ-F participants exhibited more severe symptoms, as well as significantly reduced MMN impairment compared to the SZ-C and SZ-All groups, consistent with our previous report (p's < 0.05) (Erickson et al., 2015).
3.2. Symptom and cognitive variables and MMN impairment
Of the 90 samples included in the meta-analysis, 62 included a PANSS positive or SAPS score, and 68 included a PANSS negative or SANS score. The results of the meta-regressions between positive and negative symptom severity with MMN effect size are depicted in Fig. 1a and b, respectively. There was no significant association between symptom severity and MMN effect size for positive symptoms (B = − 0.01, p = 0.51) or negative symptoms (B = 0.01, p = 0.55).
Fig. 1.
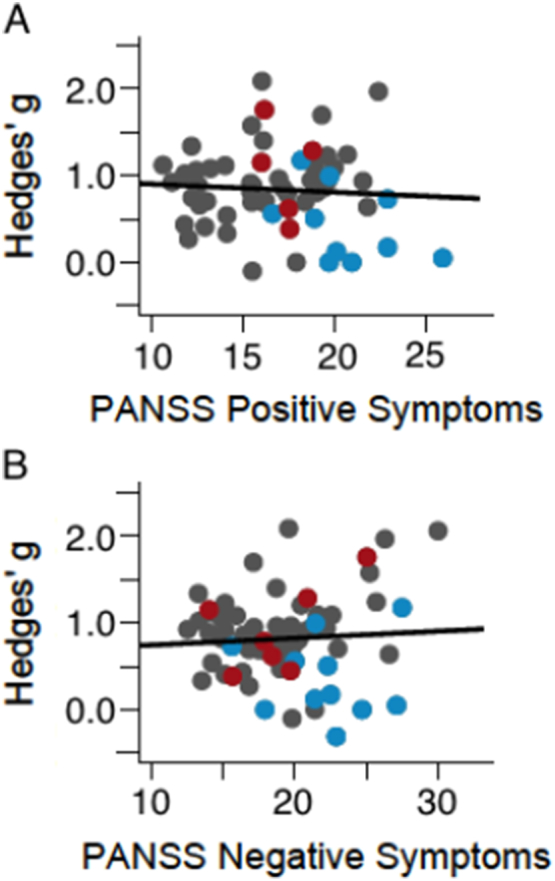
Regression of positive symptoms on Hedges' g (A) and regression of negative symptoms on Hedges' g (B). Gray = SZ-All; Red = SZ-C; Blue = SZ-F. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
By contrast, there was a trend-level association between difference in education and MMN effect size (B = 0.11, p = 0.07; Fig. 2a). That is, the larger the difference in educational achievement, the larger the difference in MMN. Given that educational achievement is typically moderately correlated with cognitive ability, we would expect to see similar effects on measures of cognitive performance (36 samples). However, the relationship between cognition and effect size was not significant (B = − 0.01, p = 0.86; Fig. 2b). Similar observations were made using the subset of 13 samples that received a more thorough cognitive assessment (B = − 0.25, p = 0.61). Given the suggestive findings on educational achievement and the widely varying quality of cognitive assessment data across studies, we urge caution in accepting this null result. Additional large sample studies with adequate cognitive testing are needed to support more definitive conclusions.
Fig. 2.
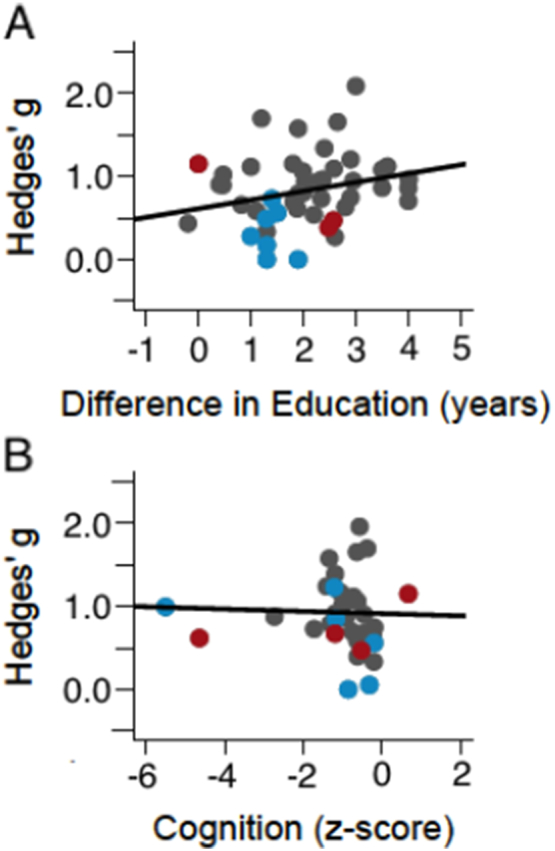
Regression of education on Hedges' g (A) and regression of cognitive performance on Hedges' g (B). Gray = SZ-All; Red = SZ-C; Blue = SZ-F. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Finally, because healthy control participants were three years younger than patient samples, on average, we conducted a final meta-regression examining the relationship between age disparity and MMN impairment (86 samples). Difference in age was significantly associated with MMN effect size (B = − 0.04, p < 0.01; Fig. 3), with larger effect sizes associated with older patient samples compared to controls.
Fig. 3.

Regression of age disparity on Hedges'g. Gray = SZ-All; Red = SZ-C; Blue = SZ-F. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The primary findings of the present study call into question the simple model of PC disruption that posits a direct relationship between MMN attenuation and the severity of psychosis. We found no significant association to suggest that the magnitude of MMN impairment is correlated with positive or negative symptoms of schizophrenia. These conclusions are underscored by the observation that SZ-F individuals exhibited more severe psychopathology in both positive and negative symptom domains, and yet had significantly smaller MMN impairment compared to the SZ-All and SZ-C participants. Furthermore, previous reports indicated that individuals at high risk for developing psychosis have robustly impaired MMN—similar to that of chronic patients—but without yet having developed psychotic symptoms (Erickson et al., 2015). These puzzling findings suggest that while MMN is severely and consistently attenuated in people with schizophrenia, this impairment is independent of the severity of clinical symptoms.
The MMN is not the only perceptual PE signal that is not robustly associated with psychosis. For example, Notredame et al., (2014) surveyed the literature and found no consistent association between weakened visual illusion susceptibility (conceptualized as a failure to develop priors) and severity of positive symptoms. In light of these findings, we suggest a modification to the current PC conceptualization that may reconcile these observations and can be tested experimentally in future studies. Put simply, the PC model of brain function and dysfunction is inherently hierarchical, yet the simple PC model of positive symptoms does not take into account this complexity. The extreme prediction of the simple model of psychosis is that failure to form predictions and generate PEs—no matter how simple or complex those predictions are—should be associated with symptom severity. In reality, however, the predictions made following a sequence of identical tones in a typical MMN paradigm are fundamentally different in precision and complexity from predictions made following a series of complex social interactions. Simple tone discrimination in the auditory cortex requires little higher order engagement and is likely to be relatively contained within early auditory processing regions. By contrast, the analysis of subtle variations in complex human behavior involves integration across time, space, and modality (Adams et al., 2013), and each step is translated by multiple priors and PEs. This complexity amplifies the potential for the PC machinery to fail, perhaps in a way that is more strongly associated with psychotic symptoms than are the simple inferences indexed by the MMN.
A more nuanced PC formulation therefore proposes that not all forms of prediction are directly relevant to psychosis. Rather, low-level PC abnormalities that occur during early perceptual events (e.g., MMN impairment) may reflect just one trait-like marker of psychiatric disturbance that does not appear to be necessary or sufficient for the emergence of positive symptoms. Instead, we suggest that psychotic symptoms reflect higher-order compensations for aberrant lower-order PE signals that may or may not be observed in conjunction with MMN deficits. That is, when low-level PE propagation fails (as in MMN impairment), higher-level, top-down inferences compensate (Adams et al., 2013). This can be observed in the case of conditioned hallucinations in psychotic patients (Kot and Serper, 2002) and in a stronger reliance on high-level priors in early psychosis and psychosis-prone individuals (Teufel et al., 2015). To test this novel conceptualization, future work will be necessary to determine the relationship between psychosis and PEs at different hierarchical levels within the same sample of patients. Even within the scope of the MMN paradigm, it will be of interest to determine whether MMN responses to complex deviants (e.g., pattern violation) exhibits a stronger relation to symptoms than do MMN responses to simple deviants (e.g., duration violation).
Although MMN impairment is not significantly related to symptom severity, it is associated with a comparatively older patient sample. As it is known that MMN amplitude decreases with age in both patients and controls (Kiang et al., 2009), this observation is not surprising. MMN impairment was also found to be associated with comparatively less educational achievement in the patient sample at the trend level. Though the present study found no significant association between MMN and a coarse measure of cognitive impairment, these results suggest that MMN impairment may be more closely linked with poor premorbid function, which is a significant risk factor for the development of psychosis (Seidman et al., 2016). Such a pattern is consistent with observations that MMN is robustly impaired in individuals who are at elevated risk for developing psychosis but have not yet begun to express symptoms (Erickson et al., 2015). Consequently, MMN impairment may serve as an important marker for identifying individuals with elevated risk for converting to psychosis.
MMN impairment, considered a key biomarker for schizophrenia, does not correlate with the severity of positive or negative symptoms. Given that MMN has been conceptualized as a PE signal, our results challenge simple PC explanations of hallucinations and delusions. They call for a deeper appreciation for the role of hierarchical representations and, taken in the context of other data, it appears that symptoms may be associated with higher levels of the hierarchy and more complex inferences than those engaged by the MMN.
Funding sources
This work was supported by the National Health and Medical Research Council of Australia (APP1090716 awarded to MA) and NIMH (R01 MH065034 awarded to JG). PRC was supported by the Connecticut Mental Health Center (CMHC) and Connecticut State Department of Mental Health and Addiction Services (DMHAS). He was funded by R01 MH067073, the NCPTSD, an IMHRO/Janssen Rising Star Translational Research Award and CTSA grant number UL1 TR000142 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of NIH or the CMHC/DMHAS.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scog.2017.05.002.
Appendix A. Supplementary data
Supplementary material
References
- Adams R.A. The computational anatomy of psychosis. Front. Psychol. 2013;4:1–26. doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T., Hirsch S.R. Mismatch negativity indexes illness-specific impairments of cortical plasticity in schizophrenia: a comparison with bipolar disorder and Alzheimer's disease. Int. J. Psychophysiol. 2015;95(2):145–155. doi: 10.1016/j.ijpsycho.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Baldeweg T. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int. J. Psychophysiol. 2002;43:111–122. doi: 10.1016/s0167-8760(01)00183-0. [DOI] [PubMed] [Google Scholar]
- Catts S.V. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am. J. Psychiatr. 1995;152:213–219. doi: 10.1176/ajp.152.2.213. [DOI] [PubMed] [Google Scholar]
- Corlett P.R., Honey G.D., Fletcher P.C. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J. Psychopharmacol. 2007;21(3):238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- Devrim-Üçok M., Keskin-Ergen H.Y., Üçok A. Mismatch negativity at acute and post-acute phases of first-episode schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258(3):179–185. doi: 10.1007/s00406-007-0772-9. [DOI] [PubMed] [Google Scholar]
- Dulude L., Labelle A., Knott V.J. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. J. Clin. Psychopharmacol. 2010;30(5):541–548. doi: 10.1097/JCP.0b013e3181f0c9c6. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Ruffle A., Gold J.M. A meta-analysis of mismatch negativity in schizophrenia from clinical risk to disease specificity and progression. Biol. Psychiatry. 2015:1–8. doi: 10.1016/j.biopsych.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp T.G.M. Converting positive and negative symptom scores between PANSS and SAPS/SANS. Schizophr. Res. 2014;152(1):289–294. doi: 10.1016/j.schres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.J. Effects of auditory hallucinations on the mismatch negativity (MMN) in schizophrenia as measured by a modified “optimal” multi-feature paradigm. Int. J. Psychophysiol. 2011;81(3):245–251. doi: 10.1016/j.ijpsycho.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Fisher D.J. Attenuation of mismatch negativity (MMN) and novelty P300 in schizophrenia patients with auditory hallucinations experiencing acute exacerbation of illness. Biol. Psychol. 2014;100:43–49. doi: 10.1016/j.biopsycho.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Frith C.D. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat. Rev. Neurosci. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Grzella I. Novelty-elicited mismatch negativity in patients with schizophrenia on admission and discharge. J. Psychiatry Neurosci. 2001;26:235–246. [PMC free article] [PubMed] [Google Scholar]
- Hedges L.V., Olkin I. Academic Press; Orlando, FL: 1985. Statistical Methods for Meta-Analysis. [Google Scholar]
- Heinz A. Dopaminergic dysfunction in alcoholism and schizophrenia – psychopathological and behavioral correlates. Eur. J. Psychiat. 2002;17:9–16. doi: 10.1016/s0924-9338(02)00628-4. [DOI] [PubMed] [Google Scholar]
- Hermens D.F. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(6):822–829. doi: 10.1016/j.pnpbp.2010.03.019. http://dx.doi.org/10.1016/j.pnpbp.2010.03.019 Available at: [DOI] [PubMed] [Google Scholar]
- Higuchi Y. Mismatch negativity and cognitive performance for the prediction of psychosis in subjects with at-risk Mental State T. Kato, ed. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horga G. Deficits in predictive coding underlie hallucinations in schizophrenia. J. Neurosci. 2014;34(24):8072–8082. doi: 10.1523/JNEUROSCI.0200-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J. MMN responsivity to manipulations of frequency and duration deviants in chronic, clozapine-treated schizophrenia patients. Schizophr. Res. 2011;126(1–3):202–211. doi: 10.1016/j.schres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Jahshan C. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol. Med. 2012;42(01):85–97. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a State of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatr. 2002;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kärgel C. Mismatch negativity latency and cognitive function in schizophrenia J. Snyder, ed. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0084536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang M. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clin. Neurophysiol. 2009;120(11):1949–1957. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. Impaired mismatch negativity is associated with current functional status rather than genetic vulnerability to schizophrenia. J. Psychiatry Res. 2014;222(1-2):100–106. doi: 10.1016/j.pscychresns.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Kot T., Serper M. Increased susceptibility to auditory conditioning in hallucinating schizophrenic patients: a preliminary investigation. J. Nerv. Ment. Dis. 2002;190:282–288. doi: 10.1097/00005053-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Light G.A. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res. 2015;163:63–72. doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H.E., Willison J. NFER-Nelson; Windsor: 1991. The National Adult Reading Test (NART) [Google Scholar]
- Notredame C.E., Pins D., Deneve S., Jardri R. What visual illusions teach us about schizophrenia. Front. Integr. Neurosci. 2014;8:1–16. doi: 10.3389/fnint.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. Psychiatr. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Perez V.B. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: forecasting psychosis risk with mismatch negativity. Biol. Psychiatry. 2014;75(6):459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosburg T., Kreitschmann-Andermahr I. The effects of ketamine on the mismatch negativity (MMN) in humans – a meta-analysis. Clin. Neurophysiol. 2016;127(2):1387–1394. doi: 10.1016/j.clinph.2015.10.062. [DOI] [PubMed] [Google Scholar]
- Salisbury D.F. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch. Gen. Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Salisbury D.F. Pitch and duration mismatch negativity and premorbid intellect in the first hospitalized schizophrenia spectrum. Schizophr. Bull. 2017 doi: 10.1093/schbul/sbw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman L.J. Association of neurocognition with transition to psychosis. JAMA Psychiat. 2016;73(12):1239–1248. doi: 10.1001/jamapsychiatry.2016.2479. http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2016.2479 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc. Natl. Acad. Sci. U. S. A. 2015;112(43):13401–13406. doi: 10.1073/pnas.1503916112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonravov D. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Res. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Umbricht D. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers. Arch. Gen. Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Wacongne C., Changeux J.-P., Dehaene S. A neuronal model of predictive coding accounting for the mismatch negativity. J. Neurosci. 2012;32(11):3665–3678. doi: 10.1523/JNEUROSCI.5003-11.2012. http://www.jneurosci.org/content/32/11/3665.full Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1997. WAIS-III: Wechsler Adult Intelligence Scale. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


