Abstract
Context
Enhanced Recovery after Surgery (ERAS) programs are multimodal care pathways that aim to decrease intra-operative blood loss, decrease postoperative complications, and reduce recovery times.
Objective
To overview the use and key elements of ERAS pathways, and define needs for future clinical trials.
Evidence acquisition
A comprehensive systematic MEDLINE search was performed for English language reports published before May 2015 using the terms “postoperative period,” “postoperative care,” “enhanced recovery after surgery,” “enhanced recovery,” “accelerated recovery,” “fast track recovery,” “recovery program,” “recovery pathway”,“ERAS ” , and “urology” or “cystectomy” or “urologic surgery.”
Evidence synthesis
We identified 18 eligible articles. Patient counseling, physical conditioning, avoiding excessive alcohol and smoking, and good nutrition appeared to protect against postoperative complications. Fasting from solid food for only 6 h and perioperative liquid – carbohydrate loading up to 2 h prior to surgery appeared to be safe and reduced recovery times. Restricted, balanced, and goal-directed fluid replacement is effective when individualized, depending on patient morbidity and surgical procedure. Decreased intraoperative blood loss may be achieved by several measures. Deep vein thrombosis prophylaxis, antibiotic prophylaxis, and thermoregulation were found to help reduce postsurgical complications, as was a multimodal approach to postoperative nausea, vomiting, and analgesia. Chewing gum, prokinetic agents, oral laxatives, and an early resumption to normal diet appear to aid faster return to normal bowel function. Further studies should compare anesthetic protocols, refine analgesia, and evaluate the importance of robot-assisted surgery and the need/timing for drains and catheters.
Conclusions
ERAS regimens are multidisciplinary, multimodal pathways that optimize postoperative recovery.
Patient summary
This review provides an overview of the use and key elements of Enhanced Recovery after Surgery programs, which are multimodal, multidisciplinary care pathways that aim to optimize postoperative recovery. Additional conclusions include identifying effective procedures within Enhanced Recovery after Surgery programs and defining needs for future clinical trials.
Keywords: Enhanced recovery after surgery, ERAS, Perioperative care
1. Introduction
Enhanced recovery after surgery (ERAS) programs are multidisciplinary, multi-element care pathways that aim to standardize and improve perioperative management [1]. The goal of ERAS is to enable a faster and more efficient recovery using evidence-based practices [1]. Studies have shown that ERAS adoption decreases postoperative complications by 50%, reduces length of stay (LOS) by 30%, and decreases readmission rates, thereby lowering health costs [2]. Cultural and bureaucratic barriers have hindered the adoption of ERAS programs in many specialties, including urology. Here, we provide a comprehensive overview of evidence-based interventions utilized in ERAS programs. Our aims are to determine the effectiveness of specific procedures and to provide a basis for future clinical trials.
2. Evidence acquisition
2.1. Search strategy and study selection
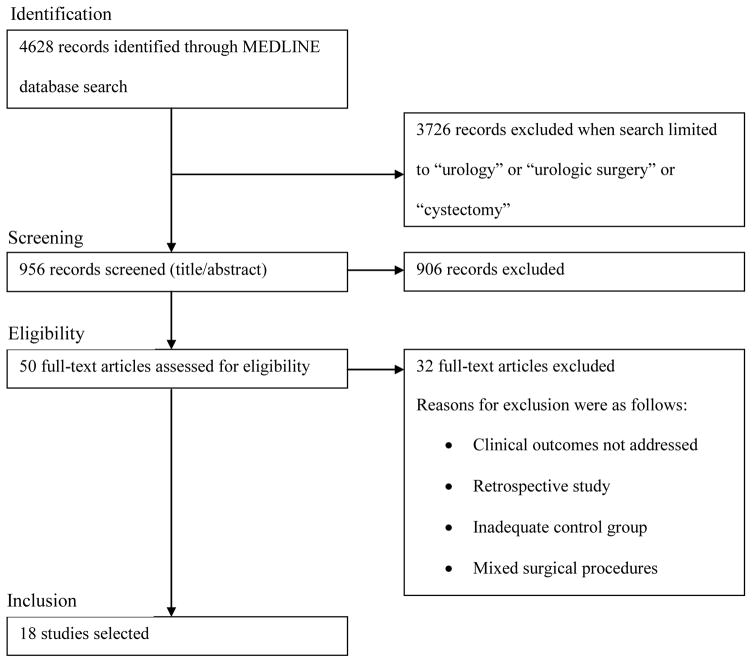
We performed a systematic literature review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Fig. 1). We used MEDLINE to identify English language articles, reviews, and editorials published prior to May 2015. The search terms and selection strategy details are provided in Figure 1. We scrutinized reference lists of recovered articles, relevant scientific meeting abstracts, and online guideline websites for additional articles. Original articles, publications within the past 5 yr, and those with the highest level of evidence were preferred. The quality of evidence from the included studies focusing on urological procedures, namely radical cystectomy (RC), was comprehensively assessed using the US Agency for Healthcare Research and Quality method (Table 1).
Fig. 1.
Selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Table 1.
Strength of evidence for components of Enhanced Recovery after Surgery pathways in urology
| Key outcomes | No. studies (N) | Risk of biasa | Directness b | Consistency c | Precision d | Reporting bias | Strength of evidence finding (ie, some reasoning) |
|---|---|---|---|---|---|---|---|
| ERAS compared with traditional pathways Preoperative counseling | 0 (0) | – | – | – | – | – | Insufficient No eligible urological studies |
| Preoperative optimization | 2 (858) [48,49] | Moderate | Direct | Consistent | Imprecise | Undetected | Low Identifies factors associated with postoperative mortality and surgical complications after radical cystectomy, but no intervention to optimize patients was [48,49]. |
| Preoperative bowel preparation | 6 (566) [9,10,50–53] | Moderate | Direct | Consistent | Precise | Undetected | Moderate No difference in outcomes when patients received mechanical bowel preparation before cystectomy or urinary diversion in two RCTs [9,10,50–53]. |
| Preoperative fasting | 0 (0) | – | – | – | – | – | Insufficient No eligible urological studies. A Cochrane review varied procedures demonstrated no adverse outcome after shortening the period of fasting from fluids [13]. |
| Preoperative carbohydrate loading | 0 (0) | – | – | – | – | – | Insufficient No eligible urological studies. |
| Preoperative alvimopan administration | 3 (474) [23,54,55 ] | Moderate | Direct | Consistent | Precise | Undetected | Moderate Studies demonstrated reduced time to return of bowel function and hospital LOS with alvimopan use in radical cystectomy, including one RCT. Also showed potential cost effectiveness [23,54,55]. |
| Preanesthesia medications | 0 (0) | – | – | – | – | – | Insufficient No eligible studies |
| Venous thromboembolism (VTE) prophylaxis | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. No RCTs or prospective studies have been performed specifically comparing complication rates with and without VTE prophylaxis in RC patients. |
| Antimicrobial prophylaxis/skin preparation | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
| Intraoperative hypothermia prophylaxis | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
| Intraoperative anesthetic protocols | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
| Intraoperative surgical approach | 2 (158) [23,24] | Moderate | Direct | Inconsistent | Precise | Undetected | Low One RCT compared robotic radical cystectomy and extracorporeal diversion with open RC and found no advantage of the robotic- assisted approach over standard open techniques. |
| Perioperative fluid management | 2 (232) [28,38] | Moderate | Direct | Consistent | Precise | Undetected | Moderate One RCT found that continuous norepinephrine administration combined with restrictive hydration significantly reduced intraoperative blood loss, the rate of blood transfusions, and the number of PRBC units required per patient undergoing open radical cystectomy with urinary diversion [28]. |
| Nasogastric intubation | 1 (43) [29] | Moderate | Direct | Consistent | Precise | Undetected | Moderate One small RCT found no significant differences in intraoperative or postoperative bowel outcomes or other complications between two groups (NGT removed 12 h after surgery vs removal after first flatus) [29]. |
| Urinary drainage/ureteral stents | 1 (54) [31] | Moderate | Direct | Consistent | Precise | Undetected | Moderate In one small RCT, stenting of the ureteroileal anastomosis was shown to significantly reduce early postoperative upper urinary tract dilatation, time to return to normal bowel function, and metabolic acidosis rate after cystectomy [31]. |
| Ileus prophylaxis and postoperative laxatives | 2 (162) [32,40] | Moderate | Direct | Consistent | Precise | Undetected | Moderate Two trials assessed the effect of gum chewing in cystectomy patients. The intervention group had shorter times to flatus and to first bowel movement compared with the control group. Both studies failed to show any difference in postoperative morbidity or LOS [32,40]. |
| Early feeding | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
| Postoperative analgesia | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
| Early postoperative mobilization | 0 (0) | – | – | – | – | – | Insufficient No eligible studies. |
ERAS = Enhanced Recovery after Surgery; LOS = length of stay; NGT = nasogastric tube; PRBC = packed red blood cells; RC = radical cystectomy; RCT = randomized control trial; VTE = venous thromboembolism.
The degree to which the study has adequate protection against bias, based primarily on study design.
Whether the evidence links the interventions directly to health outcomes. For a comparison of two treatments, directness implies that head-to-head trials measure the most important health or ultimate outcomes.
The degree to which reported effect sizes appear to have the same direction of effect, by having the same sign (ie, on the same side of “no effect”) and a narrow range.
The degree of certainty surrounding an effect estimate with respect to a given outcome (ie, for each outcome separately). If a meta-analysis was performed, this will be the confidence interval around the summary effect size.
3. Evidence synthesis
The electronic search yielded 956 potential urological articles, of which 50 were assessed for eligibility (Fig. 1). Until recently, the published ERAS literature has focused primarily on colorectal surgery outcomes. The adoption of ERAS pathways across different surgical disciplines has spread informally, although there have been some notable coordinated initiatives. For example, the UK National Health Service’s Enhanced Recovery Partnership Program acted as a catalyst for adoption among surgical specialties Recently, ERAS guidelines have been developed and published for several surgical procedures [1,3,4]. Guidelines vary by specialty but include at least 20 elements categorized into preoperative, intraoperative, and postoperative components [3].
3.1. Preoperative ERAS elements
3.1.1. Preadmission information and expectation counseling
Written, verbal, or electronic counseling about ERAS prior to surgery is important for successful implementation and may reduce patient anxiety. Counseling reduces the LOS, recovery time, and unplanned community interventions [4]. The patient should be actively engaged by preoperatively meeting members of the entire surgical team.
3.1.2. Preoperative optimization
Preoperative assessment is important for patients undergoing major surgery. It should identify and optimize risk factors/medical conditions that affect recovery. Physical conditioning and muscle training may improve recovery rates [3]. Smoking cessation and avoiding excessive alcohol intake for a minimum of 1 mo before surgery protects against postoperative complications [4]. However, smoking cessation immediately before surgery may cause greater harm than good [4]. Therefore, perioperative guidelines recommend that patients stop smoking at least 8 wk before surgery to help minimize pulmonary complications that often occur following abrupt smoking cessation by long-term smokers [5]. Nevertheless, time is not always available to stop smoking. If the patient does stop smoking and has problems with intestinal transit nicotine substitution should be considered as well as physiotherapy for the prevention of pneumonia.
Poor nutrition and diet are widely accepted risk factors for surgical morbidity. Currently, the most valuable tool for the nutritional screening of surgical patients is the Nutritional Risk Score, which is officially recommended by the European Society of Parenteral and Enteral Nutrition with Level 1 evidence validation. The Nutritional Risk Score is based on the degree of malnutrition (defined by weight loss, food intake, and body mass index) and disease severity (Table 2) [6].
Table 2.
Nutritional Risk Score 2002 scoring system [6]
| (I) Score of the severity of disease | |
| Score 1 | General malignancy Long-term hemodialysis Chronic diseases (eg, cirrhosis and COPD) Hip fracture Diabetes |
| Score 2 | Hematological malignancies Major abdominal surgery Severe pneumonia Stroke |
| Score 3 | Head and brain injury Bone marrow transplant Intensive care patients with an APACHE score higher than 10 |
| (II) Score of the impaired nutrition status | |
| Score 1 | Weight loss > 5% in 3 mo or food intake below 50–75% of normal requirement in the preceding wk |
| Score 2 | Weight loss > 5% in 2 mo or food intake below 25–50% of normal requirement in preceding week or BMI < 20.5, with poor general conditions |
| Score 3 | Weight loss > 5% in preceding month or food intake below 25% of normal requirement in preceding week or BMI < 18.5, with poor general conditions |
| (III) Score of the age | |
| Score 1 | >70 yr |
Nutrition risk screening score = Score of the severity of the disease + score of the impaired nutrition status + score of the age.
APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; COPD = chronic obstructive pulmonary disease.
Immuno-enhanced nutrients involve substrates that modulate the host immune system and inflammatory response. Randomized clinical trials (RCTs) have demonstrated that immunonutrition (a combination of arginine, fish oils, and nucleotides) positively modulates postsurgical immunosuppressive/inflammatory responses and host defense mechanisms after major surgery, even in well-nourished patients, thereby reducing LOS and infection risk [7]. A recent RCT suggested that providing immunonutrient support to RC patients can improve immunological defenses and reduce postsurgical infection [7].
3.1.3. Preoperative bowel preparation
The role of mechanical bowel preparation for ileocolic or colonic reconstruction requires further evaluation. This process can dehydrate patients and cause electrolyte imbalance, physiological stress, and prolonged ileus after colonic surgery. A systematic review and meta-analysis of colonic surgery studies concluded that there was no advantage of bowel preparation [8]. In contrast, evidence suggests that this intervention may be associated with higher rates of anastomotic leakage and incisional complications [8].
There is a lack of evidence from large RCTs to support using bowel preparation in RC patients, as many physicians have already extrapolated from the colonic surgery literature and are actively omitting this practice [9,10].
3.1.4. Preoperative fasting
A Cochrane review of 22 RCTs found that prolonged fasting prior to surgery is not necessary [11]. Consequently, most anesthesiologists recommend withholding solid food for 6 h and fluids for 2 h before surgery [12]. The European Society of Anesthesiology notes that patients who may have delayed gastric emptying (eg, obese patients), patients with gastroesophageal reflux, patients with diabetes, and pregnant women can also safely adhere to these guidelines [12].
3.1.5. Preoperative carbohydrate loading
Preoperative carbohydrate loading using clear electrolyte/carbohydrate-containing liquids helps reduce thirst, and helps maintain lean body mass and muscle strength during colorectal surgery [3], thereby decreasing recovery times[13]. A meta-analysis of preoperative liquid carbohydrate treatment in open abdominal surgery patients revealed a significant reduction in LOS compared with controls (mean difference [MD]–1.08 d, 95% confidence interval [CI]–1.87 to −0.29; seven trials; I2 = 60%) [13].
Oral and intravenous (IV) modalities are also effective at reducing insulin resistance and hyperglycemia [14]. Carbohydrate loading is a standard-of-care technique in ERAS programs that is safe in diabetic populations and can be given up to 2 h before surgery [14].
3.1.6. Preoperative alvimopan administration
Alvimopan is a peripherally active μ-opioid receptor antagonist. The use of alvimopan has been associated with a reduced LOS and faster recovery of bowel function after abdominal surgery and RC [15,16]. In a recent RCT of patients undergoing RC, patients were randomized 1:1 to receive either a single dose (12 mg) of oral alvimopan or placebo between 30 min and 5 h before surgery and then twice-daily oral doses postoperatively until hospital discharge or a maximum of 7 d. The alvimopan cohort experienced an earlier first bowel movement (5.5 d vs 6.8 d; hazard ratio: 1.8; p < 0.0001), shorter mean LOS (7.4 d vs 10.1 d; p = 0.0051), and fewer episodes of postoperative ileus-related morbidity (8.4% vs 29.1%; p < 0.001), although there were concerns regarding cardiovascular events [16].
The role of alvimopan in patients undergoing urological surgery other than RC must be evaluated in future trials (particularly in those undergoing minimal access surgery, who typically require less morphine than those undergoing open surgery).
3.1.7. Pre-anesthetic medications
Long-acting benzodiazepines can cause cognitive impairment and functional disruptions, particularly in elderly patients, for up to 4-h postsurgery, leading to reduced movement, eating, and drinking [3,4]. Short-acting benzodiazepines are preferred if necessary to reduce anxiety and facilitate patient positioning [3,4].
3.1.8. Prophylaxis against venous thromboembolism
In a landmark study, Bergqvist et al [17] observed a significant decrease in the posthospitalization venous thromboembolism rate among abdominal and pelvic surgical oncology cases in which low-molecular-weight heparin prophylaxis was continued for 19–21 d after a standard in-house anticoagulation regimen compared with placebo. No RCT or prospective study has compared complication rates with and without deep vein thrombosis prophylaxis in RC patients. Morbidity rates in these patients remain high due to the high risk of postsurgical complications.
Low-molecular-weight heparin drugs are the most tolerable, efficacious, and cost-effective drugs in this setting [17]. Other protective measures include the use of intermittent pneumatic compression devices and compression stockings during hospitalization [18].
3.1.9. Antimicrobial prophylaxis and skin preparation
Cystectomy patients benefit from prophylactic antimicrobial agents, although the best antibiotic regimen is unclear and likely depends on local antibiotic-resistance profiles. European Association of Urology guidelines recommend preoperative dosing less than 1 h prior to skin incision, continuing for up to 24 h and extending to 72 h for patients with specific infection risk factors or prolonged operations (>3 h). American Urological Association guidelines recommend a second-generation or third-generation cephalosporin or a combination of gentamicin and metronidazole for 24-h preoperatively if there are no patient risk factors. Several ERAS guidelines recommend skin preparation prior to surgery using a chlorhexidine-alcohol scrub to prevent surgical site infections (SSIs) [3,4].
3.1.10. Prevention of intraoperative hypothermia
Avoiding intraoperative hypothermia helps protect against perioperative coagulopathy and may reduce LOS [19]. The most effective warming strategies are forced-air warming blankets and warmed IV fluids [19].
3.1.11. Anesthetic protocols: systemic and regional anesthesia
The administration of intraoperative central or regional neural blockade reduces opioid use and may facilitate early enteral feeding and mobility [3]. Thoracic epidural anesthesia is widely recommended in open colorectal surgery and reduces LOS and postoperative ileus compared with patient-controlled analgesia [3].
Various studies have demonstrated the successful use of epidural anesthesia [20] or patient-controlled analgesia [21] and rectal sheath catheters [22] in open RCs. No prospective studies have compared these anesthetic protocols in RC surgeries.
3.2. Intra-operative ERAS elements
3.2.1. Surgical approach
Surgical approach (ie, open vs minimal access) may influence outcomes, complications, and recovery rates. Minimally invasive surgery requires smaller incisions, reduces analgesic use, reduces bowel handling, and decreases blood loss [23]. As such, laparoscopy may decrease postoperative complications, pain, and LOS compared with open surgery [24]. However, it is unclear whether laparoscopic resection provides better outcomes than open surgeries performed within ERAS programs.
Robot-assisted surgical approaches are increasingly utilized in urology [23,24], but the exact benefit over open surgery remains unclear. Limited evidence suggests similarities in oncology and morbidity, with reduced blood loss and analgesic use [23–25], although operating times are significantly longer. Procedure-specific RCTs that incorporate cost analysis, recovery rates, and quality of life (QoL) outcomes are needed to assess the advantages of robotic-assisted laparoscopy [1].
3.2.2. Perioperative fluid management
Fluid management in patients undergoing urology surgery can be challenging because urine output is often not measurable intraoperatively and requires careful measurement in the postoperative period. Excess fluid and hypervolemia lead to splanchnic hypoperfusion and ileus [26]. Within ERAS, both restricted and balanced fluid management protocols have been advocated [27]. Regardless, careful fluid replacement reduces bleeding, complications, and LOS.
Goal-directed fluid therapy (GDFT) attempts to add precision to fluid resuscitation by optimizing perfusion and oxygen delivery (maintaining normal physiological fluid balance and homeostasis). GDFT involves intraoperative regimens that use esophageal Doppler monitoring to optimize stroke volume [26]. GDFT decreases complication rates and LOS among patients undergoing colorectal surgery [26]. However, these studies evaluated GDFT against standard fluid management techniques, and the comparison groups often had fluid overload or unwarranted restrictions. Studies have also indicated that GDFT reduces postoperative nausea and vomiting (PONV) [26]. Restrictive intraoperative hydration combined with norepinephrine administration reduces intraoperative blood loss (and therefore transfusions), postoperative complications, and, consequently, LOS in open RCs [28].
Prospective studies are needed to compare restricted, balanced, and GDFT in patients undergoing urological surgery. The benefits of GDFT may be less significant when comparing GDFT to restrictive or balanced fluid techniques as opposed to balanced crystalloids. Additionally, the benefits of GDFT may be more individualized and more strongly influenced by patient surgical and medical risk factors.
3.2.3. Nasogastric intubation
Avoidance or early removal of a nasogastric tube (NGT) is recommended. Although most data are associated with colorectal surgery, numerous reports suggest relevance to urological procedures [29]. The use of NGTs in colonic surgery has decreased from 88% to 10%, without an increase in complications or an effect on major outcomes (bowel recovery, LOS, and morbidity) [30]. A meta-analysis of more than 33 RCTs demonstrated that avoiding NGTs decreases postoperative complication rates and the time to return of normal bowel function after abdominal surgery [30]. Lower rates of pharyngolaryngitis, respiratory infections, and vomiting have also been observed when NGTs are avoided [30]. Therefore, nasogastric suction may be limited to cases of prolonged postoperative ileus.
3.2.4. Urinary drainage
One study investigated the effect of time-to-stent removal in ileal bladder substitute and ileal conduit patients [31]. The study compared patients whose stents were removed directly following ureteroileal anastomosis with those whose stents were removed 5–10 d after surgery. Stenting improved drainage in the upper urinary tract, accelerated bowel recovery, and decreased the rate of metabolic acidosis [31]. The optimal duration of ureteral stenting must be further investigated to make safe recommendations.
3.2.5. Pelvic drainage
Studies have shown comparable outcomes in colorectal surgery patients with or without peritoneal cavity suction drains for anastomotic leaks [3], suggesting that these drains are not necessary. However, these results may not be applicable to cystectomy patients because of the possible risks of urinary leakage following surgery [1]. Postsurgical drains at the incision site significantly reduce the risk of SSI and LOS [31]. Different ERAS protocols have suggested that pelvic drains be removed as soon as possible; however, there is no clear evidence for the optimal time for removal to reduce SSI risk [32–34].
A new closing method using subcutaneous continuous aspiration drains has been associated with a reduced SSI rate after RC [35]. This method combines a dermal suture with a subcutaneous drain with a wide suction area to help reduce pressure and damage to surrounding areas during recovery.
Recently, a RCT reported that re-approximation of the dorsolateral peritoneal layer following extended pelvic lymph node dissection and cystectomy improves postoperative recovery of bowel function with less postoperative pain and fewer complications [36].
3.3. Postoperative ERAS elements
3.3.1. Postoperative nausea and vomiting
PONV are the most commonly reported adverse events after surgery (25–35% of surgical patients), the most cited reasons for patient dissatisfaction, and the primary reasons for increased LOS. PONV contribute to pulmonary aspiration and increased bleeding (through straining). The use of inhalation anesthetics, nitrous oxide, and opioids during surgery increases the likelihood of PONV [1]. PONV can be reduced or minimized by administering multimodal antiemetic prophylaxis with agents such as ondansetron [37]. Dexamethasone is also safe, efficacious, and inexpensive for such prophylaxis [37]. Combining the use of nitrous oxide and propofol also reduces PONV, and no significant interactions between these medications have been observed [37]. These medications are most effective when used as prophylaxis. Such prophylaxis can improve patient satisfaction, decrease recovery times, decrease LOS, and reduce the frequency of hospital readmission. One RCT found that intervention with intraoperative fluid optimization using esophageal Doppler monitoring of cardiovascular volumes significantly reduced PONV at 24 h and 48 h post-RC surgery [38]. A small study of 54 patients noted that the PONV rate was reduced by stenting of the ureteroileal anastomosis [31].
3.3.2. Ileus prophylaxis and use of postoperative laxatives
Ileus is a common event following RC that may also occur following prostatectomy and renal surgery. ERAS pathways highlight the importance of preventing postoperative ileus [1,4]. Prokinetic agents, such as metoclopramide, were traditionally advocated for use within ERAS programs to reduce the incidence of postoperative ileus. Although metoclopramide may not alter the time-to-first flatus or bowel opening, this agent appears to reduce PONV [39]. Gum chewing appears to be beneficial for abdominal and gastrointestinal surgery patients [35,40]. Various trials have systemically evaluated the effect of gum chewing on patient outcomes after cystectomies or gastrointestinal surgeries [35,39,40]. A recent meta-analysis found significant reductions in time to first flatus (weighted MD −12.6 h, 95% CI −21.49 to −3.72; eight arms) and to first bowel movement (weighted MD −23.11 h, 95% CI −34.32 to −11.91; seven arms) among patients who chewed gum compared with controls [40]. This effect was mediated by a reduction in postoperative paralytic ileus following gastrointestinal surgery in patients who chewed gum. Despite these findings, there was no significant difference in LOS between patients who chewed gum and controls.
Prophylactic oral laxatives have been recommended after surgery, and they are associated with an earlier return to normal bowel function and a reduction in time to defecation [3,4,41]. No prospective studies have systematically evaluated the benefits of oral laxatives in rectal or urological surgery with or without the use of ERAS pathways; such studies are necessary to ascertain the effects on patient outcomes, such as anastomotic dehiscence.
3.3.3. Early feeding
Resuming normal food intake as soon as possible following surgery is recommended. Early feeding (within 24-h postsurgery) was traditionally thought to increase the risk of bowel complications, but studies of patients who underwent gastrointestinal surgery have demonstrated positive effects on many outcomes (eg, insulin resistance, muscle function, wound healing, and risk of sepsis) [42]. A meta-analysis of major abdominal surgery patients revealed a significantly lower incidence of anastomotic dehiscence, pneumonia, and mortality among patients who ate early following surgery [42]. The benefits of early oral intake after major abdominal surgery include decreased paralytic ileus, fewer infectious complications, and a faster recovery. These benefits have been demonstrated in patients who underwent primary bowel anastomoses, with similar results to those who underwent cystectomy and urinary diversion [42].
However, vomiting is a risk of early postoperative oral intake. Active interventions for PONV must be instituted alongside early oral intake. All ERAS protocols incorporate active measures to manage nausea and reduce postoperativeileus, including sched uled antiemetics, chewing gum, cholinergic stimulants, laxatives, prokinetic agents, and limitations on narcotic administration.
Total parenteral nutrition is not routinely given to patients unless a delay in substantive enteral nutrition of greaterthan 5–7 d is expected following surgery. Given the risks of parenteral nutritionand the lack of any benefit in patients for short periods of time, total parenteral nutritionis also not initiated in any patient for whom less than 7 d of treatment is expected[ 43]. Nutritional assessments may help selectthose patients who are best suited for pre-and postoperative nutritional interventions.
Current data do not support the routine use of parenteral nutrition, and there are limited data on urological patients, particularly those undergoing cystectomy. Urinary spillage, uretero–enteric anastomosis, and large pelvic and retroperitoneal dissection differ between urological and colonic surgery; therefore, these data may not be directly comparable.
3.3.4. Postoperative analgesia
Appropriate analgesia facilitates early postoperative mobility, which in turn may counteract insulin resistance, reduce thromboembolic events and chest infection rates, increase muscle strength, and possibly reduce ileus [1,3]. Multimodal opioid-sparing analgesia, combined with regional or local anesthesia, is recommended [1] and aims to provide effective pain management while minimizing the side effects of opioids. Xu et al [44] observed that patients on an ERAS protocol (opioid-sparing analgesics) used significantly less opioid analgesics after RC.
Typically, thoracic epidural analgesia with wound infiltration or rectus sheath cannulas is used 24-h and 72-h postsurgery [48] in combination with systemic analgesics and patient-controlled opioid delivery.
Oral or IV paracetamol and/or nonsteroidal anti-inflammatory drugs have typically been used in cystectomy [20–22]. RCTs are needed to compare the effects of these pain medications on cystectomy patients. The use of adjunct medications, such as gabapentin, requires further evaluation [4].
3.3.5. Early postoperative mobilization
Early postoperative mobilization may have benefits, as previously mentioned, including counteracting insulin resistance and reducing chest complications. It can reduce pain and the likelihood of developing ileus, therefore hastening functional recovery [43]. RCTs are needed to evaluate the types and rates of improved outcomes for urological patients.
3.3.6. Discharge criteria
ERAS programs recommend that discharge should only occur when patients have resumed adequate oral intake and normal bowel function with effective oral pain management and when no other clinical or biochemical concerns remain, including stoma or neobladder competency. Patients should be well supported with regular telephone follow-ups by clinicians and access to an emergency phone number [21].
3.4. System data in ERAS
3.4.1. Audit
Auditing is an essential component of evaluating and improving the quality of healthcare practices and systems. Auditing ERAS programs can help assess compliance with recommended pathways, which is necessary to ensure successful implementation and evaluate the effect on clinical and financial outcomes [14]. Auditing can also help ensure that ERAS programs continue to be as dynamic as possible by adapting pathways that enable the development of individualized guidelines specific to different surgical modalities, disease states, or institutions [14].
3.5. Outcomes of ERAS
3.5.1. Postoperative recovery and length of stay
ERAS programs aim to improve patient recovery; however, there is no universally accepted definition of “recovery,” which encompasses multiple physiological parameters and thus complicates the evaluation of ERAS effectiveness. A systematic review of 38 studies of major elective abdominal surgeries concluded that the most commonly reported outcome measure of recovery within ERAS programs was LOS [45]. A recent trial provided clear evidence that ERAS programs in urological surgeries significantly shortened LOS after RC and urinary diversion, without increasing the hospital readmission rate [22]. In the ERAS arm of 126 patients, 82% had a bowel movement by 2-d postoperative, the median LOS was 4 d, and the 30-d readmission rate was 21% [22]. A recent prospective, randomized study compared outcomes after RC across patients treated within and outside ERAS protocols [46]. This study identified lower morbidity (fevers, wound healing disorders, and thrombosis), less demand for analgesics, less time spent in intermediate care, and higher physical and emotional QoL scores in the ERAS group compared with controls [46]. Table 3 summarizes the main outcomes of ERAS studies in the urological literature.
Table 3.
Summary of findings from published randomized control trials using Enhanced Recovery after Surgery (ERAS) protocols for radial cystectomy
| Study | Findings | |||||
|---|---|---|---|---|---|---|
| Length of stay | Complication s | Readmission s | Mortality rates | Time to BM | Other findings | |
| Arumainayagam et al (2008) [34] | Median time significantly shorter for ERAS group by 4 d | No significant difference in total complications between ERAS and control groups (ERAS n = 18; control n = 23) | No significant difference between ERAS and control groups (ERAS n = 3; control n = 5) | No significant difference between ERAS and control groups (each group n = 1) | No significant difference between ERAS and control groups (both groups median = 6 d) | None reported |
| Pruthi et al (2010) [32] | 80% discharged on postoperative days 4–5a | 39%a | 12%a | 0.01%a | M = 2.9 da | Lower rates (by 9%) of nausea and vomiting with empiric metoclopramide use More rapid recovery of bowel function with gum chewing (gum chewing M = 3.2 d, control M = 3.9 d) |
| Maffezzini et al (2012) [20] Mukhtar et al. (2013) [33] | Not reported Median time significantly shorter for ERAS group by 1.1 d | 35.3%a No significant difference between ERAS and control groups (ERAS n = 20; control n = 11) | Not reported 0% both groups | 2.9%a 3 total across groups | Not reported Significantly shorter for ERAS group by 1.3 d | Not reported Mean ICU stay significantly shorter for ERAS group by 1.4 d Time to removal of NG tube significantly shorter for ERAS group by 3.1 d Mean time to removal of IV fluids significantly shorter for ERAS group by 1.3 d Mean time to full oral diet significantly shorter for ERAS group by 1.3 d |
| Saar et al (2012) [56] | No significant difference between ERAS and control groups (ERAS M = 18.0 d; control M = 18.1 d) | No significant difference in total complications between ERAS and control groups (ERAS n = 12; control n = 15) | No significant difference between ERAS and control groups (ERAS = 2%; control = 6%) | ERAS group n = 1 | No significant difference between ERAS and control groups (ERAS M = 2.6 d; control M = 3.1 d) | Mean time to mobilization significantly shorter for ERAS group by 13.7 h Mean time to regular diet significantly shorter for ERAS group by 2.6 d Use of postoperative morphine equivalents significantly less for ERAS group by 35.1 mg Mean time of intra-operative abdominal drainaged significantly shorter for ERAS group by 3.73 d |
| Daneshmand et al (2014) [22] | Median time significantly shorter for ERAS group by 4 d | 72%a | 23%a | 1%a | M = 2 da | Median time to mobilization = 1 da
Median time to regular diet = 2 da Median oral opioid pain medication = 6.4 mg/da |
| Dutton et al (2014) [57] | M = 9.2 da | 50.1%a | 13.94%a | 1.3%a | M = 6 da | Median time to mobilization = 1–2 da |
| Karl et al (2014) [46] | No significant difference in general hospitalization time between groups ICU time significantly shorter for ERAS group by 0.8 d | No significant difference in complications between groups | Not reported | 0% in both groups | No significant difference in time between groups | ERAS group reported significantly better quality of life in most categories upon discharge compared with control group No significant difference in time to mobilization between groups (91% mobile by 3-d postoperatively ) Use of Class 1 and 2 analgesics significantly lower in ERAS group by 10% and 20%, respectively |
BM = bowel movement; ICU = intensive care unit; IV = intravenous; M = median; NGT = nasogastric
tube.
All patients received ERAS protocol (no control group).
3.5.2. Cost effectiveness
Few studies have evaluated the cost effectiveness of ERAS programs. A meta-analysis of RCTs in colorectal surgery in the US indicated a mean savings of $2000 per patient treated under ERAS [2].
One of the major criticisms of ERAS is that because patients are discharged from the hospital earlier, they may represent more frequently to the hospital after discharge. One prospective study evaluated the readmission rate among cystectomy patients and found no significant difference between the ERAS and control groups (21% vs 18%, p = 0.1); this readmission rate was comparable to that for other large centers [22].
Overall, ERAS protocols appear to be clinically efficacious and cost effective. However, randomized prospective studies to systematically evaluate cost-savings data (both in-hospital and out-of-hospital costs) for urological surgeries are lacking, and further work is needed to ensure that both the short-term and long-term cost savings of ERAS programs can be effectively captured and assessed.
3.5.3. QoL
Various authors have examined the impact of ERAS on QoL [46,47]. One study observed no improvements in QoL between ERAS and standard care [47], whereas the other study observed a nonsignificant trend in improved QoL after ERAS adoption [46]. The tools used within these studies may not have been sufficiently sensitive to detect improvements in recovery as noted by clinicians.
3.6. Adherence to ERAS protocols and barriers to implementing ERAS programs
Despite increasing evidence for the safety, potential cost savings, and improved outcomes of ERAS, institutions, surgeons, and clinicians have been slow to adopt ERAS programs. Replacing and/or adapting existing protocols and standard operating procedures can take many years, and decision makers require evidence of efficacy. One limiting factor in adoption is that much of the existing supporting evidence for ERAS has come from small-scale retrospective studies. Larger, prospective studies are necessary to provide clearer, stronger evidence for the need for and value of ERAS programs at existing institutions. Notably, the rate of adverse postoperative outcomes is directly related to percent adherence to ERAS components. Some researchers have recommended processes to facilitate ERAS adoption and adherence to ERAS elements. The successful implementation of an ERAS program requires full commitment and support of the involved parties.
4. Conclusions
4.1. Future perspectives and research initiatives in fast-track surgery
The major paradigms underlying ERAS protocols, while focused primarily on clinical recovery from surgery, also feature significant interplay with health economics, as described above. Future perspectives on ERAS will rely on additional data collection on the impact of ERAS from the patient’s perspective and on costs after hospitalization.
Out-of-hospital costs are often not considered in cost-effectiveness analyses of ERAS protocols. The focus is primarily on direct hospital-related care costs during initial hospitalization. However, postoperative communication, follow-up, and long-term complications require additional resources to prevent readmissions and enhance patient comfort and QoL. These costs and those associated with readmissions should be considered in future evaluations to better clarify the overall costs associated with patient care.
A final future perspective reemphasizes one of the core principles behind successful ERAS protocols: collaboration between surgery and anesthesia, which is essential to both the implementation of an ERAS protocol and its long-term stability and effectiveness. Evolving research is focusing on reducing opioid reliance, decreasing postoperative ileus, and optimizing or implementing GDFT management and catheterization.
As noted earlier, ERAS programs for cystectomy have been largely extrapolated from colorectal studies [1]. Given the oncological, procedural (small bowel anastomosis and urine within the peritoneal cavity), and morbidity differences between colorectal and cystectomy surgery, there is an urgent need to evaluate ERAS pathways in patients undergoing urological surgery, specifically cystectomy.
Although there is accumulating evidence supporting the use of ERAS pathways in cystectomy patients, most studies are retrospective or underpowered. Thus, high-quality prospective multicenter studies are needed to assess the different elements of ERAS protocols, such as optimal perioperative nutritional support, as well as the type and duration of pelvic and urinary catheterization, and the need to tailor ERAS elements in open- versus minimally-invasive surgery.
*Take Home Message.
The objective of this review was to provide an overview of the use and key elements of Enhanced Recovery after Surgery programs, which are multi-modal, multi-disciplinary care pathways that aim to optimize postoperative recovery. Additional objectives were to encourage the adoption of Enhanced Recovery after Surgery pathways and to identify needs for future clinical trials.
Acknowledgments
Funding/Support and role of the sponsor: None.
Footnotes
Author contributions: Raed A. Azhar had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Azhar, Bochner, Catto, Goh, Kelly, Patel, Pruthi, Thalmann, Desai.
Acquisition of data: Azhar, Goh, Patel.
Analysis and interpretation of data: Azhar, Bochner, Catto, Goh, Kelly, Patel, Pruthi, Thalmann, Desai.
Drafting of the manuscript: Azhar, Patel, Pruthi.
Critical revision of the manuscript for important intellectual content: Azhar, Bochner, Catto, Goh, Kelly, Patel, Pruthi, Thalmann, Desai.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Desai.
Other: None.
Financial disclosures: Raed A. Azhar certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel HR, Cerantola Y, Valerio M, et al. Enhanced recovery after surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy? Eur Urol. 2014;65:263–6. doi: 10.1016/j.eururo.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Lemanu DP, Singh PP, Stowers MD, Hill AG. A systematic review to assess cost effectiveness of enhanced recovery after surgery programs in colorectal surgery. Colorectal Dis. 2014;16:338–46. doi: 10.1111/codi.12505. [DOI] [PubMed] [Google Scholar]
- 3.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS) Society recommendations. Clin Nutr. 2012;31:801–16. doi: 10.1016/j.clnu.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS) society recommendations. Clin Nutr. 2013;32:879–87. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Furlong C. Smoking cessation and its effects on outcomes of surgical interventions. http://obesity.thehealthwell.info/node/27981.
- 6.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–36. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton-Reeves JM, Bechtel MD, Hand LK, et al. Effects of Immunonutrition for cystectomy on immune response and infection rates: A pilot randomized controlled clinical trial. Eur Urol. 2016;69:389–92. doi: 10.1016/j.eururo.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;7:CD001544. doi: 10.1002/14651858.CD001544. [DOI] [PubMed] [Google Scholar]
- 9.Xu R, Zhao X, Zhong Z, Zhang L. No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: a randomized controlled trial of 86 patients. Int Urol Nephrol. 2010;42:947–50. doi: 10.1007/s11255-010-9732-9. [DOI] [PubMed] [Google Scholar]
- 10.Hashad MM, Atta M, Elabbady A, Elfiky S, Khattab A, Kotb A. Safety of no bowel preparation before ileal urinary diversion. BJU Int. 2012;110:E1109–13. doi: 10.1111/j.1464-410X.2012.11415.x. [DOI] [PubMed] [Google Scholar]
- 11.Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. 2003:CD004423. doi: 10.1002/14651858.CD004423. [DOI] [PubMed] [Google Scholar]
- 12.Smith I, Kranke P, Murat I, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–69. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 13.Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32:34–44. doi: 10.1016/j.clnu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Can MF, Yagci G, Dag B, et al. Preoperative administration of oral carbohydrate-rich solutions: comparison of glucometabolic responses and tolerability between patients with and without insulin resistance. Nutrition. 2009;25:72–7. doi: 10.1016/j.nut.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Kauf TL, Svatek RS, Amiel G, et al. Alvimopan, a peripherally acting μ-opioid receptor antagonist, is associated with reduced costs after radical cystectomy: economic analysis of a phase 4 randomized, controlled trial. J Urol. 2014;191:1721–7. doi: 10.1016/j.juro.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Lee CT, Chang SS, Kamat AM, et al. Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol. 2014;66:265–72. doi: 10.1016/j.eururo.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–80. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 18.Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2010;7:CD001484. doi: 10.1002/14651858.CD001484.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 20.Maffezzini M, Campodonico F, Canepa G, Gerbi G, Parodi D. Current perioperative management of radical cystectomy with intestinal urinary reconstruction for muscle-invasive bladder cancer and reduction of the incidence of postoperative ileus. Surg Oncol. 2008;17:41–8. doi: 10.1016/j.suronc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Pruthi RS, Chun J, Richman M. Reducing time to oral diet and hospital discharge in patients undergoing radical cystectomy using a perioperative care plan. Urology. 2003;62:661–5. doi: 10.1016/s0090-4295(03)00651-4. [DOI] [PubMed] [Google Scholar]
- 22.Daneshmand S, Ahmadi H, Schuckman AK, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol. 2014;192:50–5. doi: 10.1016/j.juro.2014.01.097. [DOI] [PubMed] [Google Scholar]
- 23.Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol. 2010;57:196–201. doi: 10.1016/j.eururo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur Urol. 2015;67:1042–50. doi: 10.1016/j.eururo.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novara G, Catto JW, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015;67:376–401. doi: 10.1016/j.eururo.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed hemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 27.Bundgaard-Nielsen M, Secher NH, Kehlet H. “Liberal” vs. “restrictive” perioperative fluid therapy--a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843–51. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 28.Wuethrich PY, Studer UE, Thalmann GN, Burkhard FC. Intraoperative continuous norepinephrine infusion combined with restrictive deferred hydration significantly reduces the need for blood transfusion in patients undergoing open radical cystectomy: results of a prospective randomized trial. Eur Urol. 2014;66:352–60. doi: 10.1016/j.eururo.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 29.Donat SM, Slaton JW, Pisters LL, Swanson DA. Early nasogastric tube removal combined with metoclopramide after radical cystectomy and urinary diversion. J Urol. 1999;162:1599–602. [PubMed] [Google Scholar]
- 30.Rao W, Zhang X, Zhang J, Yan R, Hu Z, Wang Q. The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis. 2011;26:423–9. doi: 10.1007/s00384-010-1093-4. [DOI] [PubMed] [Google Scholar]
- 31.Mattei A, Birkhaeuser FD, Baermann C, Warncke SH, Studer UE. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179:582–6. doi: 10.1016/j.juro.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 32.Pruthi RS, Nielsen M, Smith A, Nix J, Schultz H, Wallen EM. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg. 2010;210:93–9. doi: 10.1016/j.jamcollsurg.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Mukhtar S, Ayres BE, Issa R, Swinn MJ, Perry MJ. Challenging boundaries: an enhanced recovery program for radical cystectomy. Ann R Coll Surg Engl. 2013;95:200–6. doi: 10.1308/003588413X13511609957579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int. 2008;101:698–701. doi: 10.1111/j.1464-410X.2007.07319.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirose Y, Naiki T, Ando R, et al. Novel closing method using subcutaneous continuous drain for preventing surgical site infections in radical cystectomy. ISRN Urol. 2014;2014:897451. doi: 10.1155/2014/897451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth B, Birkhäuser FD, Zehnder P, Burkhard FC, Thalmann GN, Studer UE. Readaptation of the peritoneum following extended pelvic lymphadenectomy and cystectomy has a significant beneficial impact on early postoperative recovery and complications: results of a prospective randomized trial. Euro Urol. 2011;59:204–10. doi: 10.1016/j.eururo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 37.López-Olaondo L, Carrascosa F, Pueyo FJ, Monedero P, Busto N, Sáez A. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth. 1996;76:835–40. doi: 10.1093/bja/76.6.835. [DOI] [PubMed] [Google Scholar]
- 38.Pillai P, McEleavy I, Gaughan M, et al. A double-blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol. 2011;186:2201–6. doi: 10.1016/j.juro.2011.07.093. [DOI] [PubMed] [Google Scholar]
- 39.Traut U, Brügger L, Kunz R, et al. Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev. 2008:CD004930. doi: 10.1002/14651858.CD004930.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald JE, Ahmed I. Systematic review and meta-analysis of chewing-gum therapy in the reduction of postoperative paralytic ileus following gastrointestinal surgery. World J Surg. 2009;33:2557–66. doi: 10.1007/s00268-009-0104-5. [DOI] [PubMed] [Google Scholar]
- 41.Zingg U, Miskovic D, Pasternak I, Meyer P, Hamel CT, Metzger U. Effect of bisacodyl on postoperative bowel motility in elective colorectal surgery: a prospective, randomized trial. Int J Colorectal Dis. 2008;23:1175–83. doi: 10.1007/s00384-008-0536-7. [DOI] [PubMed] [Google Scholar]
- 42.Lewis SJ, Andersen HK, Thomas S. Early enteral nutrition within 24 h of intestinal surgery versus later commencement of feeding: a systematic review and meta-analysis. J Gastrointest Surg. 2009;13:569–75. doi: 10.1007/s11605-008-0592-x. [DOI] [PubMed] [Google Scholar]
- 43.Klein S, Kinney J, Jeejeebhoy K, et al. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a Conference Sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr. 1997;66:683–706. doi: 10.1093/ajcn/66.3.683. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Daneshmand S, Bazargani ST, et al. Postoperative pain management after radical cystectomy: comparing traditional versus enhanced recovery protocol pathway. J Urol. 2015;194:1209–13. doi: 10.1016/j.juro.2015.05.083. [DOI] [PubMed] [Google Scholar]
- 45.Neville A, Lee L, Antonescu I, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159–70. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 46.Karl A, Buchner A, Becker A, et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a prospective randomized study. J Urol. 2014;191:335–40. doi: 10.1016/j.juro.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Stowers MD, Lemanu DP, Hill AG. Health economics in enhanced recovery after surgery programs. Can J Anaesth. 2015;62:219–30. doi: 10.1007/s12630-014-0272-0. [DOI] [PubMed] [Google Scholar]
- 48.Gregg JR, Cookson MS, Phillips S, et al. Effect of preoperative nutritional deficiency on mortality after radical cystectomy for bladder cancer. J Urol. 2011;185:90–6. doi: 10.1016/j.juro.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karl A, Rittler P, Buchner A, et al. Prospective assessment of malnutrition in urologic patients. Urology. 2009;73:1072–6. doi: 10.1016/j.urology.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 50.Large MC, Kiriluk KJ, DeCastro GJ, et al. The impact of mechanical bowel preparation on postoperative complications for patients undergoing cystectomy and urinary diversion. J Urol. 2012;188:1801–5. doi: 10.1016/j.juro.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 51.Tabibi A, Simforoosh N, Basiri A, Ezzatnejad M, Abdi H, Farrokhi F. Bowel preparation versus no preparation before ileal urinary diversion. Urology. 2007;70:654–8. doi: 10.1016/j.urology.2007.06.1107. [DOI] [PubMed] [Google Scholar]
- 52.Aslan G, Baltaci S, Akdogan B, et al. A prospective randomized multicenter study of Turkish Society of Urooncology comparing two different mechanical bowel preparation methods for radical cystectomy. Urol Oncol. 2013;31:664–70. doi: 10.1016/j.urolonc.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Tobis S, Heinlen JE, Ruel N, et al. Effect of alvimopan on return of bowel function after robot-assisted radical cystectomy. J Laparoendosc Adv Surg Tech A. 2014;24:693–7. doi: 10.1089/lap.2014.0170. [DOI] [PubMed] [Google Scholar]
- 54.Lawrentschuk N, Colombo R, Hakenberg OW, et al. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol. 2010;57:983–1001. doi: 10.1016/j.eururo.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 55.Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol. 1995;153:1763–74. [PubMed] [Google Scholar]
- 56.Saar M, Ohlmann CH, Siemer S, et al. Fast-track rehabilitation after robot-assisted laparoscopic cystectomy accelerates postoperative recovery. BJU Int. 2013;112:E99–106. doi: 10.1111/j.1464-410X.2012.11473.x. [DOI] [PubMed] [Google Scholar]
- 57.Dutton TJ, Daugherty MO, Mason RG, McGrath JS. Implementation of the Exeter enhanced recovery program for patients undergoing radical cystectomy. BJU Int. 2014;113:719–25. doi: 10.1111/bju.12533. [DOI] [PubMed] [Google Scholar]