Abstract
Parkinsonian Perry syndrome, involving mutations in the dynein motor component dynactin or p150Glued, is characterized by TDP-43 pathology in affected brain regions, including the substantia nigra. However, the molecular relationship between p150Glued and TDP-43 is largely unknown. Here, we report that a reduction in TDP-43 protein levels alleviates the synaptic defects of neurons expressing the Perry mutant p150G50R in Drosophila. Dopaminergic expression of p150G50R, which decreases dopamine release, disrupts motor ability and reduces the lifespan of Drosophila. p150G50R expression also causes aggregation of dense core vesicles (DCVs), which contain monoamines and neuropeptides, and disrupts the axonal flow of DCVs, thus decreasing synaptic strength. The above phenotypes associated with Perry syndrome are improved by the removal of a copy of Drosophila TDP-43 TBPH, thus suggesting that the stagnation of axonal transport by dynactin mutations promotes TDP-43 aggregation and interferes with the dynamics of DCVs and synaptic activities.
Keywords: Dynactin, TDP-43, Axonal transport, Dopamine, Parkinsonian syndrome, Neurodegeneration
Graphical abstract

Highlights
-
•
Fly model of Perry syndrome exhibits motor disturbance and impaired dopamine release.
-
•
Perry mutation in dynactin produces aggregation of dense core vesicles (DCVs) in axons and disrupts axonal flux of DCVs.
-
•
Removal of a copy of the TDP-43 gene improves retrograde flux of DCVs.
Parkinsonian Perry syndrome (PS), caused by mutations in a component of the retrograde transport complex, Dynactin, is pathologically characterized by the accumulation of an RNA-binding protein, TDP-43, in affected neurons. The neuronal accumulation of TDP-43 is observed in various neurodegenerative diseases including amyotrophic lateral sclerosis and Alzheimer's disease. We report that decreased TDP-43 expression improves defects in the axonal transport of dense core vesicles and in the dopamine release in a Drosophila PS model. This study provides insight into the possibility that a transient decrease in TDP-43 in neurons may be a promising therapeutic approach for treating neurodegenerative disorders associated with TDP-43 pathology, including PS.
1. Introduction
Perry syndrome (PS) is an autosomal dominant disorder characterized by parkinsonism with depression, sleep disturbance, weight loss, and central hypoventilation (Perry et al., 1975, Wider and Wszolek, 2008, Farrer et al., 2009). Genome-wide linkage analysis has identified disease-segregating missense mutations located in the dynactin (DCTN1) gene (Farrer et al., 2009). The gene product of dynactin, p150Glued, forms a complex with dynein, the microtubule-dependent retrograde motor. Disease-associated missense mutations (G71R, G71E, G71A, T72P, Q74P) are located in the cytoskeleton-associated protein Gly-rich (CAP-Gly) domain of p150Glued, which has been implicated in binding to microtubules recruiting dynein (Farrer et al., 2009, Ayloo et al., 2014, Tacik et al., 2014) and stabilizing the plus-end of microtubules (Lazarus et al., 2013). A glycine to serine substitution at residue 59 (G59S), which causes distal hereditary motor neuropathy 7B (HMN7B) (Puls et al., 2003), appears to affect the structure of the CAP-Gly domain and produces severe synaptic phenotypes, including p150Glued aggregation, dynein accumulation at nerve terminals and disruption of axonal transport (Lloyd et al., 2012, Moughamian and Holzbaur, 2012). Mutations associated with PS show milder synaptic phenotypes but cause impaired retrograde flux (Lloyd et al., 2012, Moughamian and Holzbaur, 2012).
The TAR DNA-binding protein of 43 kDa (TDP-43) is a highly conserved heterogeneous ribonucleoprotein (hnRNP) involved in the transcription, splicing, stabilization and transport of specific mRNAs (Volkening et al., 2009, Fiesel et al., 2010, Polymenidou et al., 2011, Ling et al., 2015). TDP-43 has been identified as the key component of intracellular ubiquitin-positive inclusions observed in affected brain areas of patients with amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD) and Alzheimer's disease (Arai et al., 2006, Neumann et al., 2006, Josephs et al., 2015, Tan et al., 2015, McAleese et al., 2017). A pathological feature of PS is the accumulation of TDP-43 in affected areas (Farrer et al., 2009, Wider et al., 2009). Increased TDP-43 is toxic to neurons (Li et al., 2010, Swarup et al., 2011), possibly because of its proneness to aggregation and conversion to an abnormal protein structure similar to that of prion and α-Synuclein (Nonaka et al., 2013). On the other hand, TDP-43 is indispensable to mouse development (Chiang et al., 2010, Sephton et al., 2010) and Drosophila survival (Feiguin et al., 2009, Diaper et al., 2013b, Vanden Broeck et al., 2013), thus suggesting that the control of appropriate protein levels is critical for TDP-43 function.
The molecular relationship between p150Glued and TDP-43 is largely unknown. To determine whether TDP-43 contributes to PS phenotypes, we manipulated TDP-43 protein levels in a Drosophila PS model and found that reduced TDP-43 improved defects in axonal transport and the synaptic activity of central dopaminergic neurons, as well as motor neurons, caused by the neuron-specific expression of a PS-associated p150Glued mutation.
2. Materials and Methods
2.1. Drosophila Strains
Fly culture and crosses were performed on standard fly food containing yeast, cornmeal, and molasses. Flies were raised at 25 °C. All other fly stocks and GAL4 lines used in this study were obtained from the Bloomington Drosophila Stock Center and Kyoto Stock Center and have previously been described: UAS-p150WT-HA and UAS-p150G50R-HA (Lloyd et al., 2012); TBPH∆ 23 (Feiguin et al., 2009); UAS-GFP-TBPHWT, UAS-RFP-TBPHWT and UAS-RFP-TBPHA315T (Estes et al., 2011); and UAS-VMAT-phluorin (Wu et al., 2013). Stocks were backcrossed to the w1118 wild-type background for six generations, and w1118 was used as a wild-type allele.
2.2. Survival Assay and Climbing Assay
For lifespan studies, approximately 20 adult flies per vial were maintained at 25 °C, transferred to fresh fly food, and scored for survival every 4 days. To control for isogeny, fly lines were backcrossed to the w1118 background for six generations. A climbing assay was performed as previously described (Shiba-Fukushima et al., 2014). For spontaneous locomotor behavior, male flies were preconditioned at 25 °C under a 12-h light: dark cycle for 3 days and were then recorded for another 3 days using a Drosophila activity monitoring (DAM) system (TriKinetics), which monitors the activity of individual flies (16 to 32 flies per experiment) in polycarbonate tubes (length, 65 mm; inside diameter, 3 mm).
2.3. Whole-mount Immunostaining and Transmission Electron Microscopy (TEM) Analysis
The antibodies used in immunocytochemistry were anti-Bruchpilot (1:10, Developmental Studies Hybridoma Bank (DSHB), nc82, RRID:AB_2314867), anti-GluRIIA (1:10, DSHB, 8B4D2, RRID:AB_528269), anti-Dlg (1:250, DSHB, 4F3, RRID:AB_528203), anti-GFP (1:500, Thermo Fisher Scientific, A6455, RRID:AB_221570), anti-RFP (1:100, Abcam, ab62341, RRID:AB_945213), and Alexa Fluor594- (1:200) or DyLight649- (1:500) conjugated anti-horseradish peroxidase (HRP) (Jackson ImmunoResearch, 123-585-021, RRID:AB_2338966 and 123-495-021, discontinued). The visualizations of synapse boutons, mitoGFP and active zones (AZs) in larval motor neurons were analyzed by whole-mount immunostaining as previously described (Lee et al., 2010). For image processing of synapse boutons, thirty Z-stack images were taken at 0.15- to 0.30-μm intervals and were reconstituted using ImageJ. TEM images were obtained using an electron microscope (Hitachi, HT7700) at the Laboratory of Ultrastructural Research of Juntendo University.
2.4. Immunoprecipitation and Western Blot Analysis
To analyze the binding between TBPH and p150Glued, eight 3rd-instar larvae (w1118) from which the digestive organs had been removed were lysed with 200 μl lysis buffer (Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 5% glycerol, 1 mM EDTA, 1 mM DTT) containing Complete protease inhibitor cocktail (Sigma-Aldrich) with a motor-driven pestle. Supernatant obtained after centrifugation at 16,000 × g for 10 min was subjected to immunoprecipitation with anti-p150Glued conjugated with TrueBlot Magnetic Beads (Rockland Immunochemicals Inc., RRID:AB_2610703) and was incubated at 4 °C overnight. The beads washed three times with the lysis buffer were analyzed by subsequent western blotting. For western blotting to determine protein levels, larvae from which the digestive organs had been removed were homogenized in 10 μl of RIPA buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA 1% NP-40, 1% deoxycholate, 0.1% SDS) containing Complete protease inhibitor cocktail per sample using a motor-driven pestle. After centrifugation at 16,000 × g for 10 min, the supernatants dissolved in SDS sample buffer were subjected to western blotting. The antibodies used in western blotting were anti-GluRIIA (1:50, DSHB, 8B4D2), anti-Dlg (1:1000, DSHB, 4F3), anti-Dynein (1:50, DSHB, 2C11-2, RRID:AB_2091523), anti-β-Tubulin (1:50, DSHB, E7, RRID:AB_528499), anti-HA (1:1000, Sigma-Aldrich, 3F10, RRID:AB_390919), anti-TBPH (1:1000) and anti-p150Glued (1:1000, C). Anti-TBPH and anti-p150Glued (N and C) antibodies were kind gifts from Drs. F. Hirth and V.I. Gelfand, respectively (Siller et al., 2005, Diaper et al., 2013a).
2.5. Dopamine Measurement
Five adult male fly heads were dissected and homogenized in 50 μl of 0.1 M perchloric acid by using a motor-driven pestle around 1 p.m. Dopamine levels in Drosophila brain extracts were determined by HPLC coupled to electrochemical detection using a mobile phase containing 50 mM citric acid, 50 mM sodium dihydrogen phosphate, pH 2.5, 0.1 mM EDTA, 4.4 mM 1-heptanesulfonic acid, 2.2% (vol/vol) acetonitrile, and 5.3% (vol/vol) methanol.
2.6. Live Imaging of Axonal Transport
Living 3rd-instar larvae were dissected in HL-3 saline (5 mM HEPES, pH 7.2, 70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 5 mM trehalose, and 2 mM CaCl2). Time-lapsed images (0.1 s interval during 36 s) were captured using a laser-scanning microscope system (TCS-SP5, Leica). Imaging data were processed with ImageJ software.
2.7. Electrophysiology
Third-instar larvae were dissected in HL-3, and mEJPs from NMJs were recorded using an electrophysiological setup equipped with an Eclipse FN1 microscope (Nikon), a Multiclamp 700B amplifier (Molecular Devices) and a Digidata 1550A data acquisition system (Molecular Devices). Dissected larvae were incubated in HL-3 containing 0.375 mM (for mEJP) or 2 mM (for EJP and PPR) Ca2 +, and a recording electrode filled with 3 M KCl was inserted into muscle 6 of the A3 or A4 segment containing NMJs. All data were analyzed using Mini-Analysis software (Synaptosoft). PPR was calculated as a ratio of EJP amplitudes with paired stimulation (50 msec interval). QC was calculated as the average EJP amplitude divided by the average mEJP amplitude as previously described (Lee et al., 2010).
2.8. VMAT-pHluorin Live Imaging
VMAT-pHluorin live imaging was previously described (Shiba-Fukushima et al., 2014). Briefly, the DA release rates of neuronal fibers and terminals in the whole brain region without the subesophageal ganglion were calculated by normalizing the data with the fluorescence intensity just after photobleaching using ImageJ software.
2.9. Statistical Analysis
Error bars in graphs represent the mean ± SEM. A two-tailed Student's t-test or a one-way repeated-measures analysis of variance (ANOVA) was used to determine significant differences between two or among multiple groups, respectively, unless otherwise indicated. If a significant result was determined using ANOVA (p < 0.05), the mean values of the control and the specific test group were analyzed using a Tukey-Kramer test. Steel's test and Dunnett's test were used to determine significant differences between two specific groups or among multiple groups of interest. The data distribution was assumed to be normal, although this assumption was not formally tested. Randomization was used in each genotype, and data collection and analysis were not performed blind to the conditions of the experiments. All the data obtained in the experiments were included except for the VMAT-pHluorin assay, for which the samples that showed increased fluorescence signals immediately after photobleaching were excluded.
3. Results
3.1. Removal of One Copy of TBPH Improves the Decreased Motor Ability and Lifespan Caused by PS Mutation
The amino acid residues of p150Glued affected in PS are highly conserved between humans and Drosophila (Farrer et al., 2009, Lloyd et al., 2012). To understand how PS-associated mutations of p150Glued affect neurons, we expressed wild-type p150Glued (p150WT) and a mutant, p150Glued, in which Gly50 is replaced with Arg (p150G50R; corresponding to p150G71R in humans) in Drosophila dopaminergic neurons. Forty-five-day-old normal adult flies exhibited an age-dependent decline in compulsive motor activity compared with 5-day-old flies; this decline was exacerbated in flies expressing p150G50R (Fig. 1A). The introduction of a TAR DNA-binding protein of 43 homolog (TBPH) null allele, TBPH∆ 23, caused an approximately 30% reduction in TBPH protein levels (Fig. S1A, B) (Feiguin et al., 2009, Diaper et al., 2013b) and improved motor disturbances caused by p150WT or p150G50R (Fig. 1A, lower). Whereas the lifespan of TBPH+/− flies was reduced compared to controls, the combination of TBPH+/− with p150WT or p150G50R improved the lifespan of p150WT or p150G50R flies (Fig. 1B). We did not observe decreased spontaneous locomotor activity, loss of TH-positive neurons or decreased total amount of brain dopamine in flies expressing p150WT or p150G50R even in 45-day-old flies (Fig. S1C–E). However, the removal of a copy of TBPH stimulated spontaneous locomotor activity (Fig. S1C).
Fig. 1.

Reducing endogenous TBPH rescues motor impairment caused by dopaminergic expression of p150G50R.
(A) Climbing assay at 5 and 45 days old. n = 15 trials in 20 flies per genotype, **p < 0.01 by Tukey-Kramer test.
(B) Lifespan at 25 °C. ***p < 0.001 by log-rank test.
See also Fig. S1. Genotypes in (A, B) and sample size in (B) are: TH-Gal4/+ (Control, n = 298), UAS-p150WT-HA/+; TH-Gal4/+ (p150WT, n = 188), UAS-p150G50R-HA/+; TH-Gal4/+ (p150G50R, n = 251), TBPHΔ23/+; TH-Gal4/+ (TBPH+/−, n = 202), TBPHΔ23/+; UAS-p150WT-HA; TH-Gal4/+ (p150WT; TBPH+/−, n = 149), TBPHΔ23/+; UAS-p150G50R-HA; TH-Gal4/+ (p150G50R; TBPH+/−, n = 168).
3.2. Axonal Terminals are Affected by PS Mutation
Previous studies have suggested that p150Glued is required for synapse growth and stabilization (Eaton et al., 2002, Chang et al., 2013). These events appear to be regulated by p150Glued-mediated stabilization of microtubule plus ends and to be abrogated in the Perry mutant G74P (Lazarus et al., 2013). The expression of p150G50R in larval motor neurons caused both distal and proximal synaptic boutons to swell with prominent synaptic debris (Fig. 2A, B) (Fuentes-Medel et al., 2009), but the number of synaptic boutons was not significantly changed (Fig. 2C), thus suggesting that the G50R mutation has moderate effects on p150Glued function. The swollen synaptic phenotype of proximal but not distal boutons by p150G50R was suppressed in the TBPH+/− background, whereas the synaptic phenotypes of TBPH+/− were similar to those of controls (Fig. 2A–C). In this context, TBPH+/− did not suppress the synaptic debris observed in p150G50R synapses (Fig. 2A). The number of AZs in p150G50R synapses increased, possibly as a consequence of an alteration to the signaling pathway for AZ development, accompanied by endocytic defects (Dickman et al., 2006). This phenotype was suppressed by the introduction of TBPH+/− (Fig. 2D, E). Alteration of presynaptic structures may affect the maturation and maintenance of the postsynaptic architecture (Romano et al., 2014). We therefore examined the distribution and expression of a postsynaptic scaffold protein, Dlg, and a subunit of the postsynaptic glutamate receptor, GluRIIA, in the neuromuscular junctions (NMJs) of larval motor neurons (Fig. S1A and S2A). Whereas Dlg expression appeared to be unchanged, GluRIIA tended to be reduced by p150G50R expression or removal of a copy of TBPH, thus suggesting that postsynaptic functions are partially affected.
Fig. 2.
Motor neuron-specific p150G50R expression produces swelling of terminal boutons at NMJs.
(A) Distal (d) and four proximal boutons (p) of NMJs. Arrowheads indicate synaptic debris visualized with anti-HRP. Scale bar, 5 μm.
(B, C) Size (B) and number (C) of synapse boutons. n = 16–23 NMJ terminals (B) or 8–10 NMJs (C) in 5 flies per genotype, *p < 0.05 vs. Control by Steel's test. The sample size is indicated in the graphs.
(D) Active zones (AZs, red) at the synaptic boutons stained with anti-HRP (Blue). Scale bar, 2 μm.
(E) Average number of AZs per bouton from distal and four proximal boutons. n = 20–39 NMJ terminals in 5 flies per genotype, *p < 0.05 vs. Control by Dunnett's test. The sample size is indicated in the graph.
(F) DCVs (green) at the synaptic boutons stained with anti-HRP (red). Scale bar, 5 μm.
(G) Graphs for DCV aggregates with over 0.5 μm2 in the most distal boutons (left) and average DCV density in the most distal boutons and in the proximal three boutons (right). A.U., arbitrary units. n = 10–13 NMJ terminals in 6 flies per genotype, *p < 0.05 by two-tailed Student's t-test. #p < 0.05 vs. Control; TBPH+/+, §p < 0.05 vs. Control; TBPH+/− by Dunnett's test. The sample size is indicated in the graphs.
See also Fig. S2. Genotypes used: (A–E) OK6-Gal4/+ (Control), OK6-Gal4/UAS-p150G50R-HA (p150G50R), OK6-Gal4, TBPHΔ23/+ (TBPH+/−), OK6-Gal4, TBPHΔ23/UAS-p150G50R-HA (p150G50R; TBPH+/−), (F, G) UAS-preproANF-EMD/+; +/+; D42-Gal4/+ (Control), UAS-preproANF-EMD/+; UAS-p150WT-HA/+; D42-Gal4/+ (p150WT), UAS-preproANF-EMD/+; UAS-p150G50R-HA/+; D42-Gal4/+ (p150G50R), UAS-preproANF-EMD/+; TBPHΔ23/+; D42-Gal4/+ (TBPH+/−), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150WT-HA; D42-Gal4/+ (p150WT; TBPH+/−), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150G50R-HA; D42-Gal4/+ (p150G50R; TBPH+/−).
Because mitochondrial dysfunction is thought to be part of the etiology of Parkinson's disease, we also analyzed mitochondrial distribution in NMJs (Fig. S2B) (Imai and Lu, 2011). Unexpectedly, smaller mitochondria accumulated in the most distal boutons in TBPH+/− larvae. The mitochondrial number of the terminal 6 boutons, especially the proximal 5 boutons, of the NMJs in p150G50R and p150G50R TBPH+/− larvae tended to be lower, whereas the mitochondrial density in the most distal boutons was not significantly changed among genotypes. These observations suggest that TBPH reduction did not reverse the altered mitochondrial distribution caused by p150G50R.
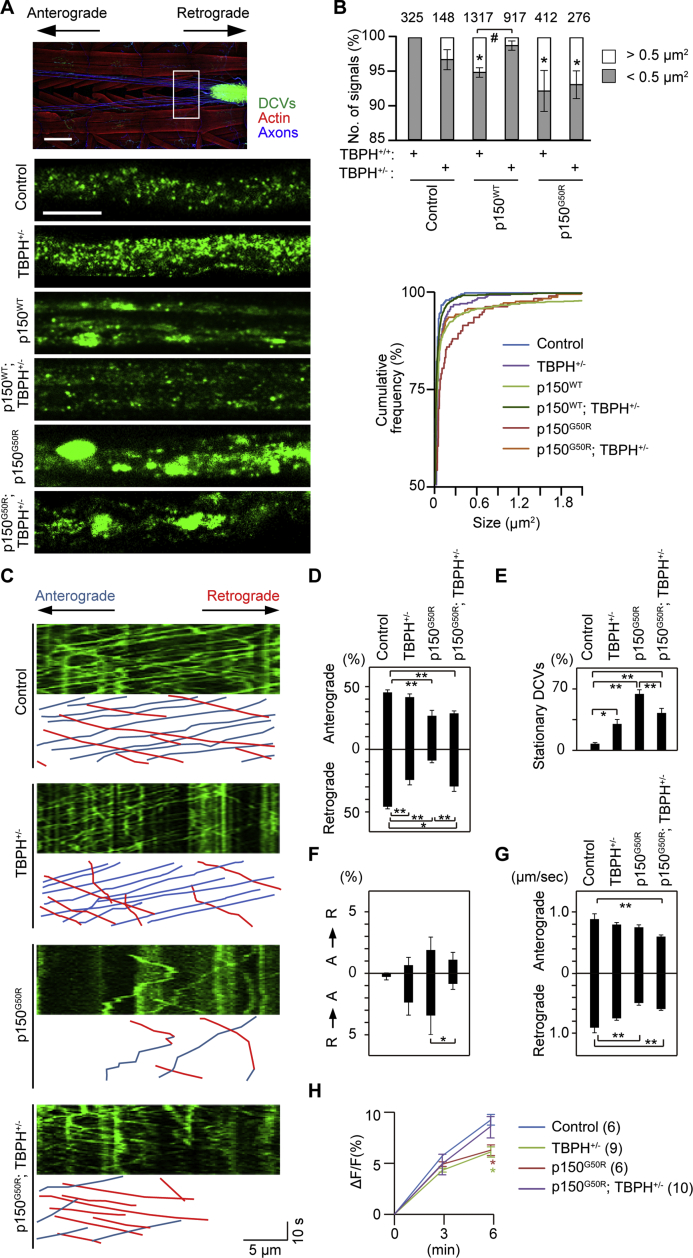
A decrease in dopamine release is an early event of dopaminergic neurodegeneration in PS as well as Parkinson's disease (Wider and Wszolek, 2008), and monoamine neurotransmitters are thought to be stored in both dense core vesicles (DCVs) and synaptic vesicles in Drosophila (Grygoruk et al., 2014). A uniform supply of DCVs in each bouton is achieved by a circulation of axonal flow and a local capture mechanism. To visualize the distribution of DCVs in the NMJs, emerald GFP-tagged atrial natriuretic factor (ANF-EMD) was expressed in motor neurons (Fig. 2F and Fig. S2C) (Rao et al., 2001). Most DCVs were approximately 0.5 μm2 in dimension in control animals, whereas the expression of p150G50R resulted in the accumulation of aggregated DCVs over 0.5 μm2 in the most distal boutons (Left graph in Fig. 2G and Fig. S2C). In contrast, the density of DCVs was increased in proximal boutons of TBPH+/− larvae (Right graph in Fig. 2G). Importantly, the accumulation of DCVs in the most distal boutons by p150G50R was suppressed, and the uniform distribution of DCVs was observed at a level similar to controls via the TBPH reduction (Fig. 2F, G and Fig. S2C). Although the expression of p150WT mildly promoted DCV aggregation in the most distal boutons, the phenotype was not changed by decreased TBPH, thus suggesting that the beneficial effect on the bouton DCV dynamics by decreased TBPH is p150G50R specific (Fig. 2F, G and Fig. S2C).
3.3. TBPH Contributes to Defects in Axonal Transport of DCVs
We next monitored the movement of DCVs in the axonal transport of larval motor neurons (Fig. 3A and Movie S1, Movie S2, Movie S3, Movie S4). Larger DCVs tended to appear in TBPH+/− animals (Fig. 3A, B and Fig. S3A). The expression of p150WT caused moderate DCV aggregates, which were suppressed by the removal of a copy of TBPH (Fig. 3A, B and Fig. S3A). The p150G50R expression resulted in the marked appearance of large DCV aggregates, which were also alleviated by the decreased TBPH (Fig. 3A, B and Fig. S3A). In sharp contrast, the morphology of axonal mitochondria was mostly unchanged by the expression of p150G50R, and mitochondria were not colocalized with TBPH, even with an ALS-associated mutant (Fig. S3B, C) (Wang et al., 2016). Whereas p150WT affected the retrograde transport of DCVs, which caused increased stationary DCVs, p150G50R markedly impaired DCV movement in both anterograde and retrograde directions and further increased stationary DCVs (Fig. 3C–E and Fig. S3D, E). In addition, the number of retrograde DCVs that reversed to an anterograde flow was significantly increased after expression of p150G50R but not p150WT, thus suggesting that retrograde transport is especially compromised by p150G50R (Fig. 3C, F and Fig. S3F). The introduction of TBPH+/− partially rescued these defects via p150WT or p150G50R (Fig. 3C–F and Fig. S3D, E), whereas the reduction in the velocity of DCVs by p150G50R was not improved (Fig. 3G). Consistently with defects in the axonal transport of DCVs, the decreased dopamine release estimated by VMAT-pHluorin in the adult brain of p150G50R flies was also alleviated by the introduction of TBPH+/− (Fig. 3H and Fig. S3G). However, we did not detect a physical interaction of TBPH with p150Glued (Fig. S3H) or decreased endogenous p150Glued and dynein after introducing TBPH+/− (Fig. S1A, B). These results suggest that the rescue effects of p150G50R phenotypes associated with decreased TBPH levels are an indirect mechanism.
Fig. 3.
p150G50R leads to a disturbance of axonal flow and aggregation of DCVs in motor neurons.
(A) DCVs in motor neuron axons. Scale bars, 200 (upper) and 5 (lower) μm.
(B) Size distribution of DCVs. The number of DCVs larger than 0.5 μm2 is increased in p150WT and p150G50R. n = 148–1317 DCVs from 5 to 10 axons in 4–5 larvae per genotype, *p < 0.05 vs. Control; TBPH+/+ by Dunnett's test, #p < 0.05 by two-tailed Student's t-test. Removal of a copy of TBPH partially suppresses the appearance of large DVCs in p150WT and p150G50R (lower).
(C) Disturbance of axonal DCV movement is partially rescued by TBPH reduction. Representative kymographs generated from axonal movies. The x-axis represents DCV position, and the y-axis is time (moving from top to bottom). Vertical lines correspond to stationary DCVs, and diagonal lines are moving DCVs. The bottom panels reveal the track of only the moving DCVs, which are shown as blue (anterograde) or red (retrograde) diagonal lines.
(D–F) The percentage of moving DCVs (D) and of stationary DCVs (E). n = 148 DCVs from 9 larvae (control), 282 DCVs from 12 larvae (TBPH+/−), 176 DCVs from 12 larvae (p150G50R), and 344 DCVs from 15 larvae (p150G50R; TBPH+/−), *p < 0.05, **p < 0.01 by Tukey-Kramer test. The percentage of DCVs that reverse the direction from anterograde (A) to retrograde (R) flow or in the opposite direction during movement (F). *p < 0.05 by two-tailed Student's t-test.
(G) The average velocity of DCVs. n = 148 DCVs from 9 larvae (control), 282 DCVs from 12 larvae (TBPH+/−), 176 DCVs from 12 larvae (p150G50R), and 344 DCVs from 15 larvae (p150G50R; TBPH+/−), **p < 0.01 by Tukey-Kramer test.
(H) Recovery ratio of VMAT-pHluorin in a 30-day-old adult brain. n = 6–10 per genotype, *p < 0.05 vs. Control by Dunnett's test. The sample size is indicated in parentheses.
See also Fig. S3 and Movie S1, Movie S2, Movie S3, Movie S4. Genotypes used: (A–G) UAS-preproANF-EMD/+; +/+; D42-Gal4/+ (Control), UAS-preproANF-EMD/+; TBPHΔ23/+; D42-Gal4/+ (TBPH+/−), UAS-preproANF-EMD/+; UAS-p150WT-HA/+; D42-Gal4/+ (p150WT), UAS-preproANF-EMD/+; UAS-p150G50R-HA/+; D42-Gal4/+ (p150G50R), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150WT-HA; D42-Gal4/+ (p150WT; TBPH+/−), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150G50R-HA; D42-Gal4/+ (p150G50R; TBPH+/−), (H) +/Y; +/+; TH-Gal4, UAS-VMAT-pHluorin/+ (Control), +/Y; UAS-p150G50R-HA/+; TH-Gal4, UAS-VMAT-pHluorin/+ (p150G50R), +/Y; TBPHΔ23/+; TH-Gal4, UAS-VMAT-pHluorin/+ (TBPH+/−), +/Y; UAS-p150G50R-HA/TBPHΔ23; TH-Gal4, UAS-VMAT-pHluorin/+ (p150G50R; TBPH+/−). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
See also Fig. S3 and Movies S1, S2, S3, S4. Genotypes used: (A–G) UAS-preproANF-EMD/+; +/+; D42-Gal4/+ (Control), UAS-preproANF-EMD/+; TBPHΔ23/+; D42-Gal4/+ (TBPH+/−), UAS-preproANF-EMD/+; UAS-p150WT-HA/+; D42-Gal4/+ (p150WT), UAS-preproANF-EMD/+; UAS-p150G50R-HA/+; D42-Gal4/+ (p150G50R), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150WT-HA; D42-Gal4/+ (p150WT; TBPH+/−), UAS-preproANF-EMD/+; TBPHΔ23/UAS-p150G50R-HA; D42-Gal4/+ (p150G50R; TBPH+/−), (H) +/Y; +/+; TH-Gal4, UAS-VMAT-pHluorin/+ (Control), +/Y; UAS-p150G50R-HA/+; TH-Gal4, UAS-VMAT-pHluorin/+ (p150G50R), +/Y; TBPHΔ23/+; TH-Gal4, UAS-VMAT-pHluorin/+ (TBPH+/−), +/Y; UAS-p150G50R-HA/TBPHΔ23; TH-Gal4, UAS-VMAT-pHluorin/+ (p150G50R; TBPH+/−).
3.4. Dysfunction of Synaptic Release by the PS Mutation Is Rescued by TBPH Reduction
Given that synaptic activity is impaired by the blockage of DCV transport in p150G50R-expressing neurons, we next analyzed the electrophysiological properties of the NMJ in larval motor neurons. The excitatory junction potential (EJP) and quantal content (QC) were reduced in p150G50R but not p150WT animals, thus suggesting impaired synaptic release in PS (Fig. 4A). Consistently with results from morphological analyses of synaptic boutons, TBPH reduction improved the EJP and QC of p150G50R animals. However, the miniature excitatory junction potential (mEJP) frequency was increased in p150WT animals, thus suggesting that p150WT overexpression partially affects synaptic activity (Fig. 4A, B and Table S1). The p150WT and TBPH+/− animals tended to exhibit larger spikes in mEJPs, and the large spikes of TBPH+/− animals were suppressed by p150G50R expression (Fig. 4B, C). The paired-pulse ratio (PPR) was not different among the five genotypes (Table S1).
Fig. 4.
Reduced TBPH rescues synaptic strength at the NMJs of p150G50R flies.
(A) Representative electrophysiological traces of EJP and mEJP in the larval NMJs. Scale bar for EJP (mEJP): y = 10 mV (3 mV), x = 50 msec (1 s). EJP amplitudes (EJP) and quantal content (QC) are reduced in p150G50R flies. *(#)p < 0.05 vs. Control by Steel's test (Dunnett's test). Black dots indicate large spikes (> 1.5 mV) in mEJP. See also Table S1 for further electrophysiological information.
(B, C) Cumulative spike numbers (B) and number of large spikes (C) during 60 s. n = 4–10 NMJs in 4–6 flies per genotype. The number of large spikes in (C) was not significantly altered among the genotypes, on the basis of Dunnett's test. The sample size is indicated in the graph of (C).
(D) Ultrastructural images of the synapse boutons in the larval motor neurons. M, mitochondria. Arrowheads, large DCVs. Abnormal vesicular structures in p150G50R flies are also indicated by asterisks. Scale bar, 500 nm.
(E) Quantification of the SV number in the unit area containing an AZ (defined in Fig. S4A). n = 8–23 AZ neighboring regions in 3 flies, *p < 0.05 vs. Control by Dunnett's test. The sample size is indicated in the graph.
See also Table S1 and Fig. S4. Genotypes used: OK6-Gal4, UAS-mitoGFP/+ (Control), OK6-Gal4, UAS-mitoGFP/UAS-p150WT-HA (p150WT), OK6-Gal4, UAS-mitoGFP/UAS-p150G50R-HA (p150G50R), OK6-Gal4, UAS-mitoGFP, TBPHΔ23/+ (TBPH+/−), OK6-Gal4, UAS-mitoGFP, TBPHΔ23/UAS-p150G50R-HA (p150G50R; TBPH+/−).
Consistently with the increased mEJP frequency, the number of synaptic vesicles (SVs) was increased in both the AZ regions and the presynaptic cytosol of TBPH+/− NMJs, thus suggesting that TBPH at synapses is important for the regulation of SVs (Fig. 4D, E and Fig. S4). The enlarged boutons of p150G50R-expressing neurons contained fewer SVs docked at AZs, but many DCVs and uncharacterized large vesicular structures with high or low electron density, which probably include aggregated DCVs and lysosomes (Fig. 4D and Fig. S4B) (Lloyd et al., 2012). Again, the appearance of abnormal vesicular structures was suppressed by the decreased TBPH in p150G50R-expressing neurons (Fig. 4D).
4. Discussion
Whereas mutations of p150Glued cause PS, in which TDP-43 pathology has been reported in the basal ganglia, including the substantia nigra, whether TDP-43 contributes to neurodegeneration in PS remained unknown. Here, we analyzed the molecular relationship between p150Glued and TDP-43 in a Drosophila PS model.
Neuronal expression of the p150G50R mutation, located in the CAP-Gly domain, led to swelling of distal boutons and the prominent aggregation of DCVs but not mitochondria in axons and synapses. DCVs travel between proximal axons and terminal boutons via axonal transport, which efficiently delivers neurotransmitters to synaptic boutons (Wong et al., 2012). Although p150G50R disrupts both the anterograde and retrograde flow of DCVs, the anterograde/retrograde ratios of moving DCVs suggested that the retrograde flow is especially affected by p150G50R (Fig. 3D), thus leading to accumulation of organelles and materials at nerve terminals (Fig. 4D). Consistently with the morphological abnormalities in synaptic phenotypes, synaptic strength, dopamine release and motor ability were decreased by p150G50R expression without detectable neuron loss. Although p150WT expression also affected the retrograde flow of DCVs and the DCV distribution at the terminal boutons, the phenotypes were milder than those of p150G50R. Given that p150WT protein was expressed at higher levels than p150G50R in our transgenic flies, in which both p150WT and p150G50R transgenes were inserted in the same genomic locus and both transcript levels were similar, p150G50R exerts highly deleterious effects on neuronal activity despite its unstable expression (Lloyd et al., 2012). Thus, our data obtained with p150G50R expression would reflect synaptic dysfunction as an early neurodegeneration event in PS.
Altered mitochondrial distribution at the boutons by p150G50R was not significantly rescued by TBPH reduction, thus suggesting that the improvement of DCV phenotypes markedly contributes to the rescue effects in neuronal functions and survival. However, we cannot exclude the possibility that mitochondrial functions may be affected in p150G50R flies, because the fluorescence intensity of mitoGFP (cytochrome C oxidase subunit VIII-GFP fusion protein), which inserts in the mitochondrial inner membrane in a membrane potential-dependent manner, was somewhat reduced by p150G50R. Ultrastructural analysis of synaptic mitochondria also suggested that mitochondrial cristae were partly damaged in p150G50R flies (Fig. 4D). Because mitochondrial shuttling between cell bodies and nerve terminals in neurons would be important to maintain mitochondrial proteins derived from the nuclear genome, which include the respiratory complex I, III, IV and V subunits, the effects of PS mutations on mitochondrial functions at nerve terminals should be examined in future studies (Abe et al., 1995).
TDP-43 accumulation in affected regions is a prominent feature of PS pathology. TDP-43 forms cytoplasmic messenger ribonucleoprotein (mRNP) granules, which also move via axonal transport to synaptic boutons to deliver mRNA for synaptic activities (Alami et al., 2014). Our discovery in Drosophila that the ablation of a copy of the TBPH gene improves the axonal aggregation of DCVs and synaptic defects provides three possible molecular mechanisms: First, a reduced concentration of TBPH in axons and nerve terminals improves axonal flow, suppressing the aggregation of TBPH. Second, TBPH reduction alters the expression of proteins regulated by TBPH at transcript levels, thus alleviating synaptic dysfunctions. Third, the above two mechanisms contribute to rescue effects. We prefer the third mechanism for the reasons listed below.
The DCV aggregates in axons and synapses were suppressed by TBPH reduction without improving the velocity of axonal transport, thus suggesting that aggregation-prone TBPH promotes DCV aggregation when the axonal flow stagnates. However, the numbers of DCVs and SVs were increased in the synaptic boutons of TBPH+/− flies, thus suggesting that TBPH negatively regulates DCV and SV production. A variety of TBPH target mRNAs have been reported in Drosophila (Hazelett et al., 2012, Vanden Broeck et al., 2013, Coyne et al., 2014), and further studies may reveal a target(s) to regulate DCVs and SVs. Regarding synaptic stabilization, microtubule-associated MAP1B/Futsch is an evolutionarily conserved target of TDP-43/TBPH, which negatively or positively regulates synaptic MAP1B/Futsch expression (Sephton et al., 2010, Godena et al., 2011, Coyne et al., 2014) and might maintain axonal transport through regulating microtubule dynamics. Although we did not detect obvious changes in the levels of Futsch protein in TBPH+/− flies (data not shown), the downregulation of Futsch/MAP1B may explain the observation that some phenotypes in TBPH+/− flies were reversed by p150G50R expression. Because p150Glued stabilizes the plus ends of microtubules, the ectopic expression of p150G50R may partially alleviate the microtubule destabilization caused by Futsch/MAP1B downregulation (Lazarus et al., 2013). Alternatively, the decreased axonal flow resulting from p150G50R expression may enable efficient synaptic capture of TDP-43-mRNP granules, which regulate local protein translation for synaptic activity including Futsch/MAP1B (Diaper et al., 2013b, Alami et al., 2014).
Autoregulation of TDP-43 mRNA has been demonstrated in mammals (Ayala et al., 2011, Polymenidou et al., 2011) and has been suggested in Drosophila (Hazelett et al., 2012, Vanden Broeck et al., 2013). Consistently with these reports, the decrease in TBPH protein was at most 30% in TBPH+/− flies. Although the genetic ablation of a copy of the TBPH gene in itself produced some synaptic phenotypes and decreased the lifespan in flies, the transient knockdown of TDP-43 would be a suitable strategy for therapeutic intervention in PS. It has been demonstrated that the transient inhibition of a truncated N-terminal huntingtin with an abnormal polyQ stretch improves the neuropathology and the motor phenotype in a Huntington's disease mouse model (Yamamoto et al., 2000). Thus, disease phenotypes caused by TDP-43 proteinopathies may be reversible under appropriate levels of TDP-43 control.
The following are the supplementary data related to this article.
Movement of DCVs in a control axon.
Movement of DCVs in a TBPH+/− axon.
Movement of DCVs in a p150G50R axon.
Movement of DCVs in a TBPH+/−; p150G50R axon.
Supplementary material
Funding Sources
This study was funded by Grants-in-Aid for Scientific Research (26293070 [Y.I.], 15H04842 [N.H.]) from MEXT in Japan, a Grant-in-Aid for Scientific Research on Innovative Areas (23111003 [N.H.]), a Health Labor Sciences Research Grant (201324123A) (N.H. and Y.I.), and a grant from Otsuka Pharmaceutical (N.H. and Y.I.).
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
Y.I. conceived and designed the study. Y.H. and T.I. performed most of the experiments and analyzed the data with support from K.S., C.C., and T.A. Y.H., Y.I., and N.H. wrote the manuscript with input from the other authors.
Acknowledgments
We thank Drs. F. Feiguin, F.E. Baralle, T.E. Lloyd, A.L. Kolodkin, D.C. Zarnescu, V.I. Gelfand, C.Q. Doe, F. Hirth, T. Chihara and M. Miura for providing materials, and we thank T. Imura, T. Kanao, Y. Umezaki, M. Yoshida and S. Kakuta for their technical assistance.
Contributor Information
Yuzuru Imai, Email: yzimai@juntendo.ac.jp.
Nobutaka Hattori, Email: nhattori@juntendo.ac.jp.
References
- Abe K., Aoki M., Kawagoe J., Yoshida T., Hattori A., Kogure K., Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A., Clare A.J., Badders N.M., Bilican B., Chaum E., Chandran S., Shaw C.E., Eggan K.C., Maniatis T., Taylor J.P. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Ayala Y.M., De Conti L., Avendano-Vazquez S.E., Dhir A., Romano M., D'Ambrogio A., Tollervey J., Ule J., Baralle M., Buratti E., Baralle F.E. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30:277–288. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayloo S., Lazarus J.E., Dodda A., Tokito M., Ostap E.M., Holzbaur E.L. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nat. Commun. 2014;5:4807. doi: 10.1038/ncomms5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Kreko T., Davison H., Cusmano T., Wu Y., Rothenfluh A., Eaton B.A. Normal dynactin complex function during synapse growth in Drosophila requires membrane binding by Arfaptin. Mol. Biol. Cell. 2013;24(1749–1764):S1741–S1745. doi: 10.1091/mbc.E12-09-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P.M., Ling J., Jeong Y.H., Price D.L., Aja S.M., Wong P.C. Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16320–16324. doi: 10.1073/pnas.1002176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne A.N., Siddegowda B.B., Estes P.S., Johannesmeyer J., Kovalik T., Daniel S.G., Pearson A., Bowser R., Zarnescu D.C. Futsch/MAP1B mRNA is a translational target of TDP-43 and is neuroprotective in a Drosophila model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34:15962–15974. doi: 10.1523/JNEUROSCI.2526-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaper D.C., Adachi Y., Lazarou L., Greenstein M., Simoes F.A., Di Domenico A., Solomon D.A., Lowe S., Alsubaie R., Cheng D., Buckley S., Humphrey D.M., Shaw C.E., Hirth F. Drosophila TDP-43 dysfunction in glia and muscle cells cause cytological and behavioural phenotypes that characterize ALS and FTLD. Hum. Mol. Genet. 2013;22:3883–3893. doi: 10.1093/hmg/ddt243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaper D.C., Adachi Y., Sutcliffe B., Humphrey D.M., Elliott C.J., Stepto A., Ludlow Z.N., Vanden Broeck L., Callaerts P., Dermaut B., Al-Chalabi A., Shaw C.E., Robinson I.M., Hirth F. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum. Mol. Genet. 2013;22:1539–1557. doi: 10.1093/hmg/ddt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman D.K., Lu Z., Meinertzhagen I.A., Schwarz T.L. Altered synaptic development and active zone spacing in endocytosis mutants. Curr. Biol. 2006;16:591–598. doi: 10.1016/j.cub.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Eaton B.A., Fetter R.D., Davis G.W. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Estes P.S., Boehringer A., Zwick R., Tang J.E., Grigsby B., Zarnescu D.C. Wild-type and A315T mutant TDP-43 exert differential neurotoxicity in a Drosophila model of ALS. Hum. Mol. Genet. 2011;20:2308–2321. doi: 10.1093/hmg/ddr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M.J., Hulihan M.M., Kachergus J.M., Dachsel J.C., Stoessl A.J., Grantier L.L., Calne S., Calne D.B., Lechevalier B., Chapon F., Tsuboi Y., Yamada T., Gutmann L., Elibol B., Bhatia K.P., Wider C., Vilarino-Guell C., Ross O.A., Brown L.A., Castanedes-Casey M., Dickson D.W., Wszolek Z.K. DCTN1 mutations in Perry syndrome. Nat. Genet. 2009;41:163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiguin F., Godena V.K., Romano G., D'Ambrogio A., Klima R., Baralle F.E. Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotive behavior. FEBS Lett. 2009;583:1586–1592. doi: 10.1016/j.febslet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Fiesel F.C., Voigt A., Weber S.S., Van den Haute C., Waldenmaier A., Gorner K., Walter M., Anderson M.L., Kern J.V., Rasse T.M., Schmidt T., Springer W., Kirchner R., Bonin M., Neumann M., Baekelandt V., Alunni-Fabbroni M., Schulz J.B., Kahle P.J. Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. EMBO J. 2010;29:209–221. doi: 10.1038/emboj.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y., Logan M.A., Ashley J., Ataman B., Budnik V., Freeman M.R. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godena V.K., Romano G., Romano M., Appocher C., Klima R., Buratti E., Baralle F.E., Feiguin F. TDP-43 regulates Drosophila neuromuscular junctions growth by modulating Futsch/MAP1B levels and synaptic microtubules organization. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygoruk A., Chen A., Martin C.A., Lawal H.O., Fei H., Gutierrez G., Biedermann T., Najibi R., Hadi R., Chouhan A.K., Murphy N.P., Schweizer F.E., Macleod G.T., Maidment N.T., Krantz D.E. The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors. J. Neurosci. 2014;34:6924–6937. doi: 10.1523/JNEUROSCI.0694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelett D.J., Chang J.C., Lakeland D.L., Morton D.B. Comparison of parallel high-throughput RNA sequencing between knockout of TDP-43 and its overexpression reveals primarily nonreciprocal and nonoverlapping gene expression changes in the central nervous system of Drosophila. G3 (Bethesda) 2012;2:789–802. doi: 10.1534/g3.112.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Lu B. Mitochondrial dynamics and mitophagy in Parkinson's disease: disordered cellular power plant becomes a big deal in a major movement disorder. Curr. Opin. Neurobiol. 2011;21:935–941. doi: 10.1016/j.conb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs K.A., Whitwell J.L., Tosakulwong N., Weigand S.D., Murray M.E., Liesinger A.M., Petrucelli L., Senjem M.L., Ivnik R.J., Parisi J.E., Petersen R.C., Dickson D.W. TAR DNA-binding protein 43 and pathological subtype of Alzheimer's disease impact clinical features. Ann. Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J.E., Moughamian A.J., Tokito M.K., Holzbaur E.L. Dynactin subunit p150(glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Liu H.P., Lin W.Y., Guo H., Lu B. LRRK2 kinase regulates synaptic morphology through distinct substrates at the presynaptic and postsynaptic compartments of the Drosophila neuromuscular junction. J. Neurosci. 2010;30:16959–16969. doi: 10.1523/JNEUROSCI.1807-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ray P., Rao E.J., Shi C., Guo W., Chen X., Woodruff E.A., 3rd, Fushimi K., Wu J.Y. A Drosophila model for TDP-43 proteinopathy. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3169–3174. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J.P., Pletnikova O., Troncoso J.C., Wong P.C. TDP-43 repression of nonconserved cryptic exons is compromised in ALS-FTD. Science. 2015;349:650–655. doi: 10.1126/science.aab0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T.E., Machamer J., O'Hara K., Kim J.H., Collins S.E., Wong M.Y., Sahin B., Imlach W., Yang Y., Levitan E.S., McCabe B.D., Kolodkin A.L. The p150(glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74:344–360. doi: 10.1016/j.neuron.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleese K.E., Walker L., Erskine D., Thomas A.J., McKeith I.G., Attems J. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017;27:472–479. doi: 10.1111/bpa.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian A.J., Holzbaur E.L. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74:331–343. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., McCluskey L.F., Miller B.L., Masliah E., Mackenzie I.R., Feldman H., Feiden W., Kretzschmar H.A., Trojanowski J.Q., Lee V.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nonaka T., Masuda-Suzukake M., Arai T., Hasegawa Y., Akatsu H., Obi T., Yoshida M., Murayama S., Mann D.M., Akiyama H., Hasegawa M. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Perry T.L., Bratty P.J., Hansen S., Kennedy J., Urquhart N., Dolman C.L. Hereditary mental depression and Parkinsonism with taurine deficiency. Arch. Neurol. 1975;32:108–113. doi: 10.1001/archneur.1975.00490440058009. [DOI] [PubMed] [Google Scholar]
- Polymenidou M., Lagier-Tourenne C., Hutt K.R., Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C., Kordasiewicz H., Sedaghat Y., Donohue J.P., Shiue L., Bennett C.F., Yeo G.W., Cleveland D.W. Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 2011;14:459–468. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J., Brown R.H., Jr., Ludlow C.L., Fischbeck K.H. Mutant dynactin in motor neuron disease. Nat. Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Rao S., Lang C., Levitan E.S., Deitcher D.L. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Romano G., Klima R., Buratti E., Verstreken P., Baralle F.E., Feiguin F. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiol. Dis. 2014;71:95–109. doi: 10.1016/j.nbd.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Sephton C.F., Good S.K., Atkin S., Dewey C.M., Mayer P., 3rd, Herz J., Yu G. TDP-43 is a developmentally regulated protein essential for early embryonic development. J. Biol. Chem. 2010;285:6826–6834. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba-Fukushima K., Inoshita T., Hattori N., Imai Y. PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila. PLoS Genet. 2014;10:e1004391. doi: 10.1371/journal.pgen.1004391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller K.H., Serr M., Steward R., Hays T.S., Doe C.Q. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol. Biol. Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V., Phaneuf D., Bareil C., Robertson J., Rouleau G.A., Kriz J., Julien J.P. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011;134:2610–2626. doi: 10.1093/brain/awr159. [DOI] [PubMed] [Google Scholar]
- Tacik P., Fiesel F.C., Fujioka S., Ross O.A., Pretelt F., Castaneda Cardona C., Kidd A., Hlavac M., Raizis A., Okun M.S., Traynor S., Strongosky A.J., Springer W., Wszolek Z.K. Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat. Disord. 2014;20:884–888. doi: 10.1016/j.parkreldis.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R.H., Kril J.J., Fatima M., McGeachie A., McCann H., Shepherd C., Forrest S.L., Affleck A., Kwok J.B., Hodges J.R., Kiernan M.C., Halliday G.M. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–3122. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- Vanden Broeck L., Naval-Sanchez M., Adachi Y., Diaper D., Dourlen P., Chapuis J., Kleinberger G., Gistelinck M., Van Broeckhoven C., Lambert J.C., Hirth F., Aerts S., Callaerts P., Dermaut B. TDP-43 loss-of-function causes neuronal loss due to defective steroid receptor-mediated gene program switching in Drosophila. Cell Rep. 2013;3:160–172. doi: 10.1016/j.celrep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Volkening K., Leystra-Lantz C., Yang W., Jaffee H., Strong M.J. Tar DNA binding protein of 43 kDa (TDP-43), 14-3-3 proteins and copper/zinc superoxide dismutase (SOD1) interact to modulate NFL mRNA stability. Implications for altered RNA processing in amyotrophic lateral sclerosis (ALS) Brain Res. 2009;1305:168–182. doi: 10.1016/j.brainres.2009.09.105. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang L., Lu J., Siedlak S.L., Fujioka H., Liang J., Jiang S., Ma X., Jiang Z., da Rocha E.L., Sheng M., Choi H., Lerou P.H., Li H., Wang X. The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat. Med. 2016;22:869–878. doi: 10.1038/nm.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C., Wszolek Z.K. Rapidly progressive familial parkinsonism with central hypoventilation, depression and weight loss (Perry syndrome)–a literature review. Parkinsonism Relat. Disord. 2008;14:1–7. doi: 10.1016/j.parkreldis.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Wider C., Dickson D.W., Stoessl A.J., Tsuboi Y., Chapon F., Gutmann L., Lechevalier B., Calne D.B., Personett D.A., Hulihan M., Kachergus J., Rademakers R., Baker M.C., Grantier L.L., Sujith O.K., Brown L., Calne S., Farrer M.J., Wszolek Z.K. Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat. Disord. 2009;15:281–286. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.Y., Zhou C., Shakiryanova D., Lloyd T.E., Deitcher D.L., Levitan E.S. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.H., Lu Y.N., Chuang C.L., Wu C.L., Chiang A.S., Krantz D.E., Chang H.Y. Loss of vesicular dopamine release precedes tauopathy in degenerative dopaminergic neurons in a Drosophila model expressing human tau. Acta Neuropathol. 2013;125:711–725. doi: 10.1007/s00401-013-1105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Lucas J.J., Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movement of DCVs in a control axon.
Movement of DCVs in a TBPH+/− axon.
Movement of DCVs in a p150G50R axon.
Movement of DCVs in a TBPH+/−; p150G50R axon.
Supplementary material