Abstract
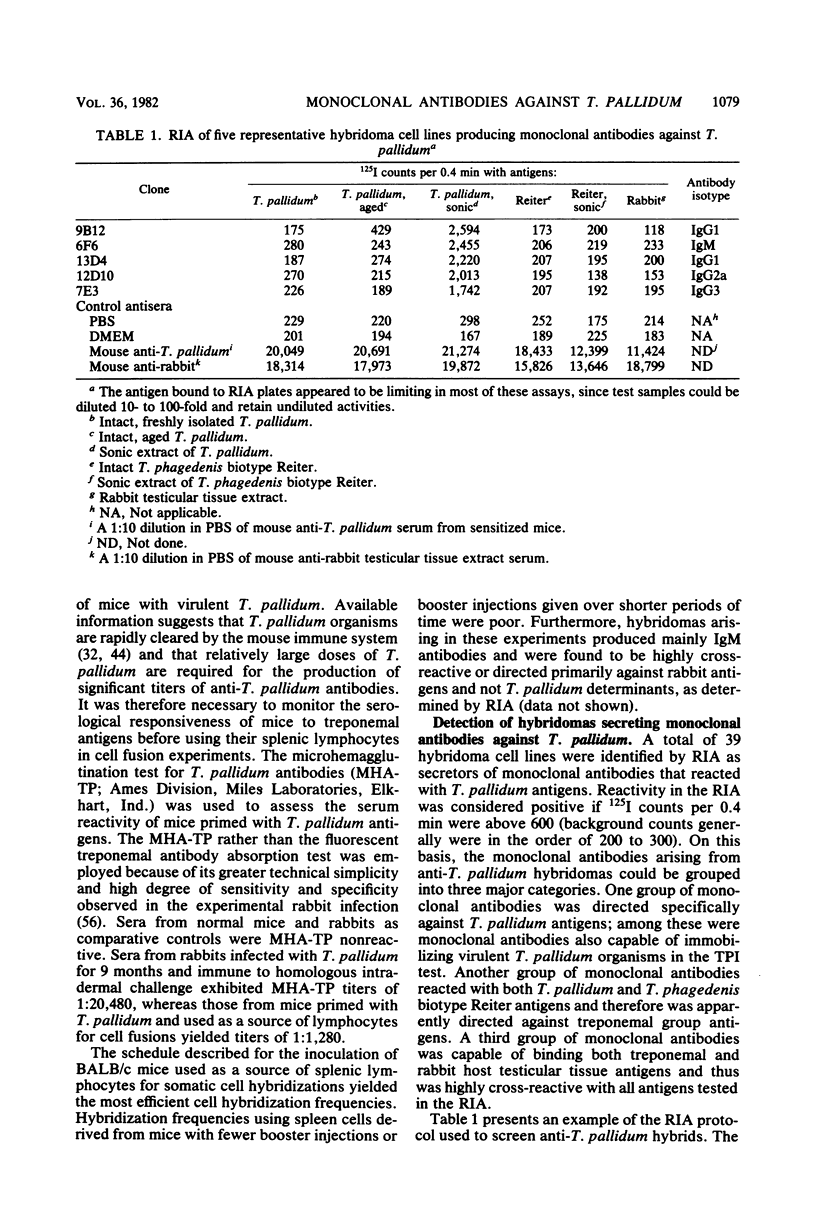
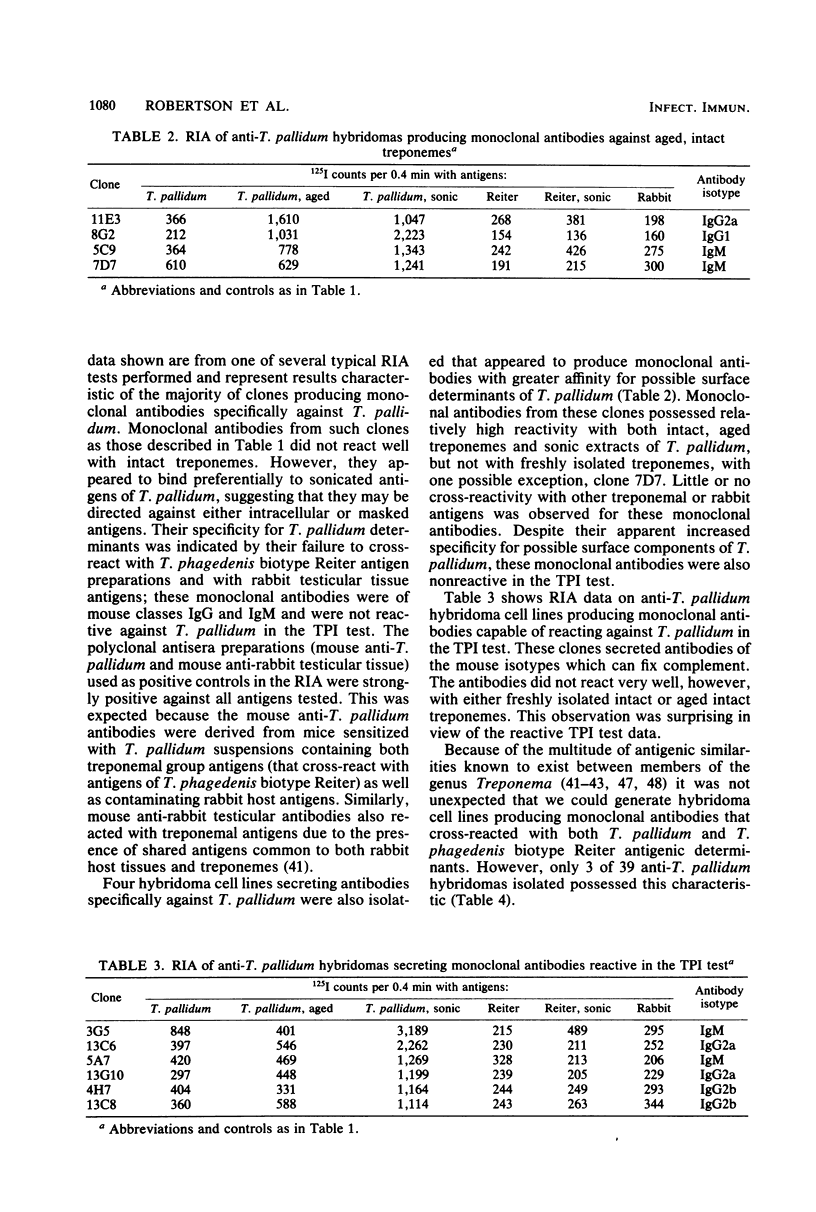
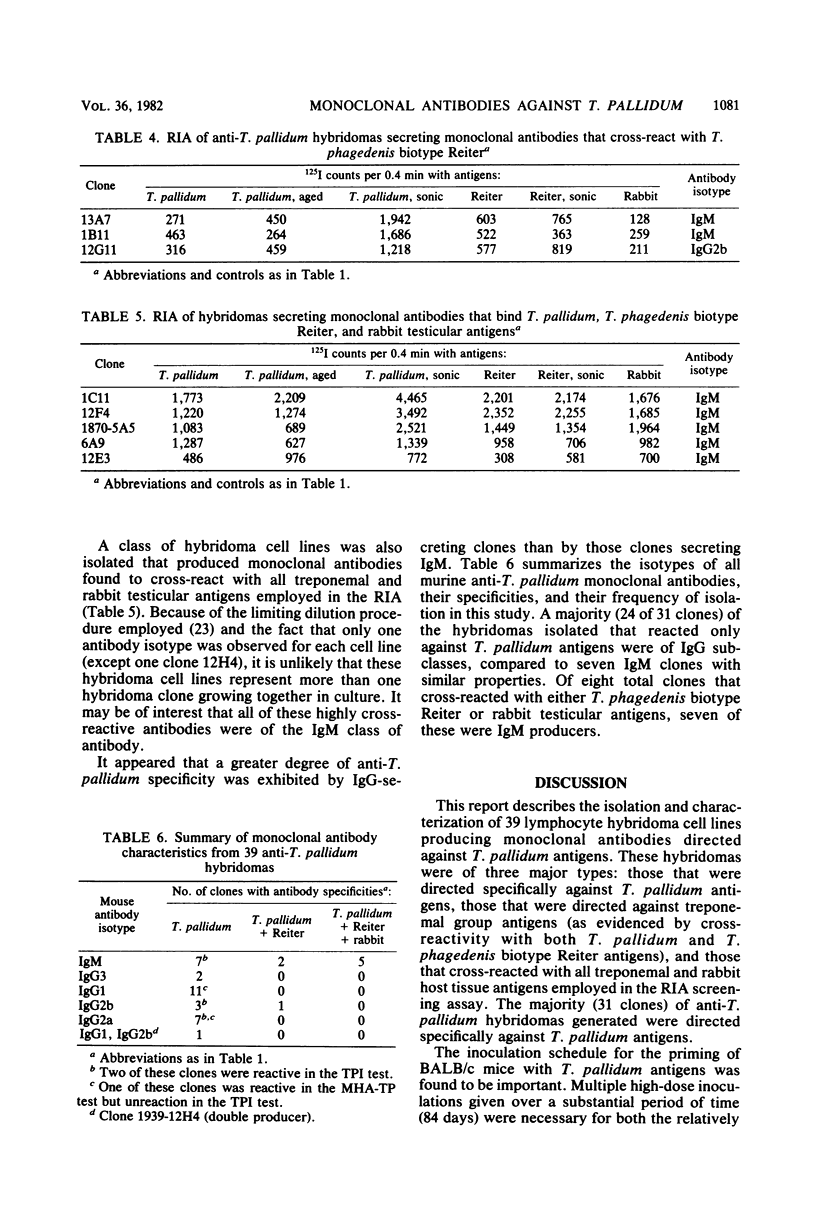
Murine anti-Treponema pallidum (Nichols) lymphocyte hybridoma cell lines secreting monoclonal antibodies against a variety of treponemal antigens have been generated. Hybridomas isolated were of three major types: those that were directed specifically against T. pallidum antigens, those that were directed against treponemal group antigens (as evidenced by their cross-reactivity with T. phagedenis biotype Reiter antigens), and those that cross-reacted with both treponemal as well as rabbit host testicular tissue antigens. The majority (31 of 39 clones) of these anti-T. pallidum hybridomas, which produced monoclonal antibodies of mouse isotypes immunoglobulin G1 (IgG1), IgG2a, IgG2b, IgG3 or IgM, were directed specifically against T. pallidum and not other treponemal or rabbit antigens tested by radioimmunoassay. Four of these T. pallidum-specific hybridomas secreted monoclonal antibodies with greater binding affinity for "aged" rather than freshly isolated intact T. pallidum cells, suggesting a possible specificity for "unmasked" surface antigens of T. pallidum. Six anti-T. pallidum hybridomas produced complement-fixing monoclonal antibodies (IgG2a, IgG2b, or IgM) that were capable of immobilizing virulent treponemes in the T. pallidum immobilization (TPI) test; these may represent biologically active monoclonal antibodies against treponemal surface antigens. Three other hybridomas secreted monoclonal antibodies which bound to both T. pallidum and T. phagedenis biotype Reiter antigens, thus demonstrating a possible specificity for treponemal group antigens. Five hybridoma cell lines were also isolated which produced IgM monoclonal antibodies that cross-reacted with all treponemal and rabbit host testicular tissue antigens employed in the radioimmunoassays. This report describes the construction and characteristics of these hybridoma cell lines. The potential applications of the anti-T. pallidum monoclonal antibodies are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface characterization of virulent Treponema pallidum. Infect Immun. 1980 Dec;30(3):814–823. doi: 10.1128/iai.30.3.814-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Zander S., Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Briles D. E., Nahm M., Schroer K., Davie J., Baker P., Kearney J., Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 streptococcus pneumoniae. J Exp Med. 1981 Mar 1;153(3):694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Ritzi D. M. Relationship of Treponema pallidum to acidic mucopolysaccharides. Infect Immun. 1979 Apr;24(1):252–260. doi: 10.1128/iai.24.1.252-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Wolff E. T. Mucopolysaccharide material resulting from the interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells. Infect Immun. 1978 Nov;22(2):575–584. doi: 10.1128/iai.22.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY P. H., Jr, NELL E. E. Study of the antigenic structure of Treponema pallidum by specific agglutination. Am J Hyg. 1957 Sep;66(2):160–172. doi: 10.1093/oxfordjournals.aje.a119893. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Graham D. J., Nell E. E., Dannenberg A. M., Jr Macrophages in immunity to syphilis: suppressive effect of concurrent infection with Mycobacterium bovis BCG on the development of syphilitic lesions and growth of Treponema pallidum in tuberculin-positive rabbits. Infect Immun. 1979 Nov;26(2):751–763. doi: 10.1128/iai.26.2.751-763.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Nevin T. A., Guest W. J., Logan L. C. Lytic effect of trypsin, lysozyme, and complement on Treponema pallidum. Br J Vener Dis. 1968 Sep;44(3):193–200. doi: 10.1136/sti.44.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Logan L. C. Rabbit globulin and antiglobulin factors associated with Treponema pallidum growth in rabbits. Br J Vener Dis. 1974 Dec;50(6):421–427. doi: 10.1136/sti.50.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Lloyd R. M., Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980 Jan;124(1):454–460. [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- MAGNUSON H. J., THOMAS E. W., OLANSKY S., KAPLAN B. I., DE MELLO L., CUTLER J. C. Inoculation syphilis in human volunteers. Medicine (Baltimore) 1956 Feb;35(1):33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- METZGER M., HARDY P. H., Jr, NELL E. E. Influence of lysozyme upon the treponeme immobilization reaction. Am J Hyg. 1961 Mar;73:236–244. doi: 10.1093/oxfordjournals.aje.a120182. [DOI] [PubMed] [Google Scholar]
- METZGER M., RUCZKOWSKA J. INFLUENCE OF LYSOZYME UPON THE REACTIVITY OF TREPONEMA PALLIDUM IN THE FLUORESCENT ANTIBODY REACTION. Arch Immunol Ther Exp (Warsz) 1964;12:702–708. [PubMed] [Google Scholar]
- MILLER J. N. THE APPEARANCE AND PERSISTENCE OF VDRL, RPCF, AND TPI ANTIBODY DURING THE COURSE AND TREATMENT OF EXPERIMENTAL SYPHILIS IN THE RABBIT. J Invest Dermatol. 1964 May;42:367–371. doi: 10.1038/jid.1964.80. [DOI] [PubMed] [Google Scholar]
- McLEOD C. P., MAGNUSON H. J. Development of treponemal immobilizing antibodies in mice following injection of killed Treponema pallidum (Nichols strain). J Vener Dis Inf. 1951 Oct;32(10):274–279. [PubMed] [Google Scholar]
- Metzger M., Michalska E., Podwińska J., Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969 Dec;45(4):299–304. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N., Bekker J. H., DeBruijn J. H., Onvlee P. C. Antigenic structure of Treponema pallidum, Nichols strain. II. Extraction of a polysaccharide antigen with "strain-specific" serological activity. J Bacteriol. 1969 Jul;99(1):132–135. doi: 10.1128/jb.99.1.132-135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N., Bekker J. H., DeBruijn J. H., Onvlee P. C. The immunologic response of goats to normal and syphilitic rabbit testicular tissue. J Immunol. 1966 Aug;97(2):184–188. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. V. The immunogenicity of Treponema pallidum attenuated by gamma-irradiation. J Immunol. 1967 Nov;99(5):1012–1016. [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Miller J. N., de Bruijn J. H., Bekker J. H., Onvlee P. C. The antigenic structure of Treponema pallidum, Nichols strain. I. The demonstration, nature and location of specific and shared antigens. J Immunol. 1966 Mar;96(3):450–456. [PubMed] [Google Scholar]
- O'Neill P. A new look at the serology of treponemal disease. Br J Vener Dis. 1976 Oct;52(5):296–299. doi: 10.1136/sti.52.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y. Treponema pallidum antibodies in syphilitic mice as determined by immunofluorescence and passive hemagglutination techniques. J Immunol. 1972 Apr;108(4):921–926. [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Jørgensen B. B., Petersen C. S. Antibodies in secondary syphilis against five of forty Reiter treponeme antigens. Scand J Immunol. 1980;11(6):629–633. doi: 10.1111/j.1365-3083.1980.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Pedersen N. S., Axelsen N. H., Petersen C. S. Antigenic analysis of Treponema pallidum: cross-reactions between individual antigens of T. pallidum and T. Reiter. Scand J Immunol. 1981;13(2):143–150. doi: 10.1111/j.1365-3083.1981.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Pepose J. S., Bishop N. H., Feigenbaum S., Miller J. N., Zeltzer P. M. The humoral immune response in rabbits infected with Treponema pallidum: Comparison of antibody levels measured by the staphylococcal protein A-IgG (SPA-TP) microassay with VDRL, FTA-Abs, and TPI antibody responses during the development of acquired resistance to challenge. Sex Transm Dis. 1980 Jul-Sep;7(3):125–129. [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Sepetjian M., Salussola D., Thivolet J. Attempt to protect rabbits against experimental syphilis by passive immunization. Br J Vener Dis. 1973 Aug;49(4):335–337. doi: 10.1136/sti.49.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- TURNER T. B., KLUTH F. C. Protective antibodies in the serum of syphilitic patients. Am J Hyg. 1948 Sep;48(2):173–181. doi: 10.1093/oxfordjournals.aje.a119233. [DOI] [PubMed] [Google Scholar]
- TURNER T. B., NELSON R. A., Jr The relationship of treponemal immobilizing antibody to immunity in syphilis. Trans Assoc Am Physicians. 1950;63:112–117. [PubMed] [Google Scholar]
- Thomas M. L., Clark J. W., Jr, Cline G. B., Anderson N. G., Russell H. Separation of Treponema pallidum from tissue substances by continuous-flow zonal centrifugation. Appl Microbiol. 1972 Apr;23(4):714–720. doi: 10.1128/am.23.4.714-720.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tight R. R., White A. C. Quantitative microhaemagglutination assay for Treponema pallidum antibodies in experimental syphilis. Br J Vener Dis. 1980 Oct;56(5):291–296. doi: 10.1136/sti.56.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- Turner T. B., Hardy P. H., Jr, Newman B., Nell E. E. Effects of passive immunization on experimental syphilis in the rabbit. Johns Hopkins Med J. 1973 Nov;133(5):241–251. [PubMed] [Google Scholar]
- Turner T. B. PROTECTIVE ANTIBODIES IN THE SERUM OF SYPHILITIC RABBITS. J Exp Med. 1939 May 31;69(6):867–890. doi: 10.1084/jem.69.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser R. S., Erickson D., Perine P. L., Pearsall N. N. Immunity to syphilis: passive transfer in rabbits using serial doses of immune serum. Infect Immun. 1976 May;13(5):1402–1407. doi: 10.1128/iai.13.5.1402-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltzer P. M., Pepose J. S., Bishop N. H., Miller J. N. Microassay for immunoglobulin G antibodies to Treponema pallidum with radioiodinated protein A from staphylococcus aureus: immunoglobulin G response in experimental syphilis in rabbits. Infect Immun. 1978 Jul;21(1):163–170. doi: 10.1128/iai.21.1.163-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



