Abstract
Background
NOD2 and smoking are risk factors for Crohn's disease. We meta-analyzed NOD2-smoking interactions in Crohn's disease (Phase 1), then explored the effect of age at diagnosis on NOD2-smoking interactions (Phase 2).
Methods
Phase 1: MEDLINE and EMBASE were searched for studies (n = 18) providing data on NOD2 and smoking in Crohn's disease. NOD2-smoking interactions were estimated using odds ratios (ORs) and 95% confidence intervals (CIs) calculated using random effects models. Phase 2: A case-only study compared the proportion of smokers and carriers of the 1007 fs variant across ages at diagnosis (≤ 16, 17–40, > 40 years).
Findings
Phase 1: Having ever smoked was less common among carriers of the 1007 fs variant of NOD2 (OR 0.74, 95%CI:0.66–0.83). There was no interaction between smoking and the G908R (OR 0.96, 95%CI:0.82–1.13) or the R702W variant (OR 0.89, 95%CI:0.76–1.05). Phase 2: The proportion of patients (n = 627) carrying the 1007 fs variant decreased with age at diagnosis (≤ 16 years: 15%; 17–40: 12%; > 40: 3%; p = 0.003). Smoking was more common in older patients (≤ 16 years: 4%; 17–40: 48%; > 40: 71%; p < 0.001).
Interpretation
The negative NOD2-smoking interaction in Crohn's disease is specific to the 1007 fs variant. However, opposing rates of this variant and smoking across age at diagnosis may explain this negative interaction.
Abbreviations: CI, confidence interval; NOD2, nucleotide binding oligomerization domain containing 2; OR, odds ratio; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses; SNP, single nucleotide polymorphism
Keywords: Crohn's disease, NOD2, Cigarette smoking, Gene-environment interactions, Age
Highlights
-
•
There is a negative interaction between NOD2 smoking in Crohn's disease and it is specific to the 1007fs variant.
-
•
With increasing age, the prevalence of the 1007fs variant decreases and exposure to cigarette smoke increases.
-
•
Contrasting trends in the 1007fs variant and cigarette smoking may explain the negative NOD2-smoking interaction.
We reviewed 18 studies evaluating NOD2-smoking interactions in Crohn's disease. Only the 1007fs variant interacted with smoking. Smokers with this mutation were less likely to develop Crohn's disease. We then conducted a study of 627 patients with Crohn's disease, which showed that the 1007fs variant was common in young patients and rare in older patients, whereas smoking was more common among older patients. The decreasing prevalence of 1007fs mutation and increasing exposure to smoking as age of diagnosis advances may explain the negative interaction between NOD2 and smoking observed in our meta-analysis. Our study highlights the challenges of identifying gene-environment interactions.
1. Introduction
Crohn's disease is a chronic inflammatory disease of the gastrointestinal tract believed to result from the interaction between genetic and environmental factors, leading to an inappropriate immune response to intestinal microbes (Knights et al., 2013). However, identifying reproducible gene-environment interactions in patients with Crohn's disease remains elusive. Previous studies examining the effects of NOD2 (nucleotide binding oligomerization domain containing 2) on Crohn's disease focus on three single nucleotide polymorphisms (SNPs): 1007fs, G908R, and R702W. Individuals with the 1007 fs variant have a 4-fold increased odds of developing Crohn's disease, while the G908R variant triples and the R702W variant doubles the odds of Crohn's disease (Economou et al., 2004). Active and ex-smokers both have an approximately 2-fold increase in the odds of developing Crohn's disease (Mahid et al., 2006).
Although NOD2 and smoking both independently increase the risk of Crohn's disease, an interactive effect is not consistently demonstrated. Some studies demonstrated a negative interaction between NOD2 and smoking (de Diego et al., 2006, Helbig et al., 2012) while others failed to demonstrate a significant interaction (Mardini et al., 2005, Mendoza et al., 2003). In part, this may be explained by age-specific effects of genetic and environmental factors. Genetic susceptibility may play a larger role in patients with early-onset Crohn's disease (i.e., diagnosis before age 16) compared to those diagnosed later in life (i.e., after age 40) (de Ridder et al., 2007). In contrast, patients with late-onset Crohn's disease may have a greater cumulative exposure to environmental factors (e.g., longer history of smoking) than those with early-onset Crohn's disease.
The aim of this study was to systematically review and summarize the current knowledge of NOD2-smoking interactions in Crohn's disease, including SNP-specific NOD2-smoking interactions, and carry out a case-only study to explore the role of age at diagnosis on the association between NOD2 and smoking.
2. Materials and Methods
2.1. Phase 1: Systematic Review and Meta-Analysis of NOD2-Smoking Interactions
Because of small sample sizes, most prior studies on NOD2-smoking interactions pooled the three most common NOD2 variants (1007fs, G908R, R702W), treating each SNP as an equivalent risk allele for the NOD2-smoking interactions. Consequently, we conducted a systematic review and meta-analysis to separately evaluate the interaction between SNP-specific NOD2 variants and smoking in patients with Crohn's disease. The systematic review and meta-analysis was based on a pre-determined protocol (Supplementary Table 1) and is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist (Moher et al., 2009).
2.1.1. Study Identification and Selection.
MEDLINE (1946–November 2015) and EMBASE (1974–November 2015) were searched to identify observational studies that examined both cigarette smoking and NOD2 variants in relation to any Crohn's disease-related outcome (i.e., disease onset, prognosis, or phenotype). The search strategy is provided in Supplementary Table 1. Studies were included if they provided data on the interaction between a NOD2 variant and smoking status in patients with Crohn's disease. Only studies reporting on each SNP independently were included. Studies reporting on the independent effects of cigarette smoking and NOD2 were included if data on the interaction between NOD2 and having ever smoked could be obtained from study authors. Additionally, authors of studies combining all SNPs in a single analysis were contacted to provide SNP-specific data and/or additional data on smoking behaviors if not included in their original publication.
References of included studies and relevant review articles were hand searched. Conference proceedings from major gastroenterological meetings (e.g., Digestive Diseases Week 2009–2015, American College of Gastroenterology Annual Scientific Meeting 2010–2014, and Congress of the European Crohn's and Colitis Organization 2011–2014) are indexed in EMBASE and were reviewed during the primary database search. When multiple manuscripts provided data on the same cohort of patients, the study providing the most complete data was included in the meta-analysis; if comparable data was presented, the most recent manuscript was included. The search was not limited by language, geography, or date of publication.
All records identified from the database search were independently screened by two study authors (MEK and SC). The full-text of selected abstracts was reviewed by two study authors (MEK and JY). Disagreements were resolved in consultation with a third author (GGK).
2.1.2. Data Extraction
Two authors (MEK and JY) independently extracted data from eligible studies using a piloted data extraction form, including: study design, quality, and location; variants of NOD2; definition of smoking and source of smoking information (questionnaire, chart review, or interview); source of cases; sample size; prevalence of smoking; NOD2 allele frequency; and the interaction between each NOD2 variant and smoking, either as contingency tables or odds ratios (ORs) and their corresponding 95% confidence intervals (CIs).
2.1.3. Outcomes
Case-only studies are commonly used to study gene-environment interactions (Thomas, 2010). We used all cases from identified studies to examine NOD2-smoking interactions in patients with Crohn's disease. We examined the association between ever smoking (current and former smoking) among individuals with a NOD2 variant as compared to patients who were wild type. The analysis was based on smoking status at the time of diagnosis or at study entry when smoking ‘at diagnosis’ was unavailable. Analyses were conducted for the three most common NOD2 variants (1007fs, G908R, and R702W) independently.
The following a priori sensitivity analyses were conducted: (1) removing studies with unclear definitions of smoking; (2) limiting analyses to studies defining smoking at diagnosis; and (3) limiting to studies where all participants were non-Jewish and White.
2.1.4. Study Quality
The quality of included studies was assessed using a modified version of the Newcastle-Ottawa Quality Assessment Scale that incorporated aspects of study quality relevant to case-only studies of gene-environment interactions (Supplementary Table 1) (Wells et al., 2017). This included the methods used to select cases and misclassification bias (e.g., ascertainment of smoking status). The risk of bias due to residual confounding could not be assessed because the assessment of NOD2-smoking interactions was not the primary objective of most included studies. Study quality was evaluated independently by two authors (MEK and JY). Disagreements were resolved by consensus and in consultation with a third author (GGK).
2.1.5. Statistical Analysis
Data were analyzed using the meta package in R version 3.3.2 (Schwarzer, 2017, Core Team, 2016). ORs and corresponding 95% CIs were calculated from contingency tables for each study based on cases of Crohn's disease only, then pooled using random effects models to account for expected heterogeneity across studies. The estimated ORs reflect the odds of being a smoker among individuals with a NOD2 mutation relative to the odds of being a smoker among patients without a NOD2 mutation.
Heterogeneity was assessed using the I2 measure and the Cochran Q-statistic; p < 0.10 was considered statistically significant. Variance between studies (τ2) was quantified using the DerSimonian-Laird estimator. Publication bias was assessed using a visual inspection of funnel plots. The Egger test was used to assess funnel plot asymmetry (Egger et al., 1997).
2.2. Phase 2: Effect of Age at Diagnosis on NOD2-Smoking Interaction
We evaluated the hypothesis that the negative interaction between the 1007 fs variant in NOD2 and cigarette smoking may be explained by the opposing prevalence of these factors across different ages at diagnosis. Based on the results of the meta-analysis we only evaluated the interaction between the 1007 fs variant of NOD2 and smoking.
We conducted a case-only study in a cohort of patients with Crohn's disease enrolled in the Alberta IBD Consortium. The Alberta IBD Consortium recruited patients from tertiary care centers in Calgary, Alberta (n = 324) and Edmonton, Alberta, Canada (n = 303) between 2007 and 2014. All genotyped patients with a completed environmental questionnaire were included in the analysis. Medical chart reviews were completed on each patient with Crohn's disease using a standardized electronic data extraction form to confirm the diagnosis of Crohn's disease and establish the age at diagnosis. Diagnosis of Crohn's disease was confirmed using standard clinical, endoscopic, radiologic, and histologic criteria (Lennard-Jones, 1989). A subset of chart reviews (n = 20) was completed in duplicate to ensure consistency across reviewers. Age at diagnosis was defined a priori according to the Montreal Classification: A1 (≤ 16 years of age); A2 (17 to 40 years); and A3 (> 40 years) (Silverberg et al., 2005, Satsangi, 2006). In order to account for population stratification, only non-Jewish Caucasian patients were included in the analysis.
A standardized environmental questionnaire was used to assess smoking status of patients and included information on frequency, timing, quitting, and duration of cigarette use. A research coordinator facilitated the administration of the environmental questionnaire to ensure accuracy and completeness of data. Determination of smoking status was blinded to NOD2 genotype. Ever smokers had smoked at least one cigarette per day for at least one year prior to being diagnosed and never smokers were lifelong non-smokers prior to diagnosis. Previous studies have shown retrospective recall of smoking habits have been demonstrated to be valid (sensitivity: 92%; specificity: 98%) (Wong et al., 2012).
DNA was extracted from venous blood of patients using the Qiagen (Germantown, MD, USA) DNAeasy kit. Samples were then sent to BGI-Shenzhen for processing. The 1007fs variant of NOD2 was genotyped using a Goldengate platform (Illumina). DNA extraction and genotyping was conducted without knowledge of the patient's smoking status. Carriers of the 1007fs variant were defined as individuals who were either homozygous or heterozygous for this variant of the NOD2 gene.
All statistical analyses were conducted using SAS Version 9.4 (Cary, NC, USA). We used the Cochran-Armitage test for trend to determine if the proportion of patients carrying the 1007fs variant and having ever smoked changed with age at diagnosis, as well as to test for differences in the city of residence and sex of patients across age groups. Age groups were determined a priori and based on the Montreal Classification (Silverberg et al., 2005, Satsangi, 2006).
To ensure the interaction between the 1007fs variant and smoking was consistent with the findings of the meta-analysis, we calculated the OR and 95% CI to estimate this interaction. This odds ratio compared the odds of carrying the 1007fs SNP among ever smokers to the odds of carrying the 1007 fs SNP (homozygotes for the risk allele and heterozygotes) among never smokers.
This study was approved by the Conjoint Research Ethics Boards at the University of Calgary and the University of Alberta. Signed informed consent was obtained from all study participants.
3. Results
3.1. Phase 1: Systematic Review and Meta-Analysis of NOD2-Smoking Interaction
The literature search yielded 248 records; 170 remained after removing duplicates. An additional 53 studies were identified after reviewing references of included studies and relevant review articles. Four studies were eligible for inclusion (Supplementary Fig. 1) (de Diego et al., 2006, Helbig et al., 2012, Mendoza et al., 2003, Mardini et al., 2005). An additional 14 studies were included after obtaining SNP-specific data from study authors (Ananthakrishnan et al., 2014, Cleynen et al., 2013, Doecke et al., 2015, Giachino et al., 2004, Henckaerts et al., 2009, Karban et al., 2011, Laghi et al., 2005, Lakatos et al., 2005, Latiano, 2008, Nagy et al., 2005, Protic et al., 2008, Renda et al., 2008, Sabate et al., 2008, Walker et al., 2004). One study providing SNP-specific data was excluded from the analysis due to small cell sizes (Lauriola et al., 2011). In total, 18 studies (9064 patients with Crohn's disease) were included in the meta-analysis. Reasons for study exclusion are outlined in Supplementary Table 2. The characteristics of included studies are provided in Table 1. The risk of bias in included studies is provided in Supplementary Table 3.
Table 1.
Characteristics of included studies.
| Study | Country | Age at diagnosis; mean (sd), years | NOD2 genotype frequenciesa | Smoking statusb | Timing of smoking | Sample size |
|---|---|---|---|---|---|---|
| Ananthakrishnan et al. (2014) | USA | Median (Q1,Q3): 24 (18, 33) | Homozygous: 7% Heterozygous: 26% Wild type: 67% |
Current: 8% Former: 30% Never: 62% |
At time of study | 697c |
| Cleynen et al. (2013) | Europe (7 countries) | 26 (11) | Homozygous: 10% Heterozygous: 29% Wild type: 62% |
Current: 21% Former: 27% Never: 52% |
At time of study | 1528c |
| de Diego et al. (2006) | Spain | Ever smokers: 33(12) Never smokers: 34 (16) Range: 12 to 77 years |
Carriers: 28% Wild type: 72% |
Ever: 70% Never: 30% |
At diagnosis | 178 |
| Doecke et al. (2015) | Australia and New Zealand | < 16: 10% 16–40: 77% >40: 13% |
Homozygous: 9% Heterozygous: 24% Wild type: 67% |
Current: 44% Former: 46% Never: 10% |
At diagnosis | 675d |
| Giachino et al. (2004) | Italy | < 40: 77% >40: 23% |
Carrier: 33% Wild type: 67% |
Current: 35% Former: 15% Never: 50% |
At time of study | 184c |
| Helbig et al. (2012) | Germany | 26 (10) | Carrier: 46% Wild type: 54% |
Current (diagnosis): 41% Non-current (diagnosis): 59% Ever (study): 58% Never (study): 42% |
At diagnosis (N = 1283) At time of study (N = 1636) |
1636 |
| Henckaerts et al. (2009) | Belgium | Median (Q1, Q3): 24 (19, 32) ≤16: 18% 17–40: 71% >40: 11% |
Carriers: 45% Wild type: 55% |
Current: 64% Former: 8% Never: 27% |
At diagnosis | 755c, e |
| Karban et al. (2011) | Israel | 25 (12) | Homozygous: 6% Heterozygous: 26% Wild type: 68% |
Current: 24% Former: 11% Never: 65% |
At time of study | 453f |
| Laghi et al. (2005) | Italy | 35 (13) | Carrier: 33% Wild type: 67% |
Ever: 53% Never: 47% |
At diagnosis | 239 |
| Lakatos et al. (2005) | Hungary | 37 (9) | Homozygous: 10% Heterozygous: 25% Wild type: 65% |
Current: 32% Former: 9% Never: 58% |
At time of study | 527 |
| Latiano (2008) | Italy | 29 (15) | Homozygous: 8% Heterozygous: 26% Wild type: 66% |
Current: 33% Former: 12% Never: 55% |
At time of study | 763c |
| Mardini et al. (2005) | USA | Median (range): 22 (11 to 41) years | G908R Heterozygous: 8% G908R Wild type: 92% 1007 fs Homozygous: 2% 1007fs Heterozygous: 12% 1007 fs Wild type: 86% R702W Homozygous: 1% R702W Heterozygous: 17% R702W Wild type: 82% |
Ever: 54% Never: 46% |
Unclear | 202 |
| Mendoza et al. (2003) | Spain | < 40 years: 78% ≥40 years: 22% |
Carrier: 33% Wild type: 67% |
Smokers: 54% Non-smokersg: 46% |
Unclear | 204 |
| Nagy et al. (2005) | Hungary |
NOD2 carrier: 30 NOD2 wild type: 33h |
Homozygous: 4% Heterozygous: 30% Wild type: 66% |
Current: 50% Former: 38% Never: 12% |
At time of study | 74 |
| Protic et al. (2008) | Serbia | Median (range): 26 (6, 59) ≤16 years: 12% 17–40 years: 72% >40 years: 16% |
Homozygous: 2% Heterozygous: 34% Wild type: 65% |
Current: 22% Former: 1% Never: 77% |
At time of study | 131 |
| Renda et al. (2008) | Italy | 30 (12) | Homozygous: 6% Heterozygous: 24% Wild type: 69% |
Current: 37% Former: 14% Never: 49% |
At diagnosis | 182c |
| Sabate et al. (2008) | France | 40 (14) | Homozygous: 5% Heterozygous: 30% Wild type: 64% |
Current: 42% Former: 6% Never: 52% |
At diagnosis | 239c |
| Walker et al. (2004) | Scotland | 28 (14) Range: 5 to 76 |
Homozygous: 2% Heterozygous: 22% Wild type: 76% |
Current: 22% Former: 47% Never: 30% |
At time of study | 228c |
Patients homozygous for a NOD2 mutation, either carrying two copies of the same mutation or one copy of a mutation and a copy of a different mutation (i.e., compound heterozygotes). Heterozygotes had one copy of a risk allele and one copy of the wild type allele. Wild type individuals had two copies of the normal allele. Carriers had at least one copy of the risk allele (i.e., homozygous or heterozygous). Genotype frequencies are expressed for all SNPs in the NOD2 gene unless otherwise noted.
Current smokers are patients that are smoking cigarettes at present. Former smokers had smoked in the past, but were no longer smoking at the time that smoking status was determined. Never smokers were lifetime non-smokers. Ever smokers were patients that had smoked at some point (i.e., includes current and former smokers).
NOD2-smoking data available on a subset of patients included in the study.
Data obtained from study authors corresponded to the cohort from Brisbane, Australia (dataset 1). There were 675 patients in this dataset.
Study was limited to patients without perianal disease at diagnosis.
Data obtained from study authors corresponded to an updated (larger) cohort of patients.
Smoking defined in the manuscript as ‘smokers’ and ‘non-smokers’ and was unclear if ‘smokers’ were ever smokers (i.e., current and former smokers) or current smokers only. Study was excluded in sensitivity analysis.
Unclear if the age of study participants was presented as a mean or median.
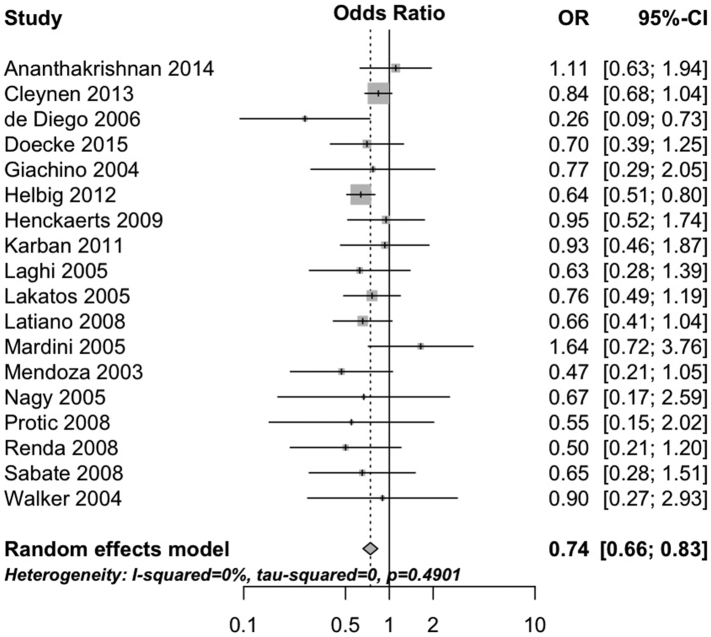
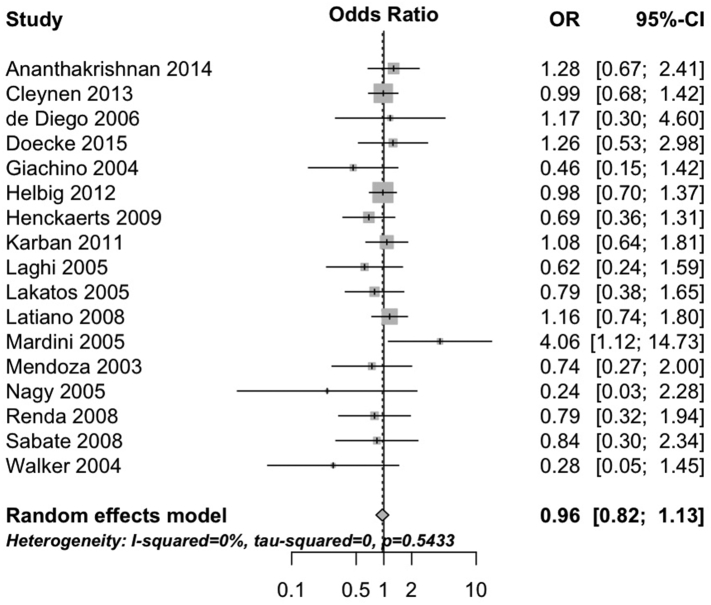
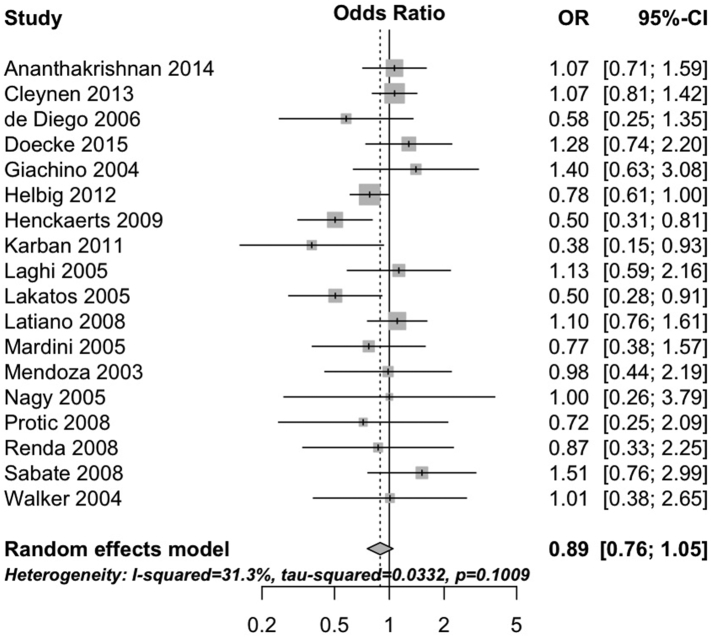
The 1007fs variant negatively interacted with having ever smoked cigarettes (pooled OR 0.74, 95%CI 0.66 to 0.83, 18 studies; heterogeneity: I2 = 0%, τ2 = 0, p = 0.49; Fig. 1). Neither the G908R variant (pooled OR 0.96, 95%CI 0.82 to 1.13, 17 studies; heterogeneity: I2 = 0%, τ2 = 0, p = 0.54; Fig. 2) nor the R702W variant (OR 0.89, 95%CI 0.76 to 1.05, 18 studies; heterogeneity: I2 = 31%, τ2 = 0.03, p = 0.10; Fig. 3) interacted with cigarette smoking. Results were consistent in sensitivity analyses excluding studies with unclear definitions of smoking (Supplementary Table 4) and when limiting analyses to studies where smoking status was defined at diagnosis (Supplementary Table 5) and all participants were non-Jewish and White (Supplementary Table 6).
Fig. 1.
Forest plot depicting the interaction between 1007fs variant of NOD2 and cigarette smoking in patients with Crohn's disease.
Fig. 2.
Forest plot depicting the interaction between G908R variant of NOD2 and cigarette smoking in patients with Crohn's disease.
Fig. 3.
Forest plot depicting the interaction between R702W variant of NOD2 and cigarette smoking in patients with Crohn's disease.
3.1.1. Publication Bias
There was no evidence of publication bias for the interaction between NOD2 and smoking, regardless of the SNP being analyzed (1007fs: p = 0.70; G908R: p = 0.22; R702W: p = 0.75) (Supplementary Fig. 2A–C).
3.2. Phase 2: Effect of Age at Diagnosis on NOD2-Smoking Interaction
We enrolled 627 patients with Crohn's disease who provided data on smoking status at diagnosis, 1007 fs NOD2 genotype, and age at diagnosis (Table 2). The proportion of patients having ever smoked significantly increased with increasing age at diagnosis (p < 0.001 for trend). Only 4% (4/91) of patients diagnosed under the age of 17 had ever smoked, compared to 48% (203/426) of those diagnosed between 17 and 40 and 71% (78/110) of patients diagnosed after 40 years of age (Table 2).
Table 2.
Characteristics of included patients.
| Age at diagnosis |
|||||
|---|---|---|---|---|---|
| Characteristic | Total | A1 (≤ 16) | A2 (17–40) | A3 (> 40) | p-value |
| Total (N) | 627 | 91 | 426 | 110 | |
| City | |||||
| Calgary | 324 (52%) | 45 (49%) | 218 (51%) | 61 (55%) | 0.87 |
| Edmonton | 303 (48%) | 46 (51%) | 208 (49%) | 49 (45%) | |
| Sex | |||||
| Male | 260 (41%) | 50 (55%) | 155 (36%) | 55 (50%) | 0.68 |
| Female | 367 (59%) | 41 (45%) | 271 (64%) | 55 (50%) | |
| NOD2 (1007fs), N (%) | |||||
| Wild type | 558 (89%) | 77 (85%) | 374 (88%) | 107 (97%) | 0.003 |
| Carriera | 69 (11%) | 14 (15%) | 52 (12%) | 3 (3%) | |
| Smoking status, N (%) | |||||
| Ever | 285 (46%) | 4 (4%) | 203 (48%) | 78 (71%) | < 0.0001 |
| Never | 342 (54%) | 87 (96%) | 223 (52%) | 32 (29%) | |
Abbreviations: Q1: 1st quartile (25th percentile); Q3: 3rd quartile (75th percentile).
Carriers of the 1007 fs variant (heterozygous or homozygous) were compared to wild type.
The proportion of patients carrying the 1007fs variant decreased significantly with increasing age at diagnosis (p = 0.003 for trend). Fifteen percent (14/91) of patients diagnosed before age 17 and 12% of patients diagnosed between 17 and 40 were carriers of this variant, whereas 3% of individuals diagnosed after 40 were carriers (3/110) (Table 2).
The odds of carrying a 1007fs variant were significantly lower among individuals who had ever smoked cigarettes compared to individuals who had never smoked (OR 0.49, 95% CI 0.27 to 0.86).
4. Discussion
We systematically reviewed the interaction between NOD2 and cigarette smoking in Crohn's disease by analyzing 18 studies with > 9000 patients, including previously unpublished data from 14 studies. Our pooled analyses demonstrated that the negative association between NOD2 and cigarette smoking was specific to the 1007 fs variant. Moreover, our case-only study showed that the prevalence of both the 1007fs mutation and cigarette smoking vary across ages at diagnosis such that with advancing age, the prevalence of the 1007 fs variant decreases and exposure to cigarette smoke increases. These findings may explain inconsistencies between studies and the elusiveness of identifying reproducible gene-environment interactions.
Our systematic review demonstrated that the majority of NOD2-smoking studies were underpowered to detect an interaction; for example, 10 identified studies included fewer than 300 participants (de Diego et al., 2006, Giachino et al., 2004, Laghi et al., 2005, Mardini et al., 2005, Mendoza et al., 2003, Protic et al., 2008, Renda et al., 2008, Sabate et al., 2008, Walker et al., 2004, Nagy et al., 2005). Because of these small samples, the three most common NOD2 variants (1007fs, G908R, and R702W) were often combined. However, this meta-analysis confirms that the NOD2-smoking interaction in Crohn's disease is SNP-specific. Thus, combining SNPs to improve power may not be methodologically sound.
The biological plausibility of a negative association between smoking and the 1007 fs variant of NOD2 is unclear. However, the complex interplay between the different NOD2 variants and cigarette smoking, and their effects on dendritic cells may underlie the negative interaction observed between smoking and the 1007 fs variant but not the NOD2 variants (Butler et al., 2007, van Heel et al., 2005, Ueno et al., 2014, Kramer et al., 2006). The 1007fs variant of NOD2 results in a protein that is truncated in the region of NOD2 responsible for intracellular detection of peptidoglycans on bacterial cell walls. The G908R and R702W variants are single base substitutions that may also alter the structure of NOD2. However, the functional impacts of each variant are not known.
Alternatively, the negative relationship between NOD2 and smoking may be explained by a methodological phenomenon. Our case-only study examined the impact of age of onset on the interaction between NOD2 and cigarette smoking in patients with Crohn's disease. Twenty percent of NOD2 carriers were diagnosed prior to age 17, whereas only 3% were diagnosed after age 40. In contrast, nearly three-quarters of patients over the age of 40 at diagnosis had a history of smoking compared to only 4% of those diagnosed under age 17. This inverse relationship between NOD2 and smoking, across ages at diagnosis results in minimal overlap of the two factors and may misleadingly appear as a gene-environment interaction. However, the negative interaction between NOD2 and smoking persists after adjusting for age at diagnosis (Helbig et al., 2012). Supplementary Fig. 3 explains the bias introduced by combining patients with varying ages of diagnosis, resulting in a negative interaction.
Consistent with prior studies, we analyzed the NOD2-smoking interaction in Crohn's disease using a case-only study design (Helbig et al., 2012). The case-only design increases resource and statistical efficiency to detect gene-environment interactions (Thomas, 2010). However, there are some assumptions of case-only designs including: (1) the absence of population stratification; and (2) the independence of the genetic and environmental risk factor in the source population for the cases (Liu et al., 2012, Pierce and Ahsan, 2010, Wang and Lee, 2008). Bias due to population stratification (i.e., confounding by ethnicity) arises when studies combine different ethnic groups with variable allele frequencies and prevalence of exposure (Wang and Lee, 2008). Sensitivity analyses limited to studies where all patients were non-Jewish and White were consistent with our main analyses. However, we were not able to assess the interaction between NOD2 and smoking in other ethnicities (e.g., African Americans) due to the paucity of studies. Secondly, we assumed gene-environment independence due to a prior study that found no association between NOD2 and smoking in their control population (Helbig et al., 2012) and NOD2 has not been identified in genome-wide association studies of smoking behaviors (Furberg et al., 2010).
The results of this study point to the importance of evaluating genetic and environmental factors in sub-phenotypes of Crohn's disease. We focused on age at diagnosis. However, the findings of NOD2-smoking interaction studies may be influenced by other disease phenotypes such as disease location or disease behavior. Both NOD2 and smoking are associated with ileal Crohn's disease. In addition, ileal and colonic Crohn's disease have been shown to be genetically distinct phenotypes (Cleynen et al., 2016). Thus, our study provides one example of how phenotypic characteristics may influence gene-environment interactions.
Our study provides the most comprehensive review of NOD2-smoking interactions in Crohn's disease, includes previously unpublished data, and evaluates the impact of age at diagnosis on this interaction. Nonetheless, limitations should be considered. Firstly, the association between NOD2 and smoking was not adjusted for potential confounders (e.g., age, family history). Also, the quality of the meta-analysis was dependent on the quality of the individual studies that were included. With regard to the case-only study we were limited by sample size, which impeded our ability to calculate age-specific ORs for the interaction between NOD2 and smoking and to investigate the role of sub-phenotypes of Crohn's disease (i.e., disease location and behavior).
Gene-environment interactions for Crohn's disease are difficult to identify. Our meta-analysis demonstrated that gene-environment interaction studies need to be designed to assess SNP-specific effects, such that only 100 fs variant of NOD2 interacted with smoking. Moreover, our case-only study showed that the negative interaction between the 1007fs variant of NOD2 and smoking may be influenced by the contrasting prevalence of NOD2 and smoking across ages at diagnosis with Crohn's disease. This is one example of how gene-environment interactions may depend on sub-phenotypes of Crohn's disease. Thus, we suggest that future gene-environment studies should be powered to assess SNP-specific interactions and be designed to evaluate interactions in specific phenotypes of patients with Crohn's disease.
Funding Sources
This project was funded by Alberta Innovates - Health Solutions and a CIHR Team Grant (Health Challenges in Chronic Inflammation). Funders had no role in the study design, data analysis, and interpretation of the study results.
Conflict of Interest
The study authors have no competing interests to declare.
Author Contributions
Guarantor of the Article: Gilaad G. Kaplan, MD MPH.
Specific Author Contributions: Study concept and design: MEK, BE, CHS, CB, GGK. Data acquisition: MEK, JY, SC, PLL, GGK. Interpretation of the data: MEK, HWB, BE, CHS, CB, RP, SG, MSS, PLL, GGK. Statistical analysis: MEK, GGK. Drafting of the manuscript: MEK, BE, CHS, CB, GGK. Critical revision of the manuscript for important intellectual content: HWB, RP, SG, MSS, PLL, JY, SC, PLB, RF, LAD, KM. Final approval of the manuscript: MEK, JY, SC, BE, CHS, CB, HWB, MSS, PLL, PLB, RF, LAD, KM, RP, SG, GGK.
Acknowledgements
The study authors would like to thank the authors of the following studies for providing additional data: Ananthakrishnan et al. (2014) (Ashwin Ananthakrishnan); Cleynen et al. (2013) (Isabelle Cleynen); Cucchiara et al. (2007) (Anna Latiano, Vito Annese); Doecke et al. (2015) (James Doecke, Graham Radford-Smith); Fowler et al. (2014) (Sharyle Fowler, Vijay Yajnik); Giachino et al. (2004) (Mario De Marchi); Henckaerts et al. (2009) (Isabelle Cleynen, Liesbet Henckaerts); Karban et al. (2011) (Amir Karban); Laghi et al. (2005) (Alberto Malesci); Lakatos et al. (2005) (Peter Lakatos); Latiano (2008) (Anna Latiano, Vito Annese); Lauriola et al. (2011) (Mattia Lauriola); Maconi et al. (2009) (Giovanni Maconi); Protic et al. (2008) (Marijana Protic); Nagy et al. (2005) (Zsuzsanna Nagy); Renda et al. (2008) (Mario Cottone); Walker et al. (2004) (Marian Aldhous, Hazel E. Drummond, Jack Satsangi). We acknowledge the support of the Alberta IBD Consortium with data derived from patient registries from the Intestinal Inflammation Tissue Bank (IITB) at the University of Calgary and the Center of Excellence for Gastrointestinal Inflammation and Immunity Research (CEGIIR) at the University of Alberta. Gil Kaplan holds a CIHR Embedded Clinician Research Award and an Alberta Innovates Population Health Award.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.06.012.
Appendix A. Supplementary data
Supplementary material
References
- Ananthakrishnan A.N. Differential effect of genetic burden on disease phenotypes in Crohn's disease and ulcerative colitis: analysis of a North American cohort. Am. J. Gastroenterol. 2014;109(3):395–400. doi: 10.1038/ajg.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. NOD2 activity modulates the phenotype of LPS-stimulated dendritic cells to promote the development of T-helper type 2-like lymphocytes — possible implications for NOD2-associated Crohn's disease. J. Crohn's Colitis. 2007;1(2):106–115. doi: 10.1016/j.crohns.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Cleynen I. Genetic factors conferring an increased susceptibility to develop Crohn's disease also influence disease phenotype: results from the IBDchip European Project. Gut. 2013;62(11):1556–1565. doi: 10.1136/gutjnl-2011-300777. [DOI] [PubMed] [Google Scholar]
- Cleynen I. Inherited determinants of Crohn's disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2016;387(10014):156–167. doi: 10.1016/S0140-6736(15)00465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiara S. Role of CARD15, DLG5 and OCTN genes polymorphisms in children with inflammatory bowel diseases. World J. Gastroenterol. 2007;13(8):1221–1229. doi: 10.3748/wjg.v13.i8.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego C. Influence of smoking habits and CARD15 mutations on the onset of Crohn's disease. Scand. J. Gastroenterol. 2006;41(10):1209–1211. doi: 10.1080/00365520600717496. [DOI] [PubMed] [Google Scholar]
- de Ridder L. Genetic susceptibility has a more important role in pediatric-onset Crohnʼs disease than in adult-onset Crohnʼs disease. Inflamm. Bowel Dis. 2007;13(9):1083–1092. doi: 10.1002/ibd.20171. [DOI] [PubMed] [Google Scholar]
- Doecke J.D. Smoking behaviour modifies IL23r-associated disease risk in patients with Crohn's disease. J. Gastroenterol. Hepatol. 2015;30(2):299–307. doi: 10.1111/jgh.12674. [DOI] [PubMed] [Google Scholar]
- Economou M. Differential effects of NOD2 variants on Crohn's disease risk and phenotype in diverse populations: a metaanalysis. Am. J. Gastroenterol. 2004;99(12):2393–2404. doi: 10.1111/j.1572-0241.2004.40304.x. [DOI] [PubMed] [Google Scholar]
- Egger M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S.A. SMAD3 gene variant is a risk factor for recurrent surgery in patients with Crohn's disease. J. Crohn's Colitis. 2014;8(8):845–851. doi: 10.1016/j.crohns.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 2010;42(5):441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino D. Analysis of the CARD15 variants R702W, G908R and L1007fs in Italian IBD patients. Eur. J. Hum. Genet. 2004;12(3):206–212. doi: 10.1038/sj.ejhg.5201130. [DOI] [PubMed] [Google Scholar]
- Helbig K.L. A case-only study of gene-environment interaction between genetic susceptibility variants in NOD2 and cigarette smoking in Crohn's disease aetiology. BMC Med. Genet. 2012;13(1):14. doi: 10.1186/1471-2350-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckaerts L. Genetic risk profiling and prediction of disease course in Crohn's disease patients. Clin. Gastroenterol. Hepatol. 2009;7(9):972–980.e2. doi: 10.1016/j.cgh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Karban A. Non-Jewish Israeli IBD patients have significantly higher Glutathione S-Transferase GSTT1-null frequency. Dig. Dis. Sci. 2011;56(7):2081–2087. doi: 10.1007/s10620-010-1543-4. [DOI] [PubMed] [Google Scholar]
- Knights D., Lassen K.G., Xavier R.J. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62(10):1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. Impaired dendritic cell function in Crohn's disease patients with NOD2 3020insC mutation. J. Leukoc. Biol. 2006;79(4):860–866. doi: 10.1189/jlb.0805484. [DOI] [PubMed] [Google Scholar]
- Laghi L. Carriage of CARD15 variants and smoking as risk factors for resective surgery in patients with Crohn's ileal disease. Aliment. Pharmacol. Ther. 2005;22(6):557–564. doi: 10.1111/j.1365-2036.2005.02629.x. [DOI] [PubMed] [Google Scholar]
- Lakatos P.L. Toll-like receptor 4 and NOD2/CARD15 mutations in Hungarian patients with Crohn's disease: phenotype-genotype correlations. World J. Gastroenterol. 2005;11(10):1489–1495. doi: 10.3748/wjg.v11.i10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latiano A. Replication of interleukin 23 receptor and autophagy-related 16-like 1 association in adult- and pediatric-onset inflammatory bowel disease in Italy. World J. Gastroenterol. 2008;14(29):4643–4649. doi: 10.3748/wjg.14.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriola M. IL23R, NOD2/CARD15, ATG16L1 and PHOX2B polymorphisms in a group of patients with Crohn's disease and correlation with sub-phenotypes. Int. J. Mol. Med. 2011;27(3):469–477. doi: 10.3892/ijmm.2010.591. [DOI] [PubMed] [Google Scholar]
- Lennard-Jones J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. 1989;24(s170):2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- Liu C.-Y. Design and analysis issues in gene and environment studies. Environ. Health. 2012;11(1):93. doi: 10.1186/1476-069X-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconi G. CARD15 gene variants and risk of reoperation in Crohn's disease patients. Am. J. Gastroenterol. 2009;104(10):2483–2491. doi: 10.1038/ajg.2009.413. [DOI] [PubMed] [Google Scholar]
- Mahid S.S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin. Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- Mardini H.E. Gastroduodenal Crohn's disease is associated with NOD2/CARD15 gene polymorphisms, particularly L1007P homozygosity. Dig. Dis. Sci. 2005;50(12):2316–2322. doi: 10.1007/s10620-005-3054-2. [DOI] [PubMed] [Google Scholar]
- Mendoza J.L. Prevalence of mutations of the NOD2/CARD15 gene and relation to phenotype in Spanish patients with Crohn disease. Scand. J. Gastroenterol. 2003;38(12):1235–1240. doi: 10.1080/00365520310006612. [DOI] [PubMed] [Google Scholar]
- Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. e1000097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z. Crohn's disease is associated with polymorphism of CARD15/NOD2 gene in a Hungarian population. Ann. N. Y. Acad. Sci. 2005;1051(1):45–51. doi: 10.1196/annals.1361.045. [DOI] [PubMed] [Google Scholar]
- Pierce B.L., Ahsan H. Case-only genome-wide interaction study of disease risk, prognosis and treatment. Genet. Epidemiol. 2010;34(1):7–15. doi: 10.1002/gepi.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protic M.B. CARD15 gene polymorphisms in Serbian patients with Crohnʼs disease: genotype–phenotype analysis. Eur. J. Gastroenterol. Hepatol. 2008;20(10):978–984. doi: 10.1097/MEG.0b013e328302f45e. [DOI] [PubMed] [Google Scholar]
- R. Core Team . 2016. R: A Language and Environment for Statistical Computing. Available at: http://www.R-project.org. [Google Scholar]
- Renda M.C. The role of CARD15 mutations and smoking in the course of Crohn's disease in a Mediterranean area. Am. J. Gastroenterol. 2008;103(3):649–655. doi: 10.1111/j.1572-0241.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- Sabate J.M. The V249I polymorphism of the CX3CR1 gene is associated with fibrostenotic disease behavior in patients with Crohn's disease. Eur. J. Gastroenterol. Hepatol. 2008;20(8):748–755. doi: 10.1097/MEG.0b013e3282f824c9. [DOI] [PubMed] [Google Scholar]
- Satsangi J. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer G. meta: General Package for Meta-Analysis. R package version 4.1–0. 2017. http://CRAN.R-project.org/package=meta Available at:
- Silverberg M.S. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- Thomas D. Methods for investigating gene-environment interactions in candidate pathway and genome-wide association studies. Annu. Rev. Public Health. 2010;31(1):21–36. doi: 10.1146/annurev.publhealth.012809.103619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A. Opposing effects of smoking in ulcerative Colitis and Crohnʼs disease may be explained by differential effects on dendritic cells. Inflamm. Bowel Dis. 2014;20(5):800–810. doi: 10.1097/MIB.0000000000000018. [DOI] [PubMed] [Google Scholar]
- van Heel D.A. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365(9473):1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- Walker L.J. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn's disease are associated with disease severity but not NOD2/CARD15 mutations. Clin. Exp. Immunol. 2004;135(3):490–496. doi: 10.1111/j.1365-2249.2003.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.Y., Lee W.C. Population stratification bias in the case-only study for gene-environment interactions. Am. J. Epidemiol. 2008;168(2):197–201. doi: 10.1093/aje/kwn130. [DOI] [PubMed] [Google Scholar]
- Wells G.A. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2017. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp ohri.ca. Available at: (Accessed January 30, 2017)
- Wong S.L. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material