Abstract
Objectives
Chronic rhinosinusitis (CRS) is often associated with persistent bacterial infection despite the use of systemic antibiotics. Topically administered antibiotics are an alternative strategy, but require effective local concentrations, prolonged mucosal contact time, minor systemic absorption, and minimal depletion. The objectives of the current study are to analyze the in vitro release rate and in vivo drug delivery tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent (CSS).
Methods
The CSS (2 mg) was created from biodegradable poly-D/L-lactic acid. After analyzing in vitro release profile, CSTs were placed unilaterally in maxillary sinuses of 16 rabbits via dorsal sinusotomy. Animals were sacrificed between 1 and 3 weeks postoperatively. Ciprofloxacin concentrations in the sinus tissue and plasmas were assessed using high-performance liquid chromatography. Radiological and histological evaluations were performed.
Results
In the in vitro release profile, an initial burst release was observed over the first 24 hrs, followed by sustained release through the 14 day time point. In the rabbit model, ciprofloxacin was continuously released from the stent up to 3 weeks at doses > 50 ng/ml. Histologic examination found no evidence of inflammation, epithelial ulceration or bony reaction upon sacrifice of the animals at 21 days. Computed tomography also demonstrated no signs of mucosal edema or opacification in the sinus.
Conclusion
The CST was safe in this preclinical model and sustained release was observed in both the in vitro and in vivo analyses. The innovative stent design coated with ciprofloxacin may provide a unique therapeutic strategy for CRS.
Keywords: Sinus stent, Ciprofloxacin, Topical drug delivery, Pharmacokinetics, Chronic sinusitis, Sinusitis, Rabbit
INTRODUCTION
Hundreds of antibiotics have been developed to treat infectious diseases since Alexander Fleming’s discovery of penicillin in 1928.1 However, overutilization and inappropriate administration has led to widespread resistance among bacterial pathogens. Infections associated with chronic rhinosinusitis (CRS) are notoriously difficult to eradicate as they typically require extended durations of therapy and sustained antibiotic exposure and concentrations. Incomplete treatment with antibiotic concentrations under the minimal inhibitory concentration (MIC) aggravates the development of antibiotic resistance.1
Several therapeutic strategies are currently in use for recalcitrant sinus infections. Extended-release antibiotic preparations improve patient compliance and reduce frequent administration by maintaining constant plasma drug concentrations over a prolonged period of time.2 Many drugs used for treating sinusitis are administered as nasal sprays/irrigation in an attempt to provide highly concentrated local delivery to the site of infection. More recently, nasal packing materials soaked with topical drugs have been utilized as a therapeutic strategy, although studies have shown release to be uncontrolled and inconsistent, thus providing erratic outcomes.3 Drug-eluting implants with prolonged mucosal contact duration and sustained drug release could provide a suitable therapeutic alternative to eliminate issues with patient compliance and erratic drug delivery.1,3,4 However, there is currently no antibiotic-coated biodegradable stent available.
Methicillin-resistant Staphylococcus aureus (MRSA) and multidrug resistant P. aeruginosa are common in the clinical isolates of CRS patients and are a cause of antibiotic treatment failures.5 Oral antibiotics, such as fluoroquinolone are frequently administered for these infections (effective for both gram-negative and gram-positive bacteria), but have recently gained increased national attention due to safety concerns regarding tendinitis and tendon rupture with systemic administration.6 However, topical delivery of fluoroquinolones could provide effective elimination of multi-drug resistant organisms at concentrations well above the MIC, while significantly decreasing systemic levels of the drug and therefore abrogating serious side effects.
We recently developed a ciprofloxacin-coated stent (CSS) from biodegradable poly-D/L-lactic acid (PLLA) that has high therapeutic potential for local antibiotic delivery in the sinuses. Ciprofloxacin was chosen as a model drug for incorporation into the stent because of its broad-spectrum activity and potent elimination of P. aeruginosa. The area under the concentration-time curve (AUC) to the MIC ratio (concentration-dependent killing) is considered the predictor for response to quinolones and the AUC:MIC > 100 is the minimum goal.7 The objective of the current study is to analyze the in vitro release profile of ciprofloxacin-coated PLLA material and measure the in vivo drug delivery tolerance and pharmacokinetics of the CSS.
METHODS
In Vitro Release Profile
PLLA stents (2 cm) were fabricated using a 3D printer. In order to coat PLLA with ciprofloxacin, the PLLA stent was rotated in an aqueous ciprofloxacin solution (10 μg/mL) overnight until completely dry. A motorized rotary coating system developed by Ho-Wook Jun, Ph.D. was used to ensure uniform coating and confirmed with scanning electron microscopy. A total of 5μg of ciprofloxacin was coated in each PLLA stent for the in vitro assay. Stents were placed in 3 mL of filtered sterilized water in a 15 mL centrifuge tube. Supernatants (200 μL) were collected at 0, 1, 3, 6, and 12 hours, as well as 1, 3, 7, and 14 days. The concentration of ciprofloxacin in each sample was then measured using a ciprofloxacin ELISA kit (REAGEN, NJ) and plotted according to the duration of incubation. Experiments were repeated five times. Results of this assay were used to determine the appropriate dose of ciprofloxacin coated on the stent and duration of stent placement in the in vivo rabbit study.
Stent Description
For in vivo animal studies, PLLA stents were fabricated using a 3D printer (Figure 1A) to conform to the size of the rabbit maxillary sinus. Standardized dimensions were obtained (0.75 cm × 0.2 cm). For efficiency, PLLA stents were printed at 1.5 cm in length and 0.2 cm in height and then divided to provide 2 stents of the aforementioned dimensions. PLLA stents were rotated in the aqueous ciprofloxacin solution (10 mg/mL) overnight until the solution had completely dried. A total of 2mg of ciprofloxacin was successfully coated on the PLLA stents for the in vivo assay.
Figure 1.
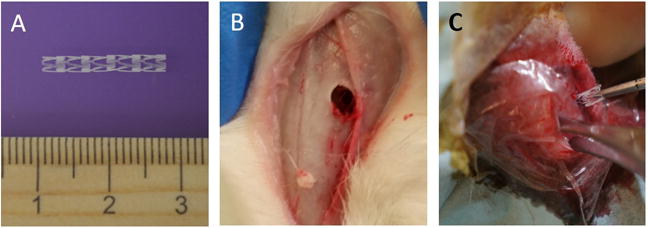
A: Original PLLA stent measured 1.5 cm in length and 0.2 cm in height, which was divided (0.75 cm × 0.2cm) and inserted into the rabbit’s maxillary sinus; B: Dorsal sinusotomy in the left side; C: Placement of PLLA stent through dorsal sinusotomy.
Animal Model
This study was approved by the institutional animal care and use committee (IACUC) at the University of Alabama at Birmingham. Rabbits are useful for preclinical studies of sinus therapeutics because 1) rabbit sinus and nasal epithelium has similar physiologic features when compared to human tissue,8,9 2) drug pharmacokinetics are easily measured,10 and 3) the rabbit maxillary sinusitis model is well established.11,12 Pasteurella-free, female, New Zealand white rabbits (2–4 kg) were used for the study. Before study initiation, rabbits were acclimatized to the animal facility for at least one week. Approximately 30 minutes before the procedure, one dose (25 mg/kg) of cefazolin (West-Ward Pharmaceutical Corp., Eatontown, NJ) was administered intramuscularly per IACUC recommendation to prevent wound infection at the incision site. No further antibiotics were administered to the rabbits for the duration of the studies. Rabbits were anesthetized with [ketamine (20 mg/kg) (MWI, Boise, ID), dextomitor (0.25 mg/kg) (Zoetis Inc., Kalamazoo, MI), buprenorphine (0.03 mg/kg) (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA), and carprofen (5 mg/kg) (Zoetis Inc., Kalamazoo, MI)] in a warm room. The surgical procedure to insert the stent was as follows: 1) a dorsal nasal, vertical, midline incision was created, 2) the left maxillary sinus was entered by creating a small 8mm × 8mm dorsal hole on left side using a trocar (Figure 1B), 3) the stent was inserted through this opening (Figure 1C), and 4) the periosteum, subcutaneous tissue, and skin were closed with 4-0 Vicryl suture (Ethicon, Inc., Somerville, NJ).
In Vivo Study #1: Tolerance
The objectives of this portion of the study were to evaluate the endoscopic, radiologic and histologic response of the sinus mucosal tissues when exposed to a ciprofloxacin-coated sinus stent. A nondrug loaded version of the stent was used as a control to differentiate the effects related to the drug from the effects related to the physical presence of the stent. Ten New Zealand white rabbits were randomized to receive either a ciprofloxacin-loaded stent (n = 5) or nondrug-loaded stent (n = 5).
Endoscopic examination – 1.7mm 30-degree endoscopy was performed in the nasal cavity before and after stent placement (week 3).
Micro Computed Tomography (CT) scanning - All rabbits were scanned before the placement of stents. After implantation, micro CT scanning was repeated at week 3. Micro CT scanning was performed at the UAB small animal imaging shared facility using SPECT/CT (X-SPECT system, Gamma Medica, Northridge, CA).
Histologic evaluation – After micro CT scanning at week 3, rabbits were euthanized and heads were harvested for histologic sectioning. The maxillary sinus was not opened to avoid injury to the sinus mucosa. Heads were divided to submit the right and left maxillary sinus separately. Specimens were provided to the UAB Comparative Pathology Laboratory (CPL) for sectioning and evaluation. Ten representative sections of the maxillary sinus were selected and stained with hematoxylin and eosin (H&E). Slides were evaluated by a veterinary pathologist blinded to the specimen and side.
In Vivo Study #2: Pharmacokinetics
We evaluated maxillary sinus tissue and plasma concentrations of ciprofloxacin after stent insertion to determine the in vivo release of ciprofloxacin over time (determined by the in vitro assay), and assess systemic exposure. Six New Zealand white rabbits were used in this study. All animals received a drug-coated stent in the left maxillary sinus. Three rabbits were euthanized at week 1 after implantation. The remaining 3 rabbits were euthanized at week 3. At each time point, tissue was procured from both maxillary sinuses and blood was collected to measure the ciprofloxacin concentration. The concentration in the sinus mucosa and plasma was determined by a highly sensitive liquid chromatography tandem mass spectrometry assay (LC/MS/MS) at UAB Targeted Metabolomics and Proteomics Laboratory (TMPL). A Gemni NX C18 column (Phenomenex, Torrance, CA) was coupled to a SCIEX 4000 Tandem Mass Spectrometer (SCIEX, Framingham MA). Sarafloxacin was used as an internal standard. Ciprofloxacin and Sarafloxacin standard curves were created ranging from 5–500 ng/ml for each chemical. Finally, 1 mL of water was added to reconstitute the dried sample, and 25 μL was injected into the LC/MS/MS for analysis. Each sample run was performed in triplicate, and the concentrations reported as an average.
Statistical Analysis
Statistical analyses were conducted using Excel 2016 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized unpaired Student t tests. Data is expressed +/− standard error of the mean.
RESULTS
In Vitro Release Profile of the CSS
An initial small burst release was observed over the first 24hr, followed by sustained release through the 14-day time point. In the CSS, 30% of coated ciprofloxacin was released within 48 hrs. The early small burst release of ciprofloxacin leads to higher initial targeted delivery of antibiotic. Subsequent sustained release was also observed and approximately 50% of the coated ciprofloxacin was discharged within 14 days. The releasing rate (μg/day) decreased more than 50% after one week: Day 3–7 = 0.09 ± 0.01 μg/day vs Day 7–14 = 0.04 ± 0.005 μg/day). Based on this in vitro releasing assay using a PLLA stent coated with 5 μg of ciprofloxacin, the ratio of AUC0–24 to MIC (MIC of ciprofloxacin for P. aeruginosa PAO1 strain H103 = 0.06 μg/ml)13 at week 1 (day 7) was around 1.5, significantly lower than the minimum goal (>100). Therefore, in the in vivo analysis, the total amount of ciprofloxacin coated in the CSS was increased to 2 mg from 5 μg in an attempt to improve the AUC:MIC > 100. In vivo tolerance and pharmacokinetic studies were designed to place the stents for a maximum of three weeks as suboptimal release (less than targeted AUC:MIC) may confer the development of antibiotic resistance and clinical treatment failures.1,14
In Vivo Study #1: Tolerance
All rabbits tolerated the procedure well with no evidence of wound infection or wound dehiscence of the midline incision.
-
Nasal Endoscopic Examination
Nasal endoscopies were performed in all rabbits (N = 10) before and after the placement of the non-drug eluting stents and CSSs (Figure 3). The right nasal cavity was used as an internal control. The inferior, middle and superior meati were inspected bilaterally. There was no evidence of purulent drainage or signs of infection before and following stent placement at Week 3 in all rabbits.
-
Micro CT
Micro CT examination was also performed in all rabbits (N = 10) before and after insertion of the non-drug eluting stents and CSS (Figure 4) at Week 3. The right side was used as an internal control. Stents were noted in the superior portion of the rabbit’s left maxillary sinus, just below the dorsal sinusotomy site. However, there was no evidence of opacification or mucosal thickening of the left or right maxillary sinus in all rabbits. Other sinuses were also clear before and after stent placement.
-
Histology
Maxillary sinus mucosa from both non-drug eluting and CSSs exhibited no evidence of submucosal edema, inflammatory epithelial hyperplasia, surface mucosa squamous metaplasia, submucosal gland hyperplasia or submucosal inflammatory cell infiltration (Figure 5). Comparisons between the right (non-implanted) and left (implanted) sinuses also revealed no obvious differences at week 3.
Figure 3. Nasal Endoscopic Examination.
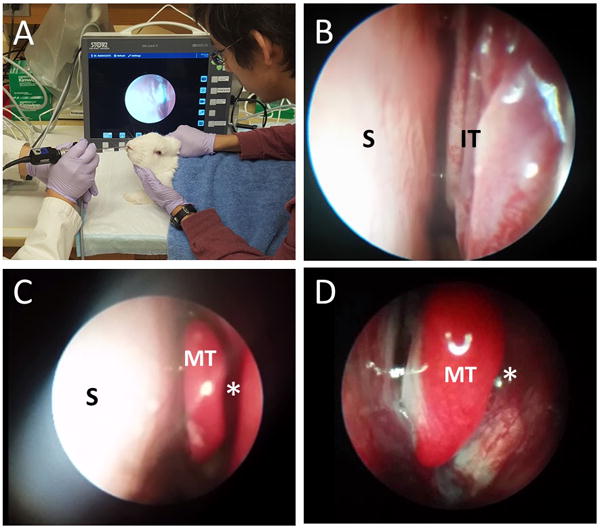
A: Endoscopic Examination Setting in the animal lab
B: Rabbit’s nasal cavity (Left) – anterior portion (S: Septum, IT: Inferior turbinates)
C: Rabbit’s nasal cavity (Left) – middle portion (S: Septum, MT: Middle turbinate)
D: Rabbit’s nasal cavity (Left) – MT: Middle turbinate, *: Middle meatus
Figure 4. Micro CT.
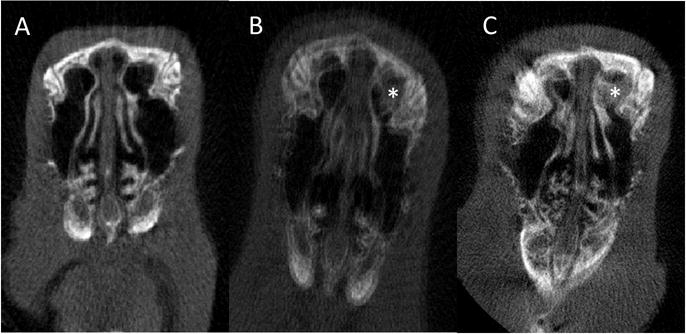
A: Pre-implantation
B: Post-implantation of non-drug eluting stent at Week 3 (*: Stent)
C: Post-implantation of Ciprofloxacin coated Sinus Stent (CSS) at Week 3 (*: Stent)
Figure 5. Histology.
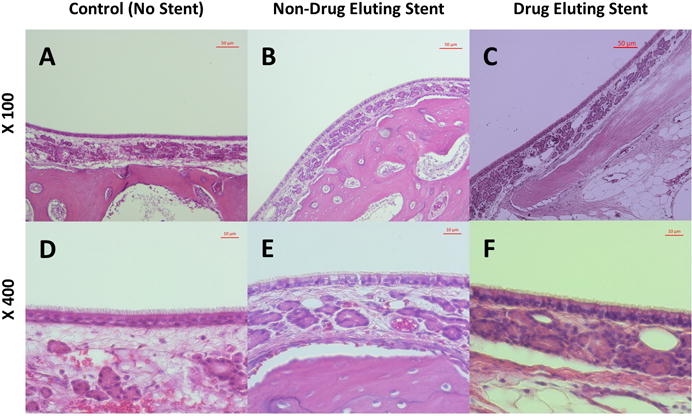
Right maxillary sinus (A and D) was used as an internal control. Maxillary sinus mucosa from both non-drug eluting (B and E) and ciprofloxacin eluting stents (C and F) exhibited no evidence of submucosal edema, inflammatory epithelial hyperplasia, surface mucosa squamous metaplasia, submucosal gland hyperplasia or submucosal inflammatory cell infiltration.
In Vivo Study #2: Pharmacokinetics
Concentrations of ciprofloxacin were measured in the right and left maxillary sinus mucosa and blood plasma at week 1 (n = 3) and week 3 (n = 3) after implantation. During control HLPC analysis at the UAB TMPL, a weak signal to noise ratio was identified in control sinus mucosa likely due to other proteins with similar absorbance pattern in the sinus tissue. Therefore, a concentration less than 20 ng/ml in the sinus mucosa was considered to be the lower limit of quantification (Figure 6, dotted line). Mucosal drug concentrations in the left maxillary sinus were decreased from week 1 to week 3 although this lacked statistical significance (week 1 = 60.73 ± 1.54 ng/ml vs week 3 = 53.53 ± 4.14 ng/ml, p = 0.18). Based on this in vivo releasing assay using a PLLA stent coated with 2 mg of ciprofloxacin, the ratio of AUC0–24 to MIC (MIC of ciprofloxacin for P. aeruginosa PAO1 strain H103 = 0.06 μg/ml)13 was approximately 135 at 1 week and 120 at 3 weeks - exceeding our minimum goal (>100). Importantly, sustained release of ciprofloxacin was noticed until the third week. Right maxillary sinus mucosal concentrations of ciprofloxacin were below the lower limit of quantification (20 ng/ml) at both week 1 and week 3 (week 1 = 16.2 ± 1.18 ng/ml vs week 3 = 17.8 ± 1.65 ng/ml), and were thus considered negative. Ciprofloxacin was undetectable in the blood plasma from four out of six rabbits and the concentrations from the remaining rabbits were below the lower limit of quantification.
Figure 6.
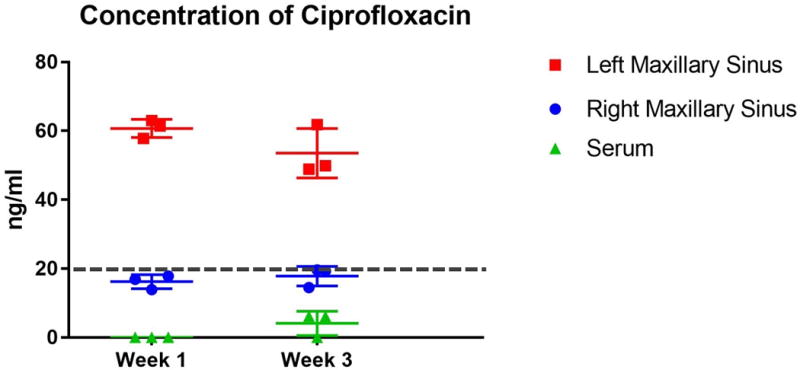
Concentrations of ciprofloxacin in the right and left maxillary sinus mucosa and blood plasma at week 1 (n = 3) and week 3 (n = 3) after implantation.
DISCUSSION
The primary objective of topical antibiotic delivery to the sinus mucosa is to provide high doses of drug directly to the site of the disease while minimizing the systemic exposure and toxicity.15 The therapeutic effect of a topical antibiotic depends on the amount of drug deposited in the airways, how well the drug distribution matches the location of the bacteria and if the local concentration of antibiotic achieved is adequate to kill the microbes. For these reasons, antibiotic-eluting stents could provide major advantages in this regard by ensuring continuous drug release over a prolonged period of time to the affected sinus mucosa.3
The bioabsorbable PLLA material is in wide use as a biomaterial with multiple approvals by the FDA for other uses. These polymeric microspheres are attractive because of their biocompatibility, biodegradability and capability of encapsulating various drugs.1 Sustained-release preparations with constant rates of discharge for a required duration have a critical role in the antibiotic delivery field. Over the past few decades, the applications of nanotechnology have been explored in many medical areas, especially in drug delivery. Owing to their ultra-small size, nanoparticle formulations have numerous advantages over traditional dosage forms. They have a prolonged action of active agent, thus they are capable of releasing drugs in a sustained and controlled manner.16 The method of action has been studied in nitric oxide-releasing cardiovascular stents, cardiac tissue regeneration with growth factor and stem cell delivery, and dental revitalization with antibiotic delivery.17,18 This nanomatrix technology should be easily translatable to local therapeutic delivery in upper airway diseases including CRS.
In the present study, the tolerance and drug delivery of the ciprofloxacin (2 mg) coated PLLA stent was tested in a preclinical animal model using nasal endoscopy, micro CT scan, histology, and high performance liquid chromatography (HPLC) assay for mucosal drug concentrations. Detailed characteristics of drug release from the stent were also measured in vitro. Initial small burst release was noticed during the in vitro assay, leading to higher initial targeted delivery, followed by prolonged release for 14 days. The initial burst release is likely attributed to diffusion of the drug through pre-existing pores and channels of PLLA material during evaporation coating and is consistent with previous drug-releasing stent studies.19 When placed into the maxillary sinuses of rabbits, histologic examination identified no evidence of inflammation, epithelial ulceration or bony reaction upon sacrifice of the animals at week 3. Computed tomography also demonstrated no signs of mucosal edema or opacification in the sinuses. Sustained ciprofloxacin delivery was noticed in the maxillary sinus until week 3 without systemic absorption according to HPLC assay.
Antibiotics are commonly divided into two major categories on the basis of their pharmacodynamics: time-dependent vs concentration-dependent antibiotics. Quinolones are concentration-dependent antibiotics and these agents achieve improved bacteria killing with increased levels of the drug. The area under the concentration-time curve (AUC) to the MIC (concentration-dependent killing) ratio incorporates both concentration intensity and exposure over time and has been advocated as the best parameter for prediction of bacteriologic response to quinolones.7 Currently, sustained-release oral drug formulations can provide controllable drug release to maximize the AUC-to-MIC ratio and minimize systemic toxicity.1 An AUC:MIC > 100 is considered the minimum goal, while an AUC:MIC > 125 is found to be predictive of microbiological and clinical cure in patients with serious gram-negative infections. Based on the HPLC analysis, a sinus stent coated with 2 mg ciprofloxacin continuously released concentrations that exceeded minimum standards for tissue concentration, but also exceeded (135 at 1 week) or neared (120 at 3 weeks) levels considered optimal for severe infections.
Unlike oral administration where blood levels of a drug are a generally accepted ‘surrogate’ for concentration at the site of action, topical drug delivery in an in vivo model poses a more complex problem in the assessment of topical drug bioavailability.20 In this case, the site of action (even when it is known) is not always accessible and a suitable ‘surrogate’ (e.g., the sinus mucosa), has not been validated. Developing valid, effective and economical approaches with which to measure topical drug bioavailability and bioequivalence are critical and must consider partition coefficient, mucociliary clearance, and effective surface area in the sinonasal cavities. To validate the efficacy of the CSS in eliminating P. aeruginosa infection, especially microbial biofilms, further studies in a preclinical rabbit model of sinusitis are planned.
Limitations of the current study include small sample sizes for both tolerance and pharmacokinetics and limited time points for pharmacokinetics. Additionally, during the specimen fixation and preparation, parts of the PLLA stents were melted by reagents and during specimen sectioning with the microtome, parts of the stent with no or minimal contact with the mucosa were occasionally dislodged. This was unavoidable because of the nature of the stent and site of implantation and explains why stent material was not always found in direct apposition to tissue.19
CONCLUSIONS
The CSS was safe in this preclinical model and sustained release was observed in both the in vitro and in vivo analyses. The innovative stent design coated with ciprofloxacin may provide a unique therapeutic strategy for eradicating recalcitrant bacterial infections in persistent CRS. Future studies using the CSS in this preclinical model with active maxillary sinusitis are planned.
Figure 2.
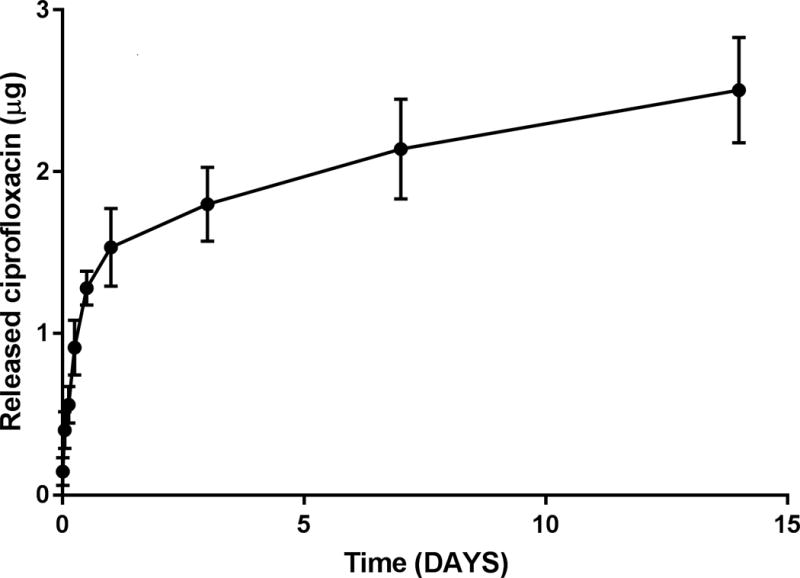
Release profile over 14 days. An initial small burst release was observed over the first 24hr, followed by sustained release through the 14-day time point.
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-01) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award) to B.A.W., and John W. Kirklin Research and Education Foundation Fellowship Award and UAB Faculty Development Research Award to D.Y.C. We would like to show our gratitude to Trenton R. Schoeb, DVM, PhD for reviewing histology slides.
Footnotes
Bradford A. Woodworth, M.D. is a consultant for Olympus and Cook Medical. He also receives grant support from Cook Medical.
Manuscript presented at the American Rhinologic Society Annual Meeting, San Diego, CA, September 19th, 2016.
Winner of the best manuscript award presented at the American Rhinologic Society’s 2016 annual meeting.
References
- 1.Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo) 2011;64:625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 2.Blasi F, Aliberti S, Tarsia P. Clinical applications of azithromycin microspheres in respiratory tract infections. Int J Nanomedicine. 2007;2:551–559. [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug-eluting nasal implants: formulation, characterization, clinical applications and challenges. Pharmaceutics. 2014;6:249–267. doi: 10.3390/pharmaceutics6020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albu S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des Devel Ther. 2012;6:125–132. doi: 10.2147/DDDT.S25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilty SJ, Desrosiers MY. The role of bacterial biofilms and the pathophysiology of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2008;8:227–233. doi: 10.1007/s11882-008-0038-2. [DOI] [PubMed] [Google Scholar]
- 6.Bidell MR, Lodise TP. Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk? Pharmacotherapy. 2016;36:679–693. doi: 10.1002/phar.1761. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamaruzaman NA, Kardia E, Kamaldin N, Latahir AZ, Yahaya BH. The rabbit as a model for studying lung disease and stem cell therapy. Biomed Res Int. 2013;2013:691830. doi: 10.1155/2013/691830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaure C, Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. doi: 10.3389/fimmu.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami J, Yamamoto K, Sawada Y, Iga T. Prediction of brain delivery of ofloxacin, a new quinolone, in the human from animal data. Journal of pharmacokinetics and biopharmaceutics. 1994;22:207–227. doi: 10.1007/BF02353329. [DOI] [PubMed] [Google Scholar]
- 11.Chiu AG, Antunes MB, Palmer JN, Cohen NA. Evaluation of the in vivo efficacy of topical tobramycin against Pseudomonas sinonasal biofilms. J Antimicrob Chemother. 2007;59:1130–1134. doi: 10.1093/jac/dkm087. [DOI] [PubMed] [Google Scholar]
- 12.Antunes MB, Feldman MD, Cohen NA, Chiu AG. Dose-dependent effects of topical tobramycin in an animal model of Pseudomonas sinusitis. American journal of rhinology. 2007;21:423–427. doi: 10.2500/ajr.2007.21.3046. [DOI] [PubMed] [Google Scholar]
- 13.Brazas MD, Hancock RE. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:3222–3227. doi: 10.1128/AAC.49.8.3222-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeseker M, Stolk L, Nieman F, et al. The ciprofloxacin target AUC : MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br J Clin Pharmacol. 2013;75:180–185. doi: 10.1111/j.1365-2125.2012.04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller DE. Aerosol antibiotics in cystic fibrosis. Respiratory care. 2009;54:658–670. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 17.Kaushik SN, Scoffield J, Andukuri A, et al. Evaluation of ciprofloxacin and metronidazole encapsulated biomimetic nanomatrix gel on and. Biomaterials research. 2015;19:9. doi: 10.1186/s40824-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ban K, Park HJ, Kim S, et al. Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS nano. 2014;8:10815–10825. doi: 10.1021/nn504617g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li PM, Downie D, Hwang PH. Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model. Am J Rhinol Allergy. 2009;23:591–596. doi: 10.2500/ajra.2009.23.3391. [DOI] [PubMed] [Google Scholar]
- 20.Herkenne C, Alberti I, Naik A, et al. In vivo methods for the assessment of topical drug bioavailability. Pharm Res. 2008;25:87–103. doi: 10.1007/s11095-007-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


