Abstract
Besides its essential role in the activation of HIV-1 gene expression, the viral Tat protein has the unusual property of trafficking in and out of cells. In contrast to Tat internalization, the mechanism involved in extracellular Tat release has so far remained elusive. Here we show that Tat secretion occurs through a Golgi-independent pathway requiring binding of Tat with three short, non-consecutive intracytoplasmic loops at the C-terminus of the cellular Na+,K+-ATPase pump alpha subunit. Ouabain, a pump inhibitor, blocked this interaction and prevented Tat secretion; virions produced in the presence of this drug were less infectious, consistent the capacity of virion-associated Tat to increase HIV-1 infectivity. Treatment of CD4 + T-cells with short peptides corresponding to the Tat-binding regions of the pump alpha subunit impaired extracellular Tat release and blocked HIV-1 replication. Thus, non canonical, extracellular Tat secretion is essential for viral infectivity.
Keywords: ATPase, HIV-1, Protein secretion, Surface plasmon resonance, Tat, Transactivation
Highlights
-
•
Extracellular secretion of HIV-1 Tat is mediated by the cellular Na+ K+-ATPase pump.
-
•
Tat release is inhibited by the Na+ K+-ATPase inhibitor ouabain
-
•
Peptides competing with the Tat docking site on the ATPase block Tat secretion.
-
•
HIV-1 virions produced by blocking extracellular Tat release are less infectious.
The Tat protein of HIV-1 is an essential regulator of viral gene expression. In addition to this role in the nucleus, here we show that this protein is secreted outside the cells through a mechanism involving its binding to a subunit of the cell membrane Na+ K+ ATPase protein. Cell treatment with peptides blocking this interaction prevents Tat release and, as a consequence, markedly diminishes infectivity of HIV-1 virions. These findings might pave the way to the development of innovative drugs that, by blocking the Tat-ATPase interaction, block HIV-1 infection.
1. Introduction
The Tat protein of the human immunodeficiency virus type 1 (HIV-1) is a small protein (101 aa in most clinical isolates, or 86 aa in the widely utilized HXB2 laboratory strain), acting as a transcriptional activator of viral gene expression. At the viral long terminal repeat (LTR) promoter, the protein binds a cis-acting RNA element (trans-activation-responsive region, TAR) present at the 5′-end of each viral transcript (Berkhout et al., 1989). Through this interaction, Tat activates HIV-1 transcription by promoting the assembly of transcriptionally active complexes at the LTR by multiple protein-protein interactions (Giacca, 2004, Ott et al., 2011).
Besides regulating HIV-1 gene expression, > 20 years ago it was first demonstrated that Tat also possesses the unusual property of entering cells when present in the extracellular milieu (Frankel and Pabo, 1988, Green and Loewenstein, 1988). This property was later extensively characterized and shown to depend on a 9-aa long, arginine-rich sequence (aa 49–57), corresponding to the Tat basic domain, which also mediates nuclear transport and TAR binding. Work performed in different laboratories has shown that short peptides corresponding to this amino acid stretch can be used as biotechnological tools for the intracellular delivery of heterologous proteins, drugs, viral vectors, siRNAs and nanoparticles (Fittipaldi and Giacca, 2005, Jones and Sayers, 2012, Schmidt et al., 2010, Zhang and Wang, 2012).
We, and others have previously shown that extracellular Tat binds heparin through its basic domain (Mann and Frankel, 1991, Rusnati et al., 1997). We also showed that membrane bound-heparan sulfate proteoglycans (HSPG) are the cell surface receptor for Tat internalization, since cells that are genetically impaired in the synthesis of these molecules fail to internalize the extracellular protein (Tyagi et al., 2001).
A few studies have also provided evidence in support of extracellular Tat release from the expressing cells (Becker-Hapak et al., 2001, Chang et al., 1997, Tasciotti and Giacca, 2005, Tyagi et al., 2001). The mechanism underlying this process, however, has remained largely elusive. The protein does not contain an N-terminal signal peptide driving its secretion from the ER-Golgi pathway and, accordingly, protein export is insensitive to drugs which disrupt the integrity of such organelles (Chang et al., 1997). Thus, Tat is a member of the small group of heterogeneous proteins that exit the cells by a process termed “unconventional” or “non-classical” protein secretion (Nickel and Rabouille, 2009). Recent data show that recruitment of Tat to the inner leaflet of the plasma membrane involves binding to membrane-associated phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) (Rayne et al., 2010), with the consequent formation of membrane pores (Zeitler et al., 2015); similar events occur for the unconventional secretion of FGF-2 (Temmerman et al., 2008). The mechanism for extracellular release of Tat and the molecular identity of the secretory machinery involved, however, remain elusive.
2. Materials and Methods
2.1. Plasmids, Peptides, Antibodies and Other Biological Reagents
Detailed description of the biological reagents used in this study is presented in the Supplementary material.
2.2. Cell Cultures and Transfections
CHO K1 and psg A-745 (Rostand and Esko, 1997) were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and maintained in Kaighn's modification of Ham's F12 medium. HEK293T, HeLa and U2OS (obtained from the ATCC) and HL3T1 cells (a HeLa cells derivative stably transfected with a silent LTR-CAT cassette), a kind gift of B. Felber, were maintained in DMEM. All culture media were supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 50 μg/ml gentamicin. U2OS cells (5 × 104) were seeded in four-well glass chamber slides (LabTek II-Nalge Nunc) and transfected with the Polyfect transfection kit (Qiagen) for 24 h. HEK293T cells were transfected using standard calcium phosphate precipitation, incubated for 36 h, and then processed for secretion or co-immunoprecipitation experiments. Primary CD4 + T cells were isolated by negative selection with a CD4 + T cell isolation kit (Miltenyi) from blood buffy coats from healthy donors, according to an approved study protocol. Procedures for lymphocyte isolation and culture were as described (Lusic et al., 2003, Manganaro et al., 2010). All cell lines were routinely tested for Mycoplasma contamination.
2.3. In Vitro Binding Assays
Binding of GST-fused α1 fragments to 35S-Tat86 and 35S-Tat86(R5A) was performed as follows. Briefly, 1 μg of recombinant proteins, after pretreatment in a solution containing DNase I 0.25 U/μl and RNase 0.2 μg/μl to remove contaminant bacterial nucleic acids, were incubated with 600 c.p.m. of in vitro translated Tat in a solution containing 0.2 mg/ml ethidium bromide. Following extensive washes, the reaction mixture was resolved by SDS–PAGE electrophoresis and analyzed by phosphoimaging.
Synthetic biotinylated peptides, corresponding to P1, P2 and P3, were used for binding assays as follows: 25 μl of streptavidin resin (UltraLink Plus streptavidin beads, Pierce), were pretreated with 3 mg of BSA for 10 min at room temperature, and subsequently incubated with 50 μg of peptide at 22 °C for 1 h; after 3 washes, the peptide-coated beads were incubated with either 600 c.p.m. of in vitro translated Tat as previously described, for 2 h at 4 °C. Following extensive washes, the reaction mixture was resolved by SDS–PAGE electrophoresis and analyzed by phosphoimaging.
2.4. HIV-1 Tat Secretion Assay
HEK293T or CHO A-745 cells were seeded in 6-cm plates (1 × 106 cells/plate) one day prior to transfection; cells were transiently transfected with either Tat11-TK, Tat86-TK or TK constructs and with the Sc-VH16-SV5 construct as a positive control of classical secretion. The calcium-phosphate-DNA complexes were incubated for 12 h, then medium was replaced with fresh DMEM, and cells were incubated for additional 24 h. The secretion assays were performed by washing the cells (3 washes of 10 min each) with Optimem containing 20 μg/ml heparin to prevent secreted Tat protein binding to the extracellular heparan sulfate proteoglycans upon transfection (Tyagi et al., 2001), and incubating the cells with 2 ml of Optimem plus heparin for the indicated times. Following incubation, cell culture supernatants were collected and concentrated using Amicon Ultra 10 concentrator (Millipore) according to the manufacturer's instructions; the concentrated fraction was then processed for western blotting. As a control of protein expression, the cellular fraction was collected, lysed in NHEN buffer (20 mM Hepes pH 7.5, 300 mM NaCl, 0.5% NP-40, 20% glycerol, 1 mM EDTA) containing protease inhibitors (Roche). Total protein concentration was assessed by the Bradford assay (BioRad), and 30 μg of each sample was processed for Western Blotting.
2.5. Transcellular Transactivation Assay
HEK293T cells were transfected with a pcDNA3 construct expressing Tat86 and a pcDNA3 expressing either wild type α1 or one of the deleted α1 mutants; after 24 h incubation, cells were washed and the medium substituted with fresh medium plus heparin 20 μg/ml and, for the secretion inhibition experiments, supplemented with 25 μM ouabain. After 4 h, the supernatant was collected and added to HL3T1 cells, containing an integrated bacterial chloramphenicol-acetyltransferase gene (CAT) gene under the control of the HIV-1 LTR, in the presence of 100 μM chloroquine. After 24 h incubation, Tat-driven CAT expression was assayed by quantifying the levels of CAT protein using a CAT ELISA kit (Roche Diagnostics, Meylan, France).
2.6. Surface Plasmon Resonance (SPR) Binding Assay
SPR measurements were performed on a BIAcore X 100 instrument (GE-healthcare). For the analysis of the direct binding of the Na+,K+-ATPase alpha1 subunit peptides with Tat, Tat86 (40 μg/ml) was immobilized to a CM5 sensorchip as described 65, leading to the immobilization of 6990 resonance units (RU) equal to 0.6 pmol/mm2 of the protein. A sensorchip activated and deactivated in the absence of any protein was used as a negative control and for blank subtraction. Increasing concentrations of synthetic P1, P2, P3 peptides or of the fusion protein GST-P1-P2-P3 in 10 mM HEPES buffer pH 7.4 containing 150 mM NaCl, 3 mM EDTA, 0.05% surfactant P20 (HBS-EP +) were injected over the Tat or control surfaces for 4 min and then washed until dissociation was observed. The dissociation constant (Kd) of the interactions were calculated by being fitted with the proper form of Scatchard's equation for the plot of the bound RU at equilibrium versus the ligand concentration in solution. The software embedded in the BIAcore X-100 instrument performed all fittings.
For competition experiments, GST-P1-P2-P3 (20 μg/ml) was immobilized to a CM4 sensorchip as described for Tat, allowing the immobilization of 6100 RU equal to 0.6 pmol of the fusion protein. Similar results were obtained for immobilization of gelatin, here used as a negative control and for blank subtraction. Synthetic Tat (300 nM) was injected over the GST-P1P2P3 surface in the presence of increasing concentrations of the peptides under test. Injection lasted for 4 min to allow the association of Tat with the sensorchip-immobilized ligand. The response (in RU) was recorded at the end of injection, and binding data were plotted as percentage of maximal bound analyte in the various experimental conditions.
2.7. Virus Production and Infection
Infectious viral stocks were generated by transfecting the viral DNA into HEK293T cells and collecting supernatants after 48 h. Viral production was quantified in the supernatants for HIV-1 p24 antigen content using the Innotest HIV antigen mAB kit (Innogenetics N.V. Gent, Belgium). Before infection, the viral stock was treated with 40 U/ml DNAse I (Life Sciences) for 1 h at 25 °C. Activated T cells (1 × 106) were then infected with 500 ng/ml of p24 for 4–5 h at 37 °C. Infection was carried out for 4 h in the presence of polybrene (Sigma). After infection, the cells were kept in culture at 1 × 106 cells/ml in complete medium supplemented with IL-2. At days 3, 5, 7, 10 and 14 post-infection, media and IL-2 were replaced and cells were kept at a density of 1 × 106/ml. The Env- molecular clone pNL4–3/Luc E− R−, a kind gift from Nathaniel Landau, harbors a frameshift mutation introduced near the 5′ end of env gene (Connor et al., 1995), and performs a single-round infection once pseudotyped with the Vesicular Stomatitis Virus–G (VSV-G) protein. Integrated viral DNA was quantified by the Alu-PCR technique using a described procedure (Manganaro et al., 2010).
2.8. Other Methods
Other, more standard methods are reported in the Supplemental Experimental Procedures.
3. Results
3.1. The Cardiac Glycoside Ouabain Blocks Extracellular Release of HIV-1 Tat
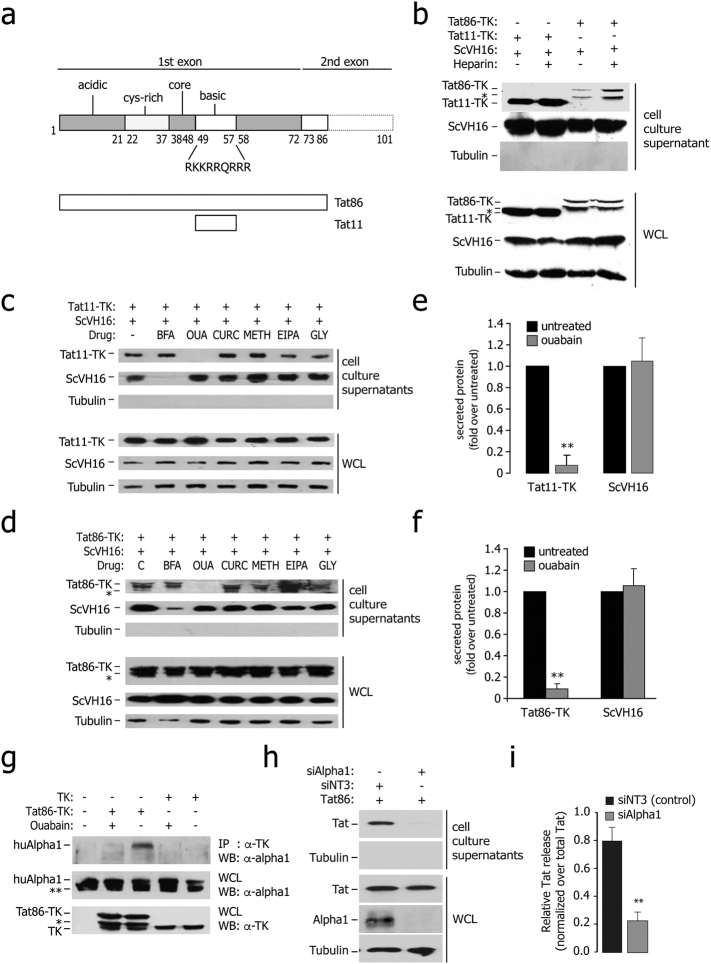
We developed an assay in which HEK293T cells are simultaneously transfected with a plasmid expressing a single-chain Fv antibody (scFv) tagged with the SV-5 epitope (ScVH16-SV5), containing and N-terminal signal peptide for ER-Golgi secretion, together with another plasmid coding for either the HIV-1HX2B 86 aa Tat (Tat86) or the Tat fragment corresponding to aa 48–59 (Tat11), encompassing the 9-aa-long, basic region of Tat (Fig. 1a); the HSV1 thymidine kinase protein (TK) served as a reporter (Tasciotti and Giacca, 2005, Tasciotti et al., 2003). At 36 h after transfection, ~ 2–10% of both Tat86-TK and Tat11-TK was found in the cell culture supernatants along with the scFv and in the absence of detectable cell lysis (Fig. 1b). The amount of free Tat-TK protein in the supernatant was increased by cell treatment with heparin, which released membrane-attached, extracellular Tat (Fig. 1c). Tat86 release depended on the integrity of the protein basic domain, since the transactivation-dead mutant Tat86(R5A), bearing alanine to arginine substitutions in the Tat basic domain (Demarchi et al., 1999), failed to be exported from the cells (Figs. S1a and S1b). Fusion proteins between Tat11 and EGFP or Cre were released similar to Tat11-TK (not shown).
Fig. 1.
Ouabain-sensitive secretion of Tat from the expressing cells.
(a) Schematic representation of the major functional domains of HIV-1 Tat (acidic, cysteine-rich, core, and basic). Tat has 101 aa in several clinical isolates and 86 aa in the laboratory strain HX2B. The amino acidic sequence of the basic domain of the protein, which imparts the protein intercellular trafficking capability, is indicated. The lower part of the panel shows a schematic representation of the two Tat proteins used in this study (Tat11, corresponding to the Tat basic domain plus two additional amino acids at both extremities, and Tat86).
(b) Tat86-TK and Tat11-TK are released from the expressing cells and bind extracellular HSPG upon secretion. The immunoblots in the upper panel show the amount of proteins released in the cell culture supernatants of cells transfected with Tat86-TK, Tat11-TK or scVH16-SV5, treated or untreated with 25 μM soluble heparin. The immunoblots in the lower part show the levels of intracellular protein expression in the same samples. WCL: whole cell lysates. The asterisk (*) indicates an additional band present in the Tat86-TK immunoblots, probably corresponding to a degradation product. Lack of tubulin immunoreactivity in the supernatants indicates the absence of appreciable cell lysis.
(c) Sensitivity of Tat11-TK and scFv secretion to the indicated drugs. HEK293T cells were co-transfected with Tat and scFV expressing plasmids and treated with the indicated metabolic drugs. The amount of secreted protein was assessed by western blot on cell culture supernatants, while protein expression and loading was checked on whole cell lysates (WCL). BFA: brefeldin A (10 μM); OUA: ouabain (25 μM); CURC: curcumin (50 μM); METH: methylamine (1 mM); EIPA: 5-(N-ethyl-N-isopropyl)amiloride (20 μM); GLY: glyburide (10 μM).
(d) Sensitivity of Tat86-TK and scFv secretion to the indicated drugs. HEK293T cells were co-transfected with Tat and scFV expressing plasmids and treated with the indicated metabolic drugs. The amount of secreted protein was assessed by western blot on cell culture supernatants, while protein expression and loading was checked on whole cell lysates (WCL). BFA: brefeldin A; OUA: ouabain; CURC: curcumin; METH: methylamine; EIPA: 5-(N-ethyl-N-isopropyl)amiloride; GLY: glyburide.
(e) Quantification of Tat11-TK and ScVH16 secretion in ouabain-treated cells. The amount of extracellular proteins, normalized over the levels of intracellular expression, was assessed after a 4 h incubation. Data are mean ± sem of three independent experiments. **P-value < 0.01.
(f) Quantification of Tat86-TK and ScVH16 secretion in ouabain-treated cells. The amount of extracellular proteins, normalized over the levels of intracellular expression, was assessed after a 4 h incubation. Data are mean ± sem of three independent experiments. **P-value < 0.01.
(g) Tat co-immunoprecipitates with the endogenous Na+,K+-ATPase α1 subunit; binding is sensitive to ouabain. HEK293T cells were transfected with Tat86-TK or TK as a control and treated with ouabain (25 μM) as indicated. The antibodies used for immunoprecipitation and subsequent western blots are indicated on the right side. Expression of endogenous α1 and either of the transfected proteins was verified in whole cell lysates (WCL). The bands marked with (*) are degradation products.
(h) Downregulation of cellular Na+,K+-ATPase α1 subunit impairs Tat release. The amount of released Tat was monitored in cell culture supernatants of Na+,K+-ATPase α1-knock down cells by immuno blotting with an anti-Tat antibody; protein expression and loading were checked in whole cell lysates (WCL).
(i) Quantification of the levels of Tat secretion after α1 RNAi knock down. Data are mean ± sem of three independent experiments.
The mechanism involved in extracellular Tat release was investigated by testing a panel of metabolic drugs. No effect was detected on either Tat release or scFv secretion by glyburide (GLY) and methylamine (METH), which block the non-classical secretion of other proteins (Hamon et al., 1997, Rubartelli et al., 1990) or 5-(N-ethyl-N-isopropyl)amiloride (EIPA), a drug interfering with macropinocytosis (West et al., 1989) and known to limit HIV-1 replication (Ewart et al., 2004). Brefeldin A (BFA), which inhibits the ER-Golgi trafficking (Misumi et al., 1986), impaired scFv antibody secretion but not Tat release, while the cardiac glycoside ouabain (OUA), which abrogates the function of the cell membrane Na+,K+-ATPase, selectively abolished Tat-fusion protein release (Fig. 1c and d for Tat11-TK and Tat86-TK respectively). Of interest, curcumine (CURC), a broad inhibitor of P-type ATPases, was instead ineffective, suggesting that the effect of ouabain was not related to the inhibition of the enzymatic function of the ATPase. Ouabain did not affect Tat protein production, since the levels of the protein in whole cell extracts were unchanged in the drug-treated cells. Quantification of the inhibitory effect of ouabain on secretion the two Tat fusion proteins is shown in Fig. 1e and f; a series of Tat exit experiments using higher concentration of the other drugs confirmed the specificity of ouabain (Fig. S1c and d). There was no apparent toxicity of OUA when used at a concentration of up to 25 μM in different cell lines (Fig. S1f–i).
3.2. Ouabain-sensitive Binding of HIV Tat to the Cellular Na+,K+-ATPase α1 Subunit
The specific cellular target of ouabain is the α subunit of the membrane Na+,K+-ATPase pump, which catalyzes ATP hydrolysis coupled with Na+ and K+ transfer through the membrane against the electrochemical gradient (Kaplan, 2002, Lingrel and Kuntzweiler, 1994). In cells transfected with Tat86, we indeed found that the endogenous Na+,K+-ATPase α1 subunit co-immunoprecipitated with Tat; cell treatment with ouabain for 4 h abolished this interaction (Fig. 1g); instead, the β1-subunit was not present in the anti-Tat immunoprecipitate (Fig. S1e). Noticeably, transient inhibition of Na+,K+-ATPase α1 expression by RNAi completely inhibited Tat release (Fig. 1h and i).
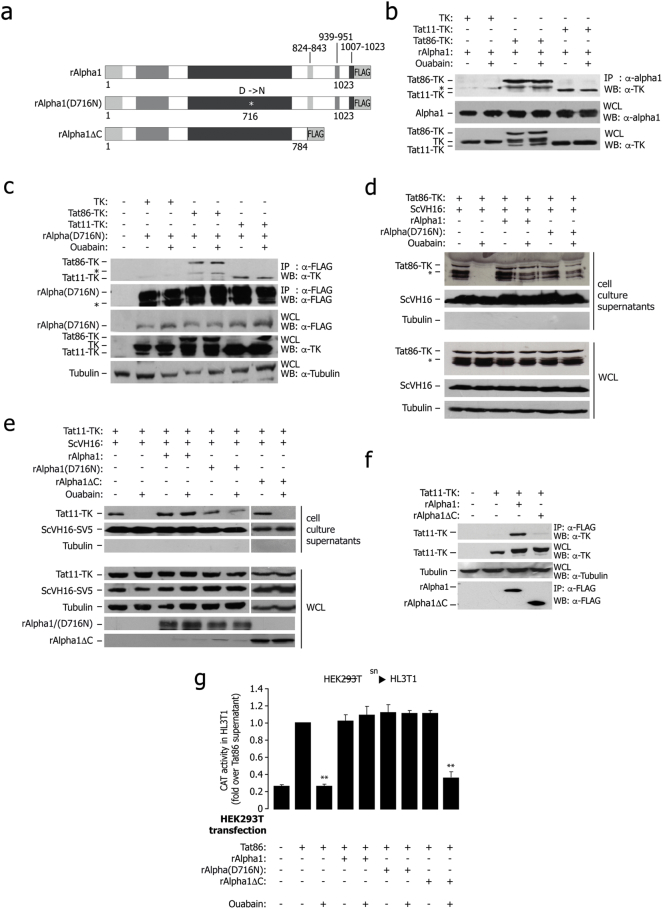
The rat Na+,K+-ATPase enzyme is insensitive to ouabain (Emanuel et al., 1988) because its α1 subunit harbors two point mutations at Gln111 and Asn122 that reduce affinity to the drug (Laursen et al., 2013, Price and Lingrel, 1988); Fig. 2a. This rat Na+,K+-ATPase α1 protein was still capable to bind both Tat11- and Tat86-TK in co-IP experiments (Fig. 2b); the same was also true for the rat α1 single point mutant D716N, in which catalytic activity is impaired (Lane et al., 1993); Fig. 2c. The latter result is consistent with the conclusion that the interaction between Tat and α1 occurs independent from the Na+,K+-ATPase enzymatic activity. Of notice, overexpression of both wt and D716N rat α1 proteins rescued the suppressive effect of ouabain on Tat secretion in human cells (shown in Fig. 2d and e for Tat86 and Tat11 respectively). Secretion of ScVH16-SV5 was unaffected by any of these treatments.
Fig. 2.
In vivo characterization of the interaction of Tat with the Na+,K+-ATPase α1.
(a) Schematic representation of the rat Na+,K+-ATPase α1 subunit constructs used to determine association with Tat inside the cells.
(b) Tat co-immunoprecipitates with transfected rat α1 protein; binding is not sensitive to ouabain. HEK 293T cells were transfected with rat α1 (rAlpha1), Tat86-TK, Tat11-TK and TK control and treated with ouabain as indicated. The antibodies used for immunoprecipitation and subsequent western blots are indicated on the right side. Expression of the transfected proteins was verified in whole cell lysates (WCL). The band marked with (*) is a degradation product.
(c) Tat co-immunoprecipitates with the transfected rat α1 wild type and catalytically inactive rat α1 D716N mutant; binding is not sensitive to ouabain. The experiment was performed as in panel (b) by transfecting FLAG-tagged, mutant rat α1 (rAlpha D716N) instead of wt α1.
(d) Both wt rat α1 and the D716N catalytically inactive mutant rescue Tat86 secretion in human cells treated with ouabain. HEK 293T cells were transfected with Tat86-TK, ScVH16 as a secretion control, and either wt rat α1or FLAG-tagged, mutant rat α1 (rAlpha D716N), and treated with ouabain as indicated. The amount of secreted protein was assessed by immunoblot on cell culture supernatants, while intracellular protein expression was assessed on whole lysates (WCL).
(e) rAlphaΔC fails to rescue Tat secretion in human cells treated with ouabain. Cells were transfected with Tat11-TK and the scFv antibody ScVH16-V5, together with wt rat α1 (rAlpha1), catalytically inactive α1 (rAlpha1(D716N)) or the truncated mutant rAlphaΔC, and treated with ouabain (25 μM) as indicated. The amount of secreted protein was assessed by immunoblot on cell culture supernatants; total protein expression was verified in whole cell lysates (WCL).
(f) The rat α1 C-terminal deletion mutant rAlphaΔC fails to co-immunoprecipitate Tat. Human HEK 293T cells were transfected with Tat11-TK and either the wt rat α1or the α1C-terminus truncated mutant. The antibodies used for immunoprecipitation and subsequent western blots are indicated on the right side.
(g) Results of transcellular transactivation assay showing that the rat Na+,K+-ATPase α1(D716N) catalytically-inactive mutant, but not the rAlphaΔC C-terminus-truncated mutant, rescues secretion of transcriptionally active Tat in human cells treated with ouabain. HEK293T cells were transfected with an expression vector for Tat86 alone, or cotransfected with wt rat α1 (rAlpha1), mutant rAlpha D716N or mutant rAlphaΔC; the cells were treated with ouabain (25 μM), as indicated, and their supernatants were then incubated with HL3T1 cells, carrying an LTR-CAT reporter cassette to measure transcellular transactivation. The levels of LTR activation were assessed by measuring CAT levels by an ELISA assay. sn: supernatant. **: P < 0.01 over Tat86.
Collectively, these results indicate that proteins containing the 11-aa long, Tat basic domain are effectively released by the expressing cells through a non canonical secretion mechanism that involves binding of Tat to the Na+,K+-ATPase α1 subunit but does not require the pump enzymatic activity.
3.3. The C-terminus of Na+,K+-ATPase α1 is Essential to Bind Tat and Mediate Its Secretion
A rat Na+,K+-ATPase α1 deletion mutant lacking the C-terminal region (rΑlpha1ΔC, carrying a deletion from aa 784 to the C-terminus of the protein; Fig. 2a) neither bound Tat (Fig. 2f) nor permitted extracellular Tat release in human cells treated with ouabain, in contrast to either wt rat α1 and the rΑlpha1(D716N) mutant (Fig. 2e); the secretion of the scFv antibody was unaffected by any of these conditions. All three rat proteins were expressed at comparable levels inside the cells (Fig. S2).
The essential role of the α1 subunit C-terminus in Tat binding and secretion was also strengthened by the results of a transcellular transactivation assay, in which the supernatants of HEK293T cells, transfected with wild type Tat86 and wild type rΑlpha1 or mutants rΑlpha1(D716N) and rΑlpha1ΔC, with or without ouabain, were subsequently incubated for 24 h with reporter HL3T1 cells, carrying the HIV-1 LTR upstream of the CAT reporter gene. Ouabain blocked transcellular transactivation mediated by the supernatant of Tat86-transfected cells. The effect of the drug was overcome by transfection of either wild type rat rΑlpha1 or rΑlpha1(D716N), but not by rΑlpha1ΔC (Fig. 2g).
Collectively, these results indicate that Tat binds the C-terminal cytoplasmic region of the Na+,K+-ATPase α1 subunit inside the cells and that integrity of this region is an essential requisite for extracellular Tat export.
3.4. The Tat Basic Domain Binds the Na+,K+-ATPase α1 Subunit C-terminal Domain Cytoplasmic Loops
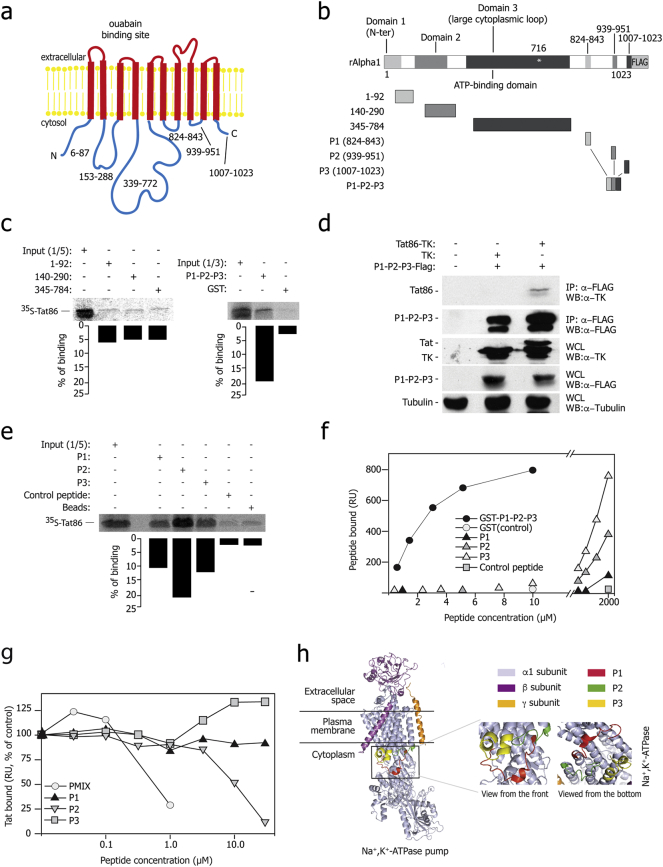
Next we wanted to define the regions of Tat and Na+,K+-ATPase α1 involved in the interaction. The α1 subunit is a large, integral membrane protein with 10 transmembrane-spanning domains plus cytoplasmic N- and C-terminal regions (Morth et al., 2007) (Fig. 3a). In GST-pulldown assays, radiolabeled Tat did not bind any of the three large, N-terminal cytoplasmic segments of rat α1; in contrast, it showed binding to a fusion protein corresponding to the three α1 C-terminal short cytoplasmic peptides, hereafter named P1-P2-P3 (residues 824–843, 939–951 and 1007–1023, respectively; Fig. 3b and c). Co-IP experiments between Tat86-TK and Flag-P1-P2-P3 fusion protein confirmed this interaction in the cells (Fig. 3d). The Tat86(R5A) mutant failed to interact with any of the analyzed α1 regions (Fig. S3a). Binding to radiolabeled Tat was detected using all three P1, P2 and P3- mostly with P2-, obtained as independent, biotinylated peptides (Fig. 3e); again, the Tat86(R5A) mutant scored negative in this assay (Fig. S3b).
Fig. 3.
Tat binds rat Na+,K+-ATPase α1 in vitro – Mapping of the interacting regions.
(a) Scheme of the Na+,K+-ATPase α1 subunit structure and membrane topology; the cytoplasmic domains are indicated.
(b) Schematic representation of the rat α1 fragments used for the pull-down assay shown in panel c.
(c) Tat protein binds the C-terminal cytoplasmic region of the rat α1 subunit in vitro. The indicated fragments of the rat α1 protein fused to GST or GST alone were incubated with invitro translated [35S]-Tat86, extensively washed, and then resolved by SDS-PAGE. Each panel shows the gel exposed to a phosphoimager from a representative experiment along with the quantification of the amount of bound proteins expressed as a percentage of radiolabeled input. These and all the subsequent pulldown experiments were performed at least in triplicate with superimposable results.
(d) Co-immunoprecipitation of Tat with the P1-P2-P3 fusion protein, corresponding to the small cytosolic loops of the Na+,K+-ATPase α1 subunit. The antibodies used for immunoprecipitation and subsequent western blots are indicated on the right side. Expression of the transfected proteins was verified in whole cell lysates (WCL).
(e) Tat individually binds three cytoplasmic peptides corresponding to the C-terminal loop of the Na+,K+-ATPase α1 subunit. Biotinylated peptides were bound to streptavidin beads, incubated with [35S]-Tat86, extensively washed, and then resolved by SDS-PAGE.
(f) Surface Plasmon resonance (SPR) analysis of Na+,K+-ATPase α1 peptides -Tat interaction. The curves were obtained using the blank subtracted values of resonance unit (RU) bound at equilibrium after injection onto sensorchip-immobilized Tat of increasing concentrations of the indicated Na+,K+-ATPase α1 peptides (P1, P2 and P3) or of a GST-protein containing the fusion of their three sequences (GST-P1-P2-P3). The P1, P2 and P3 peptides bound Tat in a dose-dependent manner, while a scrambled P3 control peptide was ineffective. The fusion protein bound Tat more effectively than the individual synthetic peptides.
(g) Peptides from the Na+,K+-ATPase α1 C-terminal domain competitively affect the binding of Tat to the fusion P1-P2-P3 protein in SPR analysis. Increasing concentrations of synthetic P1, P2 or P3 peptides or of a mix of the three peptides (PMIX) were evaluated for their capacity to prevent binding of Tat to sensorchip-immobilized GST-P1P2P3 fusion protein. The responses are plotted as percentages of Tat binding in the absence of free antagonist.
(h) 3D modeling of the Na+,K+-ATPase α1 structure, as resolved by X-ray crystallography (Yatime et al., 2011). The PDB file (PDB ID: 3N23 and 3N2F) was visualized by PyMoL tool, available at www.pymol.org; the regions corresponding to the three C-terminal cytoplasmic peptides are highlighted. The inset shows the 3D organization of the three C-terminal cytoplasmic peptides of the Na+,K+-ATPase α1subunit in the absence of ouabain.
Next we analyzed binding of the α1 P1-P2-P3 fusion protein to sensor chip-immobilized Tat by surface plasmon resonance (SPR). We found that binding occurred with a Kd value of 1.6 μM, which was saturable. Neither control GST alone bound immobilized Tat (Fig. 3f) nor P1-P2-P3 bound to a negative control surface (not shown). When tested individually, the P1, P2 and P3 peptides also bound immobilized Tat in a dose-dependent manner, however at concentrations ranging from 250 to 2000 μM (Fig. 3f). Binding of these peptides was specific, since a scrambled P3 peptide was ineffective when assayed up to 2 mM. None of the three peptides bound the control surface (data not shown).
We further investigated the capacity of an equimolar mixture of the individual P1, P2 and P3 peptides (hereafter named PMIX) to compete for the α1-Tat interaction by SPR. We found that PMIX effectively averted binding of Tat to immobilized GST-P1-P2-P3, with an ID50 equal to 0.5 μM (Fig. 3g). When the three peptides were tested individually, only P2 was effective at significantly higher concentrations (ID50 = 10 μM).
The amino acid sequences of the α1 P1, P2 and P3 loops of are highly conserved in rat, human and pig (Fig. S3c). Analysis of their spatial arrangement indicates that are very close to one another in the 3D structure of the α1 protein (Morth et al., 2007, Yatime et al., 2011), thus possibly forming an integrated docking surface for Tat protein binding (Fig. 3h). Perturbation of this structure induced by ouabain binding in a cavity of the transmembrane spans of the protein (Morth et al., 2007, Yatime et al., 2011) might explain the inhibitory effect exerted by the drug on the Tat- α1 interaction and Tat secretion.
3.5. Cellular Na +,K +-ATPase α1 Subunit is Essential for Productive HIV-1 Infection
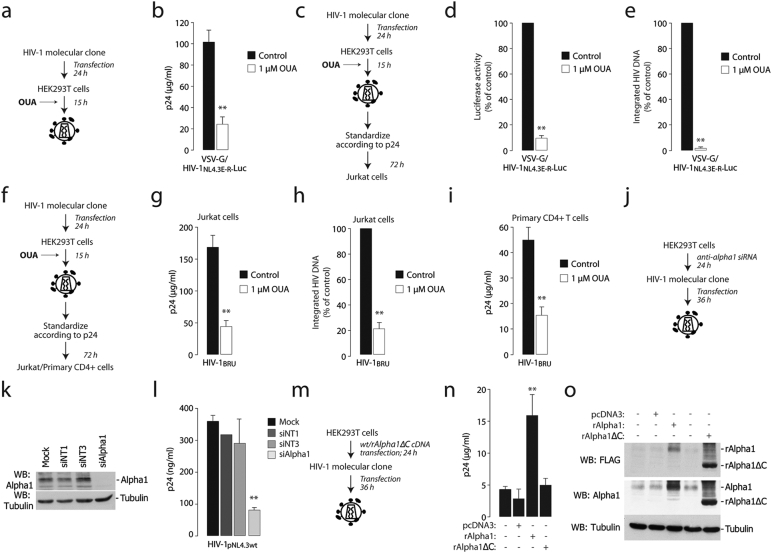
We wanted to understand the relevance of Na+,K+-ATPase–mediated Tat secretion in the context of HIV-1 infection. First, we tested effect of ouabain on virion production. We found that the drug, once added to HEK293T cells transfected with the pNL4.3.Luc.R-E HIV-1 molecular clone (Fig. 4a), significantly decreased the production of VSV-G pseudotyped virions (Fig. 4b). The same was also observed for wt HIV-1BRU virions (Figs. S4a and S4b).
Fig. 4.
Na+,K+-ATPase Inhibition by ouabain or α1 downregulation by RNAi inhibit HIV-1 production and virion infectivity.
(a) Scheme of the experiment to assess viral production from the VSV-G-pseudotyped HIV-1NL4.3E-R-Luc in the presence of ouabain.
(b) Ouabain impairs viral production. Levels of p24, measured by ELISA, produced by HEK293T cells transfected with the HIV-1NL4.3E-R-Luc molecular clone and a VSV-G-expressing plasmid in the presence of ouabain (1 μM). **: P < 0.01 over untreated cells.
(c) Scheme of the experiment to assess infectivity of HIV-1 virions produced in the presence of ouabain.
(d and e) HIV-1 produced in the presence of ouabain is less infectious. Jurkat cells were infected with the same amount of VSV-G-pseudotyped HIV-1NL4.3E-R-Luc produced in the presence or absence of ouabain (1 μM); infection was monitored after 72 h by measuring luciferase activity (d) and the levels of integrated viral DNA by Alu-PCR (e) in the infected cells. **: P < 0.01 over cells infected with a virus produced without ouabain.
(f) Scheme of the experiment to assess infectivity of HIV-1BRU virions produced in the presence of ouabain.
(g–i) HIV-1BRU produced in the presence of ouabain is less infectious. Jurkat cells (panels g and h) or primary CD4 + T cells (panel g) were infected with the same amount of HIV-1BRU produced either in the presence or absence of ouabain at the indicated concentrations; infection was monitored after 72 h by measuring p24 levels (g and i) and the amount of integrated viral DNA by Alu-PCR (h) in the infected cells. **: P < 0.01 over cells infected with a virus produced without ouabain.
(j) Scheme of the experiment to assess the effect of transient depletion of cellular Na+,K+-ATPase α1 on HIV-1 production. Na+,K+-ATPase α1-depleted and control cells were transfected with the wild type HIV-1NL4.3 molecular clone and, after 48 h, viral production was monitored by p24 ELISA in cell culture supernatant.
(k) Western blot showing silencing of Na+,K+-ATPase α1 in siRNA-transfected cells. siNT1 and siNT3 are two non-targeting siRNA controls.
(l) Downregulation of cellular Na+,K+-ATPase α1 impairs HIV-1 production. Na+,K+-ATPase α1-knock down and control cells were transfected with the HIV-1NL4.3 molecular clone and, after 48 h, virus production was monitored by p24 ELISA in the cell culture supernatants.
(m) Scheme of the experiment to assess the effect of full length and mutant rat α1 overexpression on HIV-1 production.
(n) Western blotting showing overexpression of full length and rAlphaΔC in HEK293T cell, visualized by both anti-FLAG and anti- α1 antibodies.
(o) Overexpression of wild type rat α1, but not of its truncated mutant rAlphaΔC, increases HIV-1 production. The levels of p24 present in the supernatants of HEK293T cells transfected with either of the two constructs and later with the HIV-1NL4.3 molecular clone were assessed by ELISA. Data are mean ± sd of three experiments.
More remarkably, virions produced in the presence of the drug were markedly less infectious than those produced in untreated cells. This conclusion was reached by testing the effect of the same number of VSVG-pseudotyped virions (2 μg p24 per 1 × 106 cells), produced in the presence or absence of ouabain, on Jurkat cells (Fig. 4c and d) as well of wt HIV-1BRU on both Jurkat cells (Fig. 4f and g) and primary CD4 + lymphocytes (Fig. 4i). For both VSV-G and wt viruses, decreased infectivity correlated with reduced viral integration in the target cells, as assessed by Alu-LTR measurements (Fig. 4e and h respectively). Ouabain did not exert significant cell toxicity when administered to cells during the 15 h-long virus production period (Fig. S4c). The effect of ouabain appeared to specifically affect infectivity of virions at the level of virus production and not infection per se, since HIV-1 virions produced without ouabain, when used to infect Jurkat cells, were unaffected by the presence of the drug (Fig. S4d and Ee).
Next, we tested whether the downregulation of cellular Na+,K+-ATPase α1 by RNAi mimicked the inhibitory effect of ouabain on viral production (Fig. 4j). Treatment of HEK293T cells with an anti- α1 siRNA pool markedly impaired HIV-1 production; two control, non-targeting siRNAs (siNT1 and siNT3) had no apparent effect (Fig. 4k and l). Finally, we observed that, on the contrary, transfection of an expression vector for rAlpha1, but not for the C-terminal mutant rAlpha1ΔC, determined a > 3-fold increase in HIV-1 p24 protein in the supernatants (Fig. 4m and n); both wt and mutant proteins were overexpressed at comparable levels (Fig. 4o).
3.6. Exogenous Tat Reconstitutes Infectivity of HIV-1 Virions Produced in the Presence of Ouabain
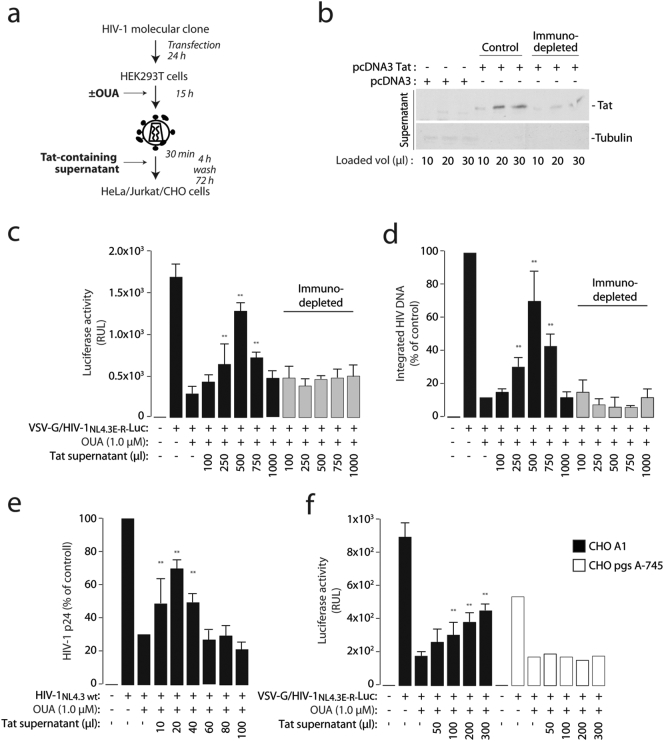
Previous work had indicated that secreted Tat is found on the surface of HIV-1 virions and takes part in virion infectivity by enhancing surface attachment and entry into the target cells (Gratton et al., 2003, Marchiò et al., 2005). We therefore tested whether exogenous addition of Tat-containing supernatants to virions produced in the presence of ouabain could rescue infectivity; the same supernatants, depleted of Tat using an anti-Tat specific antibody, served as a control (Fig. 5a and b). We found that the Tat-containing supernatants, but not those after Tat immunodepletion, rescued infectivity of the virions in a dose-dependent manner, as measured by both luciferase activity and levels of integrated HIV-1 DNA in the target cells (Fig. 5c and d respectively). The Tat-containing supernatant exerted no toxic effects on Jurkat or CHO cells (Fig. S5a and b). Rescue of infectivity was also obtained using a recombinant GST-Tat protein (Fig. S5c and d). Of interest, in both cases supplementation with scalar doses generated a bell-shaped infectivity curve, being less effective at higher concentrations. In addition to VSV-G-pseudotyped virions exogenous addition of Tat also enhanced infectivity of wt HIV-1 produced in the presence of ouabain, again with a bell-shaped response (Fig. 5e).
Fig. 5.
Exogenously added Tat rescues infectivity of HIV-1 virions produced by ouabain-treated cells.
(a) Scheme of the procedure to assess reconstitution of the infectivity of virus produced in the presence of ouabain by exogenously added Tat protein-containing supernatant. VSV-G pseudotyped HIV-1NL4.3E-R-luc was produced in the presence of ouabain 1 μM; at the time of infection of HeLa cells, virions were incubated scalar amounts of Tat-containing supernatant.
(b) Western blot analysis of the cell culture supernatants containing Tat before (control) and after immunodepletion with an anti-Tat monoclonal antibody (for 30 min at 37 °C).
(c and d) Rescue of infectivity of virions produced in the presence of ouabain by supernatants containing Tat. VSV-G pseudotyped HIV-1NL4.3E-R-luc produced in the presence of ouabain 1 μM; at the time of infection of HeLa cells, virions were incubated with the indicated amounts of the Tat-containing supernatant or the same after immunodepletion using an anti-Tat monoclonal antibody. The levels of luciferase measured in the infected cells at 48 h after infection are shown in panel C while the amount of integrated viral DNA in panel D. Data are mean ± sd of three experiments; **: P < 0.01
(e) Rescue of infectivity of wild type HIV-1NL4.3 virions produced in the presence of ouabain by supernatants containing Tat. Infection was performed in Jurkat cells; the graphs shows the levels of p24 produced. Data are mean ± sd of three experiments; **: P < 0.01.
(f) Exogenous Tat fails to rescue infectivity of VSV-G pseudotyped HIV-1NL4.3E-R-luc produced in the presence of ouabain in the CHO psg A-745 cell clone, which is defective in proteoglycan synthesis. Rescue experiments were performed as above in wt CHO K1 cells and the mutant. Data are mean ± sd of three experiments; **: P < 0.01.
Finally, we previously observed that the main cell surface receptors for extracellular Tat are the cell membrane-bound heparan-sulfate proteoglycans and that cells lacking these molecules fail to bind and internalize the protein (Fittipaldi et al., 2003). We therefore wanted to test the effect of pseudotyped virion-associated Tat on the infection of the CHO A-745 cell mutant, which is impaired in glycosaminoglycan biosynthesis (Esko et al., 1985). We found that, in contrast to wt CHO K1 cells, infectivity of pseudotyped virions produced in the presence of ouabain was not rescued by exogenous Tat addition (Fig. 5f).
Taken together, these observations are consistent with the conclusion that a major function of virion-bound Tat is to modify the interaction of the virus particle with the cells to be infected, with the ultimate effect of increasing infectivity.
3.7. Peptides Corresponding to the α1 C-terminal Loops Inhibit Tat release and HIV-1 Infection
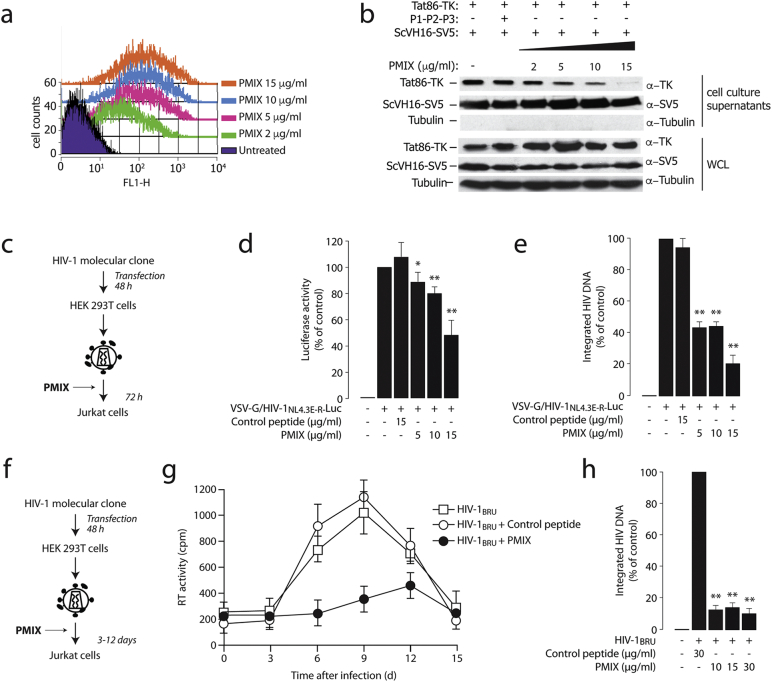
Our protein binding data indicated that the P1, P2 and P3 peptides, corresponding to the C-terminal α1 intracytoplasmic loops, bound Tat. We therefore tested whether these peptides, by competing for endogenous α1 binding, might also affect extracellular Tat release and HIV-1 replication. Initially, we observed that all three peptides, once conjugated to fluorescein, were internalized in a dose-dependent manner, as detected by flow cytometry at 4 h after addition to the cells, following extensive cell trypsinization and washings to remove extracellular material (Fig. 6a and Figs. S6a and S6b for the equimolar mixture of the three peptides, PMIX, and the individual peptides, respectively). Indeed, when cells transfected with Tat and the ScVH16-SV5 antibody were treated with the individual peptides or with PMIX, extracellular Tat release was inhibited, leaving secretion of the scFv antibody unaffected (Fig. 6b and S6c).
Fig. 6.
Synthetic peptides corresponding to the Na+,K+-ATPase α1 C-terminal loops inhibit Tat secretion and HIV-1 infection
(a) Peptides mixture (PMIX) of intracellular loops of the Na+,K+-ATPase α1 subunit C-terminal domain are internalized by the cells. Different amounts of an equimolar mixture of fluorescein-tagged peptides (PMIX) were added, at the indicated concentrations, to HEK293T cells cultured in OPTIMEM medium. After 4 h, cells were tryspinized, extensively washed, and analyzed by flow cytometry. The overlay plots show the cell mean fluorescence for increasing concentrations of the peptide mix.
(b) Cell treatment with PMIX peptides blocks Tat86-TK release. Cells were transfected with Tat86-TK and the ScVH16 scFv antibody and, after 36 h, washed with heparin and then treated with equimolar amounts of the three peptides (PMIX); presence of Tat86-TK and the scFv antibody in the cell culture supernatant was analyzed by western blotting after a 4 h incubation. The levels of intracellular protein expression were verified on whole cell lysates (WCL).
(c) Scheme of the procedure to assess the effect of PMIX in a single-round HIV-1 infection.
(d) Treatment with the PMIX peptides impairs HIV-1 infection in a single-round cell infection assay. Jurkat cells were infected with HIV-1NL4.3E-R-Luc pseudotyped with the VSV-G envelope. Before infection, cells were incubated with different concentrations of the PMIX peptides at 37 °C for 1 h. Then, cells were infected with the virus for 4 h, washed and fresh medium with peptides was added; after additional 72 h, cells were harvested and luciferase expression level was measured. *: P < 0.05 and **: P < 0.01 over untreated cells, respectively.
(e) Quantification of viral integration after infection in the presence of PMIX. DNA from Jurkat cells infected as in panel (d) was analyzed for the levels of proviral integration by Alu-PCR. *: P < 0.05 and **: P < 0.01 over untreated cells, respectively.
(f) Scheme of the experiment to assess the effect of PMIX on multiple rounds of infection by wild type HIV-1.
(g) The PMIX peptides inhibit HIV-1 replication. Jurkat cells were infected with wild type HIV-1BRU virus for 4 h in the presence of the PMIX peptides or of a control peptide (both at 5 μM). After infection, the medium containing the virus was washed and substituted with fresh medium, containing the corresponding peptide preparation. At time = 0 and, subsequently, every 3 days until the 15th day, the supernatants were tested for reverse transcriptase (RT) activity, while the infected cells were diluted 1:2 and fresh peptides were added to the culture media.
(h) Quantification of viral DNA integration after inhibition of viral replication by PMIX. DNA from Jurkat cells treated as in panel (g) was analyzed for the levels of proviral integration by Alu-PCR. *: P < 0.05 and **: P < 0.01 over untreated cells, respectively.
Next, a single-round infection experiment was performed by using VSV-G-pseudotyped HIV-1NL4.3E-R-Luc to infect Jurkat cells that were pre-incubated with different concentrations of PMIX or a control peptide (Fig. 6c). At 72 h after infection, A dose-dependent decrease in luciferase activity was clearly detected (Fig. 6d). This was paralleled by decreased levels of integrated viral DNA (Fig. S6e).
We also analyzed the effect of PMIX on the infection of Jurkat cells with wild type HIV-1BRU virus. Over the course of 15 days after infection, half of the cell medium was replaced every third day with fresh medium containing either PMIX or a control peptide (Fig. 6f). PMIX markedly suppressed viral infection (Fig. 6g). Finally, PMIX also suppressed HIV-1BRU integration at 72 h after infection (Fig. 6h). Analogous results were also obtained by testing the effects of PMIX on HIV-1BRU infection of primary CD4 + T cells (Fig. S6d and e). No apparent cellular toxicity of PMIX was detected (Fig. S6f).
Collectively, these results indicate that suppression of extracellular Tat release by peptides derived from the Na+,K+-ATPase α1 subunit C-terminal domain impair HIV-1 infection.
4. Discussion
Our experiments indicate that the α subunit of the cellular Na+,K+-ATPase mediates unconventional Tat secretion in a ouabain-sensitive manner. Extracellular Tat release was affected neither by methylamine (a drug which blocks endosomal recycling and impairs IL1B and FGF-2 non canonical secretion, implying a vesicular intermediate in their release (Hamon et al., 1997, Rubartelli et al., 1990, Zhou et al., 2002)), nor glyburide (a sulfonylurea interfering with the ABC-1 transporter essential for the secretion of Galectin-1 (Flieger et al., 2003, Hamon et al., 1997)). In contrast, Tat secretion was markedly sensitive to ouabain, an inhibitor of the Na+,K+-ATPase. The observations that Tat secretion still occurred in the presence of curcumin, an inhibitor of all P-type ATPases (including the Na+,K+-ATPase) and that the rat D716N α1 mutant, which is impaired in catalytic function (Lane et al., 1993), still rescued Tat secretion in human cells treated with ouabain, are concordant in indicating that the effect of the Na+,K+-ATPase on extracellular Tat release is independent from its enzymatic activity while still demands physical binding of α1 to Tat.
These characteristics of Tat secretion are remarkably overlapping with those of cellular FGF-2, which is also sensitive to ouabain and involves binding of the factor to the Na+,K+-ATPase (Dahl et al., 2000, Florkiewicz et al., 1998, Smith et al., 2001, Trudel et al., 2000, Zeitler et al., 2015), while does not require integrity of the enzymatic function of the pump (Zacherl et al., 2015). Work from other laboratories has also shown that unconventional secretion of both FGF-2 (Temmerman et al., 2008) and HIV-1 Tat (Rayne et al., 2010) require interaction of the two proteins with acidic domains in the phospholipid components of the inner membrane leaflet. Thus, the Na+,K+ ATPase α subunit might represent a preferential landing site for the association of Tat to the inner membrane leaflet, which cooperates with PI(4,5)P2 in docking Tat to the plasma membrane to favor its extracellular export, similar to what proposed for FGF-2 (Zacherl et al., 2015).
The ouabain binding site is a cavity in the transmembrane domain at the extracellular interface of the protein (Morth et al., 2007, Yatime et al., 2011). It can be speculated that this interaction determines some spatial modification in the α1 structure, such as to abolish interaction of Tat to the C-terminus. Consistent with this possibility, analysis of the protein structure obtained in the presence of ouabain (Yatime et al., 2011) indicates that the drug indeed induces a modification of the surface formed by the C-terminal cytosolic loops.
What is molecular function of extracellular Tat release? Past work from several laboratories has indicated that extracellular Tat exerts a number of pleiotropic activities when released outside the producing cells, ranging from stimulation of gene expression to inhibition of the immune response (reviewed in: (Fittipaldi and Giacca, 2005)). A non mutually exclusive, but perhaps more intriguing possibility is that a major function of extracellular Tat is to increase virion infectivity. Indeed, our experiments show that HIV-1 viral preparations, either pseudotyped or carrying a natural Env protein, are significantly less infectious when produced in the presence of ouabain; infectivity could be rescued by the addition of recombinant Tat or supernatants containing the protein. The results of these experiments indicate that a major function of secreted Tat is to increase HIV-1 virion infectivity on its primary CD4 + T cell targets. This effect is likely to be mediated by the association of Tat, on the surface of virions, with cell surface-associated heparan sulfate proteoglycans (Rusnati et al., 1998, Tyagi et al., 2001); the observation that infection of the CHO psg A-745 mutant, which lack proteoglycan biosynthesis, by HIV-1 virions produced in the presence of ouabain cannot be rescued by exogenous Tat addition is consistent with this possibility.
Of note, cell treatment with peptides corresponding to the three short cytoplasmic sequences in the C-terminal domain of the α1 protein both inhibited non canonical Tat secretion and suppressed HIV-1 infection. While no drugs are currently available in the clinics targeting Tat or its transactivation functions in the nucleus, the efficacy of these peptides suggests that the competitive inhibition of the Tat-Na+,K+-ATPase α1 subunit interaction might represent a novel strategy for the development of alternative anti-HIV-1 compounds. The design of derivatives of these peptides, or of their peptidomimetics, or the identification of small chemical molecules able to interfere with Tat-α1 binding might represent exciting avenues for future pharmacological development.
Funding Sources
This work was supported by the Intramural Funding Programme of the ICGEB to the Molecular Medicine Laboratory in Trieste, Italy.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
S.A., C.V., A.F. and E.T. performed most biochemical assays, H.A. performed all the virus infectious assays, A.B. and M.R. performed the SPR experiments, A.C. and M.L. supervised all experiments, M.L, H.A. and M.G. wrote the paper, M.G. coordinated the research.
Acknowledgments
The authors are very grateful to P. Devarajan, O. Burrone and B. Felber for the kind gifts of reagents and to A. Sabò, C. Guarnaccia, A. Albanese and S. Tosi for insightful comments and suggestions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.06.011.
Appendix A. Supplementary Data
Supplementary materials and methods; supplemental figures.
References
- Becker-Hapak M., McAllister S.S., Dowdy S.F. Tat-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- Berkhout B., Silverman R.H., Jeang K.T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Chang H.C., Samaniego F., Nair B.C., Buonaguro L., Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS. 1997;11:1421–1431. doi: 10.1097/00002030-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Connor R.I., Chen B.K., Choe S., Landau N.R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Dahl J.P., Binda A., Canfield V.A., Levenson R. Participation of Na,K-ATPase in FGF-2 secretion: rescue of ouabain-inhibitable FGF-2 secretion by ouabain-resistant Na,K-ATPase alpha subunits. Biochemistry. 2000;39:14877–14883. doi: 10.1021/bi001073y. [DOI] [PubMed] [Google Scholar]
- Demarchi F., Gutierrez M.I., Giacca M. Human Immunodeficiency Virus type 1 Tat protein activates transcription factor NF-kappaB through the cellular interferon-inducible, double-stranded RNA-dependent protein kinase, PKR. J. Virol. 1999;73:7080–7086. doi: 10.1128/jvi.73.8.7080-7086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel J.R., Schulz J., Zhou X.M., Kent R.B., Housman D., Cantley L., Levenson R. Expression of an ouabain-resistant Na,K-ATPase in CV-1 cells after transfection with a cDNA encoding the rat Na,K-ATPase alpha 1 subunit. J. Biol. Chem. 1988;263:7726–7733. [PubMed] [Google Scholar]
- Esko J.D., Stewart T.E., Taylor W.H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart G.D., Nasr N., Naif H., Cox G.B., Cunningham A.L., Gage P.W. Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages. Antimicrob. Agents Chemother. 2004;48:2325–2330. doi: 10.1128/AAC.48.6.2325-2330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi A., Giacca M. Transcellular protein transduction using the Tat protein of HIV-1. Adv. Drug Deliv. Rev. 2005;57:597–608. doi: 10.1016/j.addr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Fittipaldi A., Ferrari A., Zoppe M., Arcangeli C., Pellegrini V., Beltram F., Giacca M. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J. Biol. Chem. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- Flieger O., Engling A., Bucala R., Lue H., Nickel W., Bernhagen J. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 2003;551:78–86. doi: 10.1016/s0014-5793(03)00900-1. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R.Z., Anchin J., Baird A. The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na +,K +-ATPase. J. Biol. Chem. 1998;273:544–551. doi: 10.1074/jbc.273.1.544. [DOI] [PubMed] [Google Scholar]
- Frankel A.D., Pabo C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Giacca M. The HIV-1 Tat protein: a multifaceted target for novel therapeutic opportunities. Curr. Drug Targets Immune Endocr. Metabol Disord. 2004;4:277–285. doi: 10.2174/1568008043339767. [DOI] [PubMed] [Google Scholar]
- Gratton J.P., Yu J., Griffith J.W., Babbitt R.W., Scotland R.S., Hickey R., Giordano F.J., Sessa W.C. Cell-permeable peptides improve cellular uptake and therapeutic gene delivery of replication-deficient viruses in cells and in vivo. Nat. Med. 2003;9:357–363. doi: 10.1038/nm835. [DOI] [PubMed] [Google Scholar]
- Green M., Loewenstein P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Hamon Y., Luciani M.F., Becq F., Verrier B., Rubartelli A., Chimini G. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- Jones A.T., Sayers E.J. Cell entry of cell penetrating peptides: tales of tails wagging dogs. J. Control. Release. 2012;161:582–591. doi: 10.1016/j.jconrel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kaplan J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Lane L.K., Feldmann J.M., Flarsheim C.E., Rybczynski C.L. Expression of rat alpha 1 Na,K-ATPase containing substitutions of “essential” amino acids in the catalytic center. J. Biol. Chem. 1993;268:17930–17934. [PubMed] [Google Scholar]
- Laursen M., Yatime L., Nissen P., Fedosova N.U. Crystal structure of the high-affinity Na + K +-ATPase-ouabain complex with Mg2 + bound in the cation binding site. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10958–10963. doi: 10.1073/pnas.1222308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel J.B., Kuntzweiler T. Na +,K(+)-ATPase. J. Biol. Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- Lusic M., Marcello A., Cereseto A., Giacca M. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 2003;22:6550–6561. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganaro L., Lusic M., Gutierrez M.I., Cereseto A., Del Sal G., Giacca M. Concerted action of cellular JNK and Pin1 restricts HIV-1 genome integration to activated CD4 + T lymphocytes. Nat. Med. 2010;16:329–333. doi: 10.1038/nm.2102. [DOI] [PubMed] [Google Scholar]
- Mann D.A., Frankel A.D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiò S., Alfano M., Primo L., Gramaglia D., Butini L., Gennero L., De Vivo E., Arap W., Giacca M., Pasqualini R. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood. 2005;105:2802–2811. doi: 10.1182/blood-2004-06-2212. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 1986;261:11398–11403. [PubMed] [Google Scholar]
- Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sorensen T.L., Petersen J., Andersen J.P., Vilsen B., Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- Nickel W., Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- Ott M., Geyer M., Zhou Q. The control of HIV transcription: keeping RNA polymerase II on track. Cell Host Microbe. 2011;10:426–435. doi: 10.1016/j.chom.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E.M., Lingrel J.B. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Rayne F., Debaisieux S., Yezid H., Lin Y.L., Mettling C., Konate K., Chazal N., Arold S.T., Pugniere M., Sanchez F. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010;29:1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostand K.S., Esko J.D. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M., Coltrini D., Oreste P., Zoppetti G., Albini A., Noonan D., d'Adda di Fagagna F., Giacca M., Presta M. Interaction of HIV-1 Tat protein with heparin. J. Biol. Chem. 1997;272:11313–11320. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- Rusnati M., Tulipano G., Urbinati C., Tanghetti E., Giuliani R., Giacca M., Ciomei M., Corallini A., Presta M. The basic domain in HIV-1 Tat protein as a target for polysulfated heparin-mimicking extra-cellular Tat antagonists. J. Biol. Chem. 1998;273:16027–16037. doi: 10.1074/jbc.273.26.16027. [DOI] [PubMed] [Google Scholar]
- Schmidt N., Mishra A., Lai G.H., Wong G.C. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Madden T., Vijjeswarapu M., Newman R.A. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem. Pharmacol. 2001;62:469–472. doi: 10.1016/s0006-2952(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Tasciotti E., Giacca M. Fusion of the human immunodeficiency virus type 1 tat protein transduction domain to thymidine kinase increases bystander effect and induces enhanced tumor killing in vivo. Hum. Gene Ther. 2005;16:1389–1403. doi: 10.1089/hum.2005.16.1389. [DOI] [PubMed] [Google Scholar]
- Tasciotti E., Zoppe M., Giacca M. Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 tat. Cancer Gene Ther. 2003;10:64–74. doi: 10.1038/sj.cgt.7700526. [DOI] [PubMed] [Google Scholar]
- Temmerman K., Ebert A.D., Muller H.M., Sinning I., Tews I., Nickel W. A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic. 2008;9:1204–1217. doi: 10.1111/j.1600-0854.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Trudel C., Faure-Desire V., Florkiewicz R.Z., Baird A. Translocation of FGF2 to the cell surface without release into conditioned media. J. Cell. Physiol. 2000;185:260–268. doi: 10.1002/1097-4652(200011)185:2<260::AID-JCP11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tyagi M., Rusnati M., Presta M., Giacca M. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- West M.A., Bretscher M.S., Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatime L., Laursen M., Morth J.P., Esmann M., Nissen P., Fedosova N.U. Structural insights into the high affinity binding of cardiotonic steroids to the Na +,K +-ATPase. J. Struct. Biol. 2011;174:296–306. doi: 10.1016/j.jsb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Zacherl S., La Venuta G., Muller H.M., Wegehingel S., Dimou E., Sehr P., Lewis J.D., Erfle H., Pepperkok R., Nickel W. A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2. J. Biol. Chem. 2015;290:3654–3665. doi: 10.1074/jbc.M114.590067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler M., Steringer J.P., Muller H.M., Mayer M.P., Nickel W. HIV-tat protein forms phosphoinositide-dependent membrane pores implicated in unconventional protein secretion. J. Biol. Chem. 2015;290:21976–21984. doi: 10.1074/jbc.M115.667097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang F. Intracellular transduction and potential of Tat PTD and its analogs: from basic drug delivery mechanism to application. Expert Opin. Drug Deliv. 2012;9:457–472. doi: 10.1517/17425247.2012.663351. [DOI] [PubMed] [Google Scholar]
- Zhou X., Engel T., Goepfert C., Erren M., Assmann G., von Eckardstein A. The ATP binding cassette transporter A1 contributes to the secretion of interleukin 1beta from macrophages but not from monocytes. Biochem. Biophys. Res. Commun. 2002;291:598–604. doi: 10.1006/bbrc.2002.6473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods; supplemental figures.