Abstract
Background
Contradictory findings on the differential effects of second-hand smoke (SHS) on lung function in girls and boys may result from masked relationships between host and environmental factors. Allergic sensitization may augment the relationship between SHS and decreased lung function, although its role in relation to the inconsistent gender differences in children has not been elucidated.
Hypothesis
We hypothesize that there will be differences between boys and girls related to early-life allergic sensitization and exposure to SHS on pulmonary function later in childhood.
Methods
Participants in this study (n = 486) were drawn from the Cincinnati Childhood Allergy and Air Pollution (CCAAPS) birth cohort study consisting of 46% girls. Allergic sensitization was assessed by skin prick test (SPT) to 15 aeroallergens at ages 2, 4, and 7, while pulmonary function and asthma diagnosis occurred at age 7. SHS exposure was measured by hair cotinine at ages 2 and/or 4. Gender differences of SHS exposure on pulmonary function among children with positive SPTs at ages 2, 4, and 7 as well as first- and higher-order interactions were examined by multiple linear regression. Interactions significant in the multivariate models were also examined via stratification. Comparisons within and between stratified groups were assessed by examining the slope of the parameter estimates/beta coefficients and associated p-values and confidence intervals.
Results
Increased cotinine levels were significantly associated with decreases in FEV1 (−0.03 l, p < 0.05), peak expiratory flow (−0.07 l/s, p < 0.05), and FEF 25–75% (−0.06 l/s, p < 0.01). The interaction between cotinine and sensitization at age 2 was borderline significant (p = 0.10) in the FEF25–75% model and showed an exposure response effect according to the number of positive SPTs at age 2; zero (−0.06 l/s, p < 0.01), one (−0.09 l/s, p < 0.05), or two or more positive SPTs (−0.30 l/s, p < 0.01). Despite increased polysensitization among boys, the association between cotinine and FEF25–75% among girls, with two or more positive SPTs at age 2, showed the greatest deficits in FEF25–75% (−0.34 l/s vs. −0.05 l/s and − 0.06 l/s for non-sensitized girls and boys, respectively. Girls with two or more positive SPTs showed a twofold greater decrease in FEF25–5% (−0.34 l/s; 95% CI: −0.55, −0.13) compared to boys with the same degree of allergic sensitization (−0.18 l/s; 95% CI: −0.41, 0.06), although this difference was not statistically significant.
Conclusions
Reductions in lung function were observed among children exposed to SHS, and the number of aeroallergen-positive SPTs at age 2 modifies this relationship. Girls experiencing early childhood allergic sensitization and high SHS exposure are at greater risk of decreased lung function later in childhood compared to non-sensitized girls and boys and demonstrate greater deficits compared to boys with similar degrees of sensitization.
Keywords: allergic sensitization, gender, child, spirometry, second-hand smoke
Second-hand smoke exposure (SHS) is widespread among children in the United States, with nearly one in four children living in a home with at least one smoker and having cotinine concentrations (a metabolite of nicotine) more than twice as high as those among non-smoking adults (1). An extensive body of evidence has documented that SHS exposure during childhood is associated with respiratory illness (2), asthma development and exacerbation (2), and decreased lung function (3). Biologic evidence for causal relationships may involve changes in the nervous (4) or immune (5) systems, airway responsiveness (3), and/or structural changes of the lungs and airways (4).
The effects of SHS exposure on lung function, however, may not be uniform among boys and girls or among those with allergen sensitization and allergic diseases, as these conditions likely increase the susceptibility of the airways to non-allergenic irritants such as SHS (6, 7). Among genetically predisposed children, atopy has been shown increase a child’s risk of asthma and bronchial hyper-responsiveness (BHR) associated with SHS exposure (8). This finding has also varied by gender. For example, boys without a family history of atopy exposed to SHS in utero, in the first 2 yr of life, or currently were at greater risk of asthma or wheeze. The same study found that the opposite was true among children with a family history of atopy, demonstrating that girls were more susceptible to SHS exposure, regardless of the timing of exposure, showing stronger associations for asthma, allergic rhinitis, and wheeze (7). Further, it has been shown that only boys with asthma and girls without asthma exposed to past SHS experienced decreased flow rates (6).
These varied findings highlight the complex relationships among host and environmental factors that are seldom examined concurrently. Because approximately 50% of children between the ages of 6 and 9 are sensitized (9) and early and persistent allergic sensitization is recognized as a risk factor for asthma development (10), the purpose of this study is to disentangle the relationships related to early childhood sensitization, gender, environmental exposure to SHS as measured by internal dose of hair cotinine, and the potential long-term consequences on pulmonary function.
Methods
Study population
The objective of the Cincinnati Childhood Allergy and Air Pollution Study (CCAAPS) cohort was to determine whether exposure to indoor and outdoor pollutants during early childhood is associated with the development of allergic sensitization and allergic diseases and to determine gender susceptibility. Newborns in the Cincinnati metropolitan area were identified from birth records from 2001 to 2003 (11). Infants were eligible for study recruitment if their residence at birth was either <400 m or >1500 m from a major road defined as ≥1000 trucks per day (11). Enrollment criteria included being born to at least one atopic parent, confirmed by allergy symptoms and a current skin prick test (SPT) to 15 aeroallergens (11). Parents were also administered a questionnaire to gather parental and child health information in the previous year and environmental exposures, including address history for traffic-related particle exposure. The University of Cincinnati Institutional Review Board approved the study protocol prior to subject recruitment, and written informed consent was obtained prior to participating in any study-related procedures.
Children had a physical examination by trained clinicians annually at age 1 through 4 and at age 7, including a skin test for 15 aeroallergens plus cow’s milk and egg. The aeroallergens tested include seasonal (outdoor) allergens: meadow fescue and timothy grass pollens, white oak, maple mix, American elm, red cedar, and short ragweed; and perennial (indoor) allergens: Alternaria, Aspergillus fumigatus, Penicillium mix, Cladosporium, dust mite mix, German cockroach, cat, and dog. A positive SPT was defined as a reaction producing a wheal at least 3 mm greater than a saline control after 15 min. Although many studies define atopy as having one positive SPT, a recent study found that among patients ranging from 5 to 65 yr of age, having two or more positive SPTs was associated with moderate to severe rhinitis and asthma (12). Therefore, for the purpose of this study, children were categorized based on the number of positive SPTs at ages 2, 4, and 7 as follows: negative to all aeroallergens, positive to one, or positive to two or more.
Traffic-related particle exposure
Estimated traffic-related air pollution was adjusted for in this study, as this is a major focus of CCAAPS. An average daily exposure to traffic-related particles was calculated for each child using a land-use regression model as previously described (13). From December 2001 through December 2006, ambient air sampling was performed at 27 sampling sites in the greater Cincinnati area. The average daily concentration of elemental carbon attributable to traffic (ECAT), a marker of traffic-related particles, was determined for each site (13). A land-use regression model was then developed to relate ECAT with land-use and traffic variables including elevation, truck intensity, and length of bus routes. The final land-use regression model had an R2 = 0.73.
An individual time-weighted average daily exposure was calculated for each year of a child’s life for all subjects enrolled in CCAAPS. This exposure was determined by geocoding all addresses where the child was reported to have spent more than 8 h/wk in the previous 12 months and calculating a time-weighted estimate of exposure (14). Geocoding and geographic information systems were conducted using EZLocate (TeleAtlas) and ArcGIS 9.3 (Environmental Systems Research Institute, Redlands, CA, USA).
Pulmonary function and asthma diagnosis
At age 7, all children had spirometry testing according to ATS-ERS guidelines (15). Spirometers had their volume accuracy verified daily, and each child performed at least four acceptable maneuvers. A random sample of five percent of the pulmonary function tests was examined for any significant measurement artifact that would invalidate the reported forced vital capacity (FVC) or forced expiratory volume in 1-s (FEV1) or significantly influence the reported forced expiratory flow between 25% and 75% of FVC (FEF25–75%). The best FEV1, FVC and peak expiratory flow (PEF) were recorded, whereas FEF25–75% was derived from the best curve, defined as the greatest sum of FEV1 and FVC. In addition, FEV1 as a percentage of FVC was calculated (FEV1/FVC).
A diagnosis of asthma first required parental report of asthma symptoms in the previous 12 months (tight or clogged chest or throat, difficulty breathing or wheezy after exercise, wheezing or whistling in the chest, or a prior physician diagnosis of asthma). Second, the child had to exhibit BHR. BHR was first defined by a ≥12% increase in baseline FEV1 following bronchodilator administration of 2.5 mg of levalbuterol (15). Children who did not have a ≥12% increase in FEV1 were tested for BHR by methacholine challenge testing (MCCT). The four-dose protocol began with a saline control followed by sequential doses of 0.0625, 0.25, 1.0, and 4.0 mg/ml of methacholine chloride. BHR by MCCT was defined as a 20% or greater decline in baseline FEV1 postsaline diluents challenge. If there was a <20% decline in FEV1 at 4 mg/ml, the MCCT was considered negative and not diagnostic for BHR.
Hair collection and analysis
At ages 2 and 4, hair was collected by cutting approximately 20 strands of hair from the root end in the occipital region of the scalp. The samples were washed prior to analysis to remove surface nicotine, adjusted by weight, and analyzed for the quantity of cotinine by radioimmunoassay at the Hospital for Sick Children in Toronto, Canada (16). Children with concentrations below the limit of detection (0.02 ng/mg) were assigned a cotinine value of 0.01 ng/mg (17). Cotinine values for the two time periods were highly correlated (r2 = 0.71). For this reason and to decrease missing values, the average value of age 2 and 4 was calculated, and if one time period was available, that was used.
Statistical analysis
Explanatory analyses were performed to identify important variables for predicting lung function among boys and girls. Univariate associations with the primary exposure variable (cotinine) and possible predictor variables were evaluated by chi-square contingency tables for categorical variables and by t-tests for continuous variables. Predictor variables included race [African American (AA), non-African American (NAA)], sex, age (yr), height (cm), weight (kg), chest circumference (cm), child asthma status (yes/no), aeroallergen sensitization (0, 1, 2, or more positive SPTs) and food (milk and egg) sensitization (yes/no) at ages 2, 4, and 7, ECAT (μg/m3), riding in a school bus (yes/no), gas stove present in home (yes/no), prenatal SHS exposure (yes/no), and maternal education (non-high school graduate, graduated high school, attended college, or was a college graduate). The correlation and distribution of hair cotinine were examined at ages 2 and 4. Cotinine was skewed and subsequently log transformed using the natural log.
Linear regression was performed to assess univariate associations between each pulmonary function outcome and cotinine, modified by other predictor variables. Accordingly, first- and higher-order interactions between cotinine, gender, and allergic sensitization were modeled. Multiple linear regression models were then built for each pulmonary function outcome separately. A priori, hair cotinine, ECAT, and variables related to pulmonary function testing (PFT) outcomes (race, gender, and height) were included in all multivariate models. ECAT was included as it is a source of particulate matter exposure and could be a potential confounder. Other predictor variables and interactions significant at the 15% level were initially included in multivariate models.
A deterministic approach to model reduction was used to reduce the initial multivariate model of each pulmonary function outcome. Variables were removed one at a time, beginning with the highest p-value. If a change in the parameter estimate of cotinine exceeded 10% after removal, the variable remained in the model. The final models included variables and interactions significant at the 15% level. The variance inflation factor (VIF) values of each final model were then assessed to quantitate multicollinearity. If an interaction was significant, the model was repeated after stratification by the variable that interacted with cotinine. The slope of the parameter estimates/beta coefficients and associated p-values and confidence intervals were used to assess differences between stratified models. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Population description
Of the 762 children enrolled at age 1, 81% (n = 617) were evaluated at age 7, and 591 successfully completed the PFT. Sufficient hair samples were available for 82% (n = 486) of those children and were included in this analysis. There were no significant differences between those who did or did not complete the pulmonary function with respect to race, gender, or allergic sensitization nor did gender or allergic sensitization differ for those with or without hair cotinine results. AA, however, were less likely to provide hair samples (data not shown). Of the final cohort used for analysis, 46% were girls, 14.5% AA, and 14.9% met the clinical criteria for asthma.
The overall geometric mean cotinine concentration in hair (±1 s.d.) was 0.15 ng/mg (±0.28) ranging from 0.01 to 2.80 ng/mg. Girls (0.16 ng/mg) and boys (0.15 ng/mg) had similar concentrations (Table 1). Asthmatic (0.20 ng/mg) compared to non-asthmatic children (0.14 ng/mg) had higher cotinine concentrations although this difference was not statistically significant. Cotinine values did not differ between asthmatic and non-asthmatic children by gender. AA had, on average, four times the concentration of cotinine, 0.49 ng/mg compared to 0.09 ng/mg for NAA (p < 0.05), but this difference did not vary by gender. Based on our questionnaire data, AA also had more SHS exposure compared to NAA while riding in a car.
Table 1.
Participant characteristics by gender (n = 486)
| Total n = 486 | Girls n = 224 | Boys n = 262 | |
|---|---|---|---|
| African American n (%) | 70 (14.5) | 32 (14.3) | 38 (14.5) |
| Asthmatic n (%) | 72 (14.9) | 28 (12.5) | 44 (16.8) |
| Allergic sensitization age 2 (n = 461) | |||
| 0 SPT+ n (%) | 287 (62.2) | 132 (58.9) | 155 (59.2) |
| 1 SPT+ n (%) | 96 (20.8) | 48 (21.4) | 48 (18.3) |
| 2 or more SPT+ n (%) | 78 (16.9) | 33 (14.7) | 45 (17.1) |
| Allergic sensitization age 4 (n = 464) | |||
| 0 SPT+ n (%) | 220 (47.3) | 102 (45.5) | 118 (45.0) |
| 1 SPT+ n (%) | 121 (26.1) | 56 (25.0) | 65 (24.8) |
| 2 or more SPT+ n (%) | 123 (26.5) | 53 (23.7) | 70 (26.7) |
| Allergic sensitization age 7 (n = 472) | |||
| 0 SPT+ n (%) | 260 (55) | 122 (54.5) | 138 (52.7)* |
| 1 SPT+ n (%) | 74 (15.7) | 47 (20.9) | 27 (10.3)* |
| 2 or more SPT+ n (%) | 138 (29.2) | 46 (20.5) | 92 (35.1)* |
| Maternal education | |||
| Non-high school graduate n (%) | 23 (4.85) | 12 (5.43) | 11 (4.35) |
| High school graduate n (%) | 68 (14.35) | 29 (13.12) | 39 (15.42) |
| Attended college or college graduate n (%) | 383 (80.8) | 180 (81.45) | 203 (80.24) |
| Mean age in years (s.d.) | 6.9 (0.47) | 6.9 (.46) | 6.9 (0.48) |
| Mean height in cm (s.d.) | 124.0 (7.36) | 123.3 (7.47) | 124.6 (7.23) |
| Mean weight in kg (s.d.) | 25.29 (4.76) | 25.00 (4.27) | 25.53 (51.4) |
| Mean chest circumference in cm (s.d.) | 61.91 (5.14) | 61.23 (4.96) | 62.49 (5.22)† |
| Mean ECAT in μg/m3 | 0.36 (0.12) | 0.36 (0.14) | 0.35 (0.10) |
| Mean cotinine in ng/mg (s.d.) | 0.15 (0.28) | 0.16 (0.31) | 0.15 (0.26) |
| African American | 0.49 (0.54)* | 0.54 (0.57) | 0.43 (0.51) |
| Non-African American | 0.09 (0.14)* | 0.08 (0.13) | 0.09 (0.15) |
| Asthmatics | 0.20 (0.25) | 0.24 (0.32) | 0.16 (0.19) |
| Non-asthmatic | 0.14 (0.29) | 0.14 (0.30) | 0.13 (0.28) |
| Mean FEV1 in l (s.d.) | 1.42 (0.23) | 1.38 (0.22) | 1.46 (0.23)† |
| Mean FVC in l (s.d.) | 1.60 (0.27) | 1.54 (0.24) | 1.65 (0.27)† |
| Mean FEV1/FVC % (s.d.) | 0.89 (0.06) | 0.89 (0.06) | 0.89 (0.06) |
| Mean FEF25–75% in l/s (s.d.) | 1.74 (0.42) | 1.72 (0.41) | 1.75 (0.44) |
| Mean PEF in l/s (s.d.) | 3.16 (0.55) | 3.09 (0.51) | 3.23 (0.58)† |
SPT, skin prick test; 0 SPT+, tested negative to all aeroallergens; 1 SPT+, one positive skin prick test; 2 or more SPT+, two or more positive skin prick tests; ECAT, elemental carbon attributable to traffic; FEV1, Forced expiratory volume in 1 s; FEF25–75%, Forced expiratory flow between 25% and 75% of forced vital capacity; PEF, peak expiratory flow; FVC, Forced vital capacity; FEV1/FVC%, FEV1 as a percentage of FVC; s.d., standard deviation.
Bolded values represent significant differences by gender (*p < 0.05; †p < 0.01) using chi-square contingency tables for categorical variables and by T-tests for continuous variables.
Allergic sensitization
At age 2, 4, and 7, 37.7%, 52.6%, and 44.9% of children were SPT positive, respectively (Table 1). The number of children with only one positive SPT initially increased from age 2 (20.8%) to 4 (26.1%) but then decreased at age 7 (15.7%). A continual increase in those with two or more SPTs occurred as the children aged from 2 (16.9%) to 4 (26.5%) and to 7 (29.2%). Overall, the number of positive SPTs was evenly distributed between genders at ages 2 and 4. At age 7, there was a significant association between the number of positive SPTs and gender (p = 0.03) as 35% of boys and only 21% of girls had two or more positive SPTs (Table 1).
The most common aeroallergen sensitization at age 2 was timothy grass (8.5%) (Table 2). Similarly at age 4 and 7, timothy grass remained one of the most common allergies (11.6% and 15.7%) along with maple mix (12.1% and 17.2%), respectively, with dust mite (12.1%) and cat (10.8%) sensitization increasing at age 4. A shift in the pattern of allergic sensitization was observed at age 7, with short ragweed and white oak allergies becoming more prevalent (Table 2). Among children with two or more positive SPTs, timothy grass and maple pollen allergies were the most common at all ages (Table 2). While the prevalence of egg and milk allergies was the highest at age 2 (7.9% and 1.9%, respectively), their frequency sharply decreased with increasing age (Table 2). Further, food allergies were not associated with decreases in lung function (data not shown).
Table 2.
Prevalence of sensitization to individual aeroallergens and food allergens at ages 2, 4, and 7*
| Category | Individual allergens | % Yr 2 Total (n = 461) |
% Yr 2 2 or more SPT+ (n = 78) |
% Yr 4 Total (n = 464) |
% Yr 4 2 or more SPT+ (n = 123) |
% Yr 7 Total (n = 472) |
% Yr 7 2 or more SPT+ (n = 138) |
|---|---|---|---|---|---|---|---|
| Pollen | Meadow fescue | 3.7 | 21.8 | 7.3 | 22.8 | 13.1 | 43.5 |
| Timothy | 8.5 | 37.2 | 11.6 | 36.6 | 15.7 | 51.4 | |
| White oak | 4.6 | 21.8 | 9.7 | 30.1 | 14.8 | 48.6 | |
| Maple mix | 5.6 | 26.9 | 12.1 | 36.6 | 17.2 | 51.4 | |
| American elm | 3.7 | 16.7 | 8.4 | 27.6 | 12.7 | 41.3 | |
| Red cedar | 7.4 | 25.6 | 5.0 | 16.3 | 4.4 | 12.3 | |
| Short ragweed | 3.7 | 14.3 | 7.8 | 24.4 | 15.7 | 50.0 | |
| Mold | Alternaria | 3.7 | 14.3 | 5.8 | 16.3 | 2.8 | 7.2 |
| Aspergillus fumigatus | 4.6 | 17.9 | 1.9 | 5.7 | 2.3 | 7.2 | |
| Penicillium | 2.6 | 14.1 | 3.7 | 11.4 | 2.1 | 5.8 | |
| Cladosporium | 3.0 | 17.9 | 3.0 | 8.1 | 2.1 | 5.8 | |
| Dust | Dust mite | 6.5 | 20.5 | 12.1 | 26.0 | 12.3 | 25.4 |
| German cockroach | 4.3 | 19.2 | 6.9 | 17.1 | 4.0 | 13.8 | |
| Animal | Cat | 6.1 | 19.2 | 10.8 | 26.8 | 11.2 | 30.4 |
| Dog | 3.9 | 12.8 | 5.8 | 17.9 | 4.9 | 15.2 | |
| Food | Milk | 1.9 | 5.1 | 1.1 | 1.3 | 0.42 | 1.2 |
| Egg | 7.9 | 11.7 | 3.4 | 5.3 | 2.3 | 2.6 |
SPT, skin prick test.
All prevalence values are per total number tested at each year.
Pulmonary function
Significant reductions in FEV1 (−0.03 l; 95% CI: −0.04, −0.01), FEF25–75% (−0.06 l/s; 95% CI: −0.09, −0.03), and PEF (−0.07 l/s; 95% CI: −0.11, −0.03) were observed for every unit change of log cotinine, but this was not true for FVC and FEV1/FVC (Table 3). Neither gender nor allergic sensitization at age 2, 4, or 7 independently modified the relationship between cotinine and FEV1, FVC or FEV1/FVC outcomes.
Table 3.
Effects of cotinine on pulmonary function outcomes at ages 2, 4, and 7 in total population and stratified by gender and allergic sensitization
| FEV1 (95% CI) | FVC (95% CI) | FEV1/FVC (95% CI) | FEF25–75% (95% CI) | PEF (95% CI) | |
|---|---|---|---|---|---|
| Total population | −0.03 (−0.04, −0.01)* | −0.02 (−0.04, 0.003) | −0.26 (−0.78, 0.25) | −0.06 (−0.09, −0.03)† | −0.07 (−0.11, −0.03)† |
| Gender | |||||
| Girls | −0.02 (−0.05, 0.004) | −0.02 (−0.05, 0.01) | −0.81 (−1.63, 0.013) | −0.06 (−0.11, −0.01)* | −0.08 (−0.13,−0.02)* |
| Boys | −0.01 (−0.03, 0.003) | −0.01 (−0.04, 0.02) | −0.02 (−0.69, 0.64) | −0.05 (−0.09, −0.01)† | −0.07 (−0.12, −0.01)* |
| Allergic sensitization age 2 | |||||
| 0 SPT+ | −0.02 (−0.04, 0.004) | −0.02 (−0.04, 0.001) | −0.11 (−0.71, 0.50) | −0.06 (−0.09, −0.02)† | −0.07 (−0.11, −0.02)† |
| 1 SPT+ | −0.03 (−0.10, 0.04) | −0.02 (−0.10, 0.05) | −0.59 (−2.78, 1.61) | −0.09 (−0.17, −0.01)* | −0.15 (−0.33, 0.04) |
| ≥2 SPT+ | −0.05 (−0.11, 0.01) | −0.06 (−0.12, 0.01) | −1.36 (−3.30, 0.59) | −0.30 (−0.41, −0.18)† | −0.20 (−0.35, −0.04)* |
| Allergic sensitization age 4 | |||||
| 0 SPT+ | −0.004 (−0.019, 0.012) | −0.007 (−0.022, 0.008) | −0.0004 (−0.0084, 0.0074) | −0.015 (−0.19, 0.16) | −0.04 (−0.11, 0.04) |
| 1 SPT+ | −0.01 (−0.02, 0.01) | −0.005 (−0.021, 0.011) | −0.008 (−0.0183, 0.0019) | −0.04 (−0.09, 0.01) | −0.06 (−0.12, 0.01) |
| ≥2 SPT+ | −0.03 (−0.06, 0.01) | −0.02 (−0.04, 0.01) | −0.006 (−0.017, 0.005) | −0.04 (−0.10, 0.02) | −0.09 (−0.25, 0.07) |
| Allergic sensitization age 7 | |||||
| 0 SPT+ | −0.01 (−0.04, 0.02) | −0.01 (−0.05, 0.03) | 0.24 (−0.48, 0.95) | −0.04 (−0.09, 0.01) | −0.11 (−0.16, −0.05)† |
| 1 SPT+ | −0.02 (−0.05, 0.01) | −0.02 (−0.05, 0.002) | −0.75 (−1.99, 0.50) | −0.04 (−0.12, 0.04) | −0.04 (−0.13, 0.04) |
| ≥2 SPT+ | −0.03 (−0.06, 0.003) | −0.02 (−0.06, 0.02) | −0.23 (−1.4, 0.90) | −0.07 (−0.14, 0.01) | 0.01 (−0.10, 0.11) |
FEV1, Forced expiratory volume in 1 s measured in l; FEF25–75%, Forced expiratory flow between 25% and 75% of forced vital capacity measured in l/s; PEF, peak expiratory flow measured in l/s; FVC, Forced vital capacity measured in l; FEV1/FVC, FEV1 as a percentage of FVC; SPT, skin prick test.
Bolded values represent significant associations:
p-value < 0.05;
p-value < 0.01;
p-values represent the effect of cotinine on outcome within each model.
Models are adjusted for age, race, gender, height, elemental carbon attributable to traffic and any covariates remaining in the model with a p < 0.15. Parameter estimates represent the change in the pulmonary function outcome per 1 unit change in log cotinine. 95% Confidence intervals of each outcome’s predicted mean values are shown for each model.
The interaction between cotinine and allergic sensitization at age 2 was borderline significant in the FEF25–75% (p = 0.10) and PEF model (p = 0.15). For both genders of the non-sensitized children (−0.06 l/s; 95% CI: −0.09, −0.02) and those children with two or more positive SPTs (−0.30 l/s; 95% CI: −0.41, −0.18), the effect of cotinine on FEF25–75% was highly significant (p < 0.01) (Table 3). These data also showed a dose–response effect of allergic sensitization at age 2 on FEF25–75% and PEF among children with zero, one, or two or more positive SPTs (Table 3). Reductions in FEF25–75% (−0.06, −0.09, and −0.30 l/s, respectively) were three and five times greater among children positive to one or two or more aeroallergens compared to non-sensitized children (p < 0.05) (Table 3). A similar dose–response reduction in PEF related to the number of positive SPTs at age 2 was observed from those who were non-sensitized (−0.07 l/s; 95% CI: −0.11, −0.02), had one (−0.15 l/s; −0.33, 0.04), or had two or more positive SPTs (−0.20 l/s; 95% CI: −0.35, −0.04). Although not statistically significant, those children with two or more positive SPTs had a three times greater decrease in PEF compared to non-sensitized children (Table 3). Allergic sensitization at age 4 or 7, however, did not modify the relationship between hair cotinine and FEF25–75% or PEF.
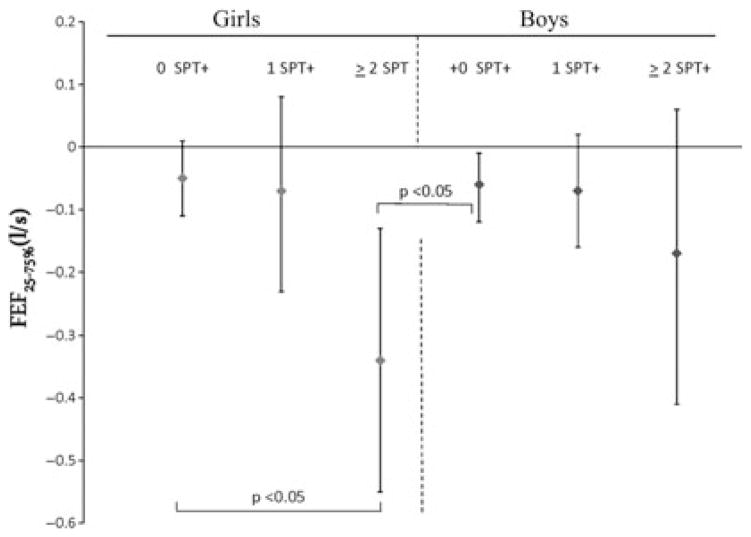
The interaction between gender and cotinine was significant (p = 0.03) in the FEF25–75% model. After stratification by gender, it was evident that similar and significant reductions in FEF25–75% were observed between boys and girls as hair cotinine concentrations increased, suggesting that cotinine was driving the observed interaction between gender and cotinine (Table 3). Interestingly, the combined interaction between cotinine, gender, and allergic sensitization at age 2 was also significant in the FEF25–75% model (p = 0.05). Figure 1 shows the six to sevenfold increased effects of cotinine on FEF25–75% when stratified by both gender and allergic sensitization while controlling for other covariates. The greatest significant effect was observed for girls with two or more positive SPTs (−0.34 l/s; 95% CI: −0.55, −0.13) compared to non-sensitized girls (−0.05 l/s; 95% CI: −0.11, 0.01) and non-sensitized boys (−0.06 l/s; 95% CI: −0.01, −0.12) (Fig. 1). The difference between girls with one or two or more positive SPTs or between boys with one or two or more positive SPTs was not significant (Fig. 1). A trend, however, was observed among girls and boys with two or more positive SPTs, as girls showed a twofold greater decrease in FEF25–75% compared to boys with a similar degree of sensitization.
Figure 1.
Combined effect of gender and allergic sensitization at age 2 on the reduction of FEF25–75% at age 7. FEF25–75%, Forced expiratory flow between 25% and 75% of forced vital capacity; 95% Confidence intervals for FEF25–75% predicted mean values are shown. Models are adjusted for age, race, height, elemental carbon attributable to traffic and any covariates remaining in the model with a p < 0.15.
Discussion
This study is the first to investigate the differential gender effects of SHS exposure using hair cotinine, while exploring the importance of timing and extent of allergic sensitization on lung function. Significant associations were observed between hair cotinine levels and reductions in FEV1, FEF25–75%, and PEF. The results varied by allergic sensitization status, suggesting that sensitization as early as age 2 increases a child’s susceptibility for reductions in FEF25–75% at age 7. This was not observed for allergic sensitization at age 4 or 7. The extent of allergic sensitization also plays a vital dose–response role in susceptibility. The reduction in FEF25–75% among children with two or more positive SPTs at age 2 was five times greater compared to non-sensitized children and three times greater compared to children with one positive SPT. We support previous findings that allergic sensitization in the first 3 yr of life is associated with a loss of lung function at age 6 that persisted into puberty (18).
Although the exact mechanism of this modification is unclear, it is possible that this finding reflects a heightened Th2 immune response. Independently, both SHS and poly-sensitization create a Th-2-biased environment. For instance, it has been shown that SHS enhances the immune response to allergens and that multi-allergic children experience sub-clinical asthma-like changes in their lung function (19, 20). SHS exposure among the more sensitized children may potentiate the effect SHS has on pulmonary function reflecting a combined adjuvant effect on the allergic inflammation response to the specific allergens or a heighted general irritant effect impacting the airways. Further, there are three mechanisms that may explain why the modification effect was only observed at age 2. First, it has been suggested that the most important steps toward the development of mature systemic immune responses occur prior to age 3 (21). Second, the timing of atopy, which directly ties into the development of the systemic immune response, appears to have an important role in not only this study but also others (18). Lastly, between 36 wk and prior to age 3, proliferation of alveolar is completed (4). Our results confirm the importance of this critical window and suggest that very early-life allergic sensitization, especially if positive to two or more aeroallergens, increases a child’s risk of pulmonary function loss at age 7 when exposed to SHS. This finding appears to be particularly true among girls.
Although nearly 45% of girls and boys in the CCAAPS cohort, with a family history of atopy, had at least one positive SPT prior to age 7, boys were more sensitized than girls at all ages; this finding has been previously confirmed (22). Interestingly, although boys and girls had similar exposures, the effect of SHS on FEF25–75% loss was six times greater among girls with two or more positive SPTs compared to non-atopic girls and boys. Prior studies have found conflicting results but also provide very intriguing hypotheses needing further exploration. For instance, Chen et al. (23) concluded that current or recent tobacco smoke exposure, as measured by questionnaire, had a larger effect on FEV1 and FEF25–75% in girls than in boys. Earlier findings, also based on questionnaire data, documented opposing gender effects showing boys experiencing greater lung function deficits as a result of SHS exposure (24). Recently, it has become evident that girls with a family history of atopy may be at greater risk of respiratory diseases and respiratory symptoms resulting from exposure to SHS (7). Further, Li et al. (6) found that boys with asthma and reported SHS exposure showed significant decreases in FEV1, while only girls without asthma showed decreases. It is possible that the gender discrepancy in the relationship between SHS exposure and flow rates of airways may have a physiological foundation because females tend to have smaller absolute airways after adjusting for height (25). However, another interesting hypothesis is the possible role of sex hormones. Although CCAAPS is unable to address this hypothesis, there have been intriguing studies that support this theory. Girls prior to menarche have been shown to have detectable levels of progesterone in their saliva (26). Prenatal and SHS exposure has been associated with earlier menarche (27), and progesterone (a hormone involved in the female menstrual cycle) has been shown to illicit a Th2 immune response similar to that seen in allergic asthma; this effect is enhanced by exposure to SHS (28). Despite the inconsistencies in the literature, it is compelling that gender plays a fundamental role especially in a girl’s risk of decreased lung function with SHS exposure. Whether or not hormones are responsible is appealing but remains unknown.
This study’s major findings center on FEF25–75%, suggesting that the small airways may be a particular target for SHS. What is clear is that airway development and final alveolarization occur at different stages. Therefore, depending on when SHS exposure occurs, there may be differing dimensions of lung damage. It is noted, however, that the guidelines of the American Thoracic Society (15) do not suggest the use of FEF25–75% to define airway obstruction because of its variability, dependence on length of forced expiratory time, and level of FVC achieved. Confidence can be found in our data, however, as those children diagnosed with asthma also had a lower FEF25–75%. Further, we found no signs of measurement artifact that would invalidate the reported FVC, FEV1, or FEF25–75% after review by a pulmonary toxicologist (RM). Other recent studies (29, 30) have demonstrated that FEF25–75% measurement in children with allergic disease and/or asthma may be beneficial where FEV1 is not yet affected. FEF25–75% has been demonstrated to correlate better with air trapping and bronchodilator responsiveness than FEV1 in asthmatic children (30). FEF25–75% has also been suggested as a possible early indicator of bronchial impairment, and early allergic or inflammatory involvement of the small airways in subjects with allergic disease (29). Further, FEF25–75% has predicted the presence of clinically relevant reversible airflow obstruction in children (30). Therefore, changes in FEF25–75% within population studies, especially for children, may give insight toward airflow modifications or remodeling in the small airways that are undetected by parameters such as FEV1 or FEV1/FVC, which are affected primarily by alteration of central and larger airway function. Further research exploring the clinical predictability of FEF25–75% in detecting early or smaller airway obstruction, especially in children, is warranted.
We feel that these findings are also generalizable to a non-high-risk cohort. Two of the major risk factors for childhood asthma are family history of asthma and allergies and early and persistent allergic sensitization to environmental allergens (10). It has been shown previously that nearly 50% of children between the ages of 6 and 9 are atopic (9). In the CCAAPS cohort, the frequency of SPT positivity to at least one aeroallergen (15 evaluated) is approximately 45%. Hence, these results are likely applicable to a large portion of children in the United States presenting with allergic sensitization. This study addresses the limitations of previous studies using questionnaire data rather than internal dose data for SHS exposure as it controls for potential sources of bias and confounding associated with parental reporting of smoking, passive smoking outside of the child’s home, and exposure misclassification. It is acknowledged that prenatal exposure to SHS and other sources of indoor/outdoor particulate matter is relevant to lung function and may be potential confounders. However, during univariate analyses, prenatal exposure to SHS, riding a school bus, and the use of a gas stove in the home, all according to parental report, were examined and were not associated with the pulmonary function outcomes at age 7 and therefore were not included in the models.
Based on our results, and those of others, gender and the extent of allergic sensitization are significant factors in susceptibility to SHS. Our study identified sensitized girls as being a high-risk group for the damaging effects of SHS on FEF25–75%. The discordant results on lung function deficits presented in the literature, however, complicate and highlight the complexity of the underlying mechanisms for gender differences in children exposed to SHS. It is likely that the multifarious relationship between SHS and pulmonary function loss among boys and girls is ultimately dependent on not only timing of exposure but also the child’s ‘total load’ in relationship to cumulative risks (i.e., exposures + allergic sensitization + asthma status + genetic susceptibility + sex hormones). Hence, it is overdue for researchers to extend analyses to include these more complex interactions between exposures and multiple simultaneous determinants to fully understand a child’s risk for lung damage especially during the early stages of lung development.
Acknowledgments
The authors thank Bridget Whitehead, Christopher Schaffer, and the clinic staff for their efforts in study coordination, recruitment, data management, and data collection. We also thank all of the CCAAPS families for their time and commitment.
References
- 1.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the US population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114:853–8. doi: 10.1289/ehp.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–46. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 3.Stocks J, Dezateux C. The effect of parental smoking on lung function and development during infancy. Respirology. 2003;8:266–85. doi: 10.1046/j.1440-1843.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 4.Pinkerton KE, Joad JP. Influence of air pollution on respiratory health during perinatal development. Clin Exp Pharmacol Physiol. 2006;33:269–72. doi: 10.1111/j.1440-1681.2006.04357.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Joad JP, Abel K, et al. Effects of environmental tobacco smoke on the developing immune system of infant monkeys. J Allergy Clin Immunol. 2007;120:445–51. doi: 10.1016/j.jaci.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Li Y-F, Gilliland FD, Berhane K, et al. Effects of in utero and environmental tobacco smoke exposure on lung function in boys and girls with and without asthma. Am J Respir Crit Care Med. 2000;162:2097–104. doi: 10.1164/ajrccm.162.6.2004178. [DOI] [PubMed] [Google Scholar]
- 7.Dong G, Wang D, Yang Z, et al. Gender-specific differences in effects of prenatal and postnatal environmental tobacco smoke exposure on respiratory symptoms in 23,474 children with and without allergic predisposition: results from 25 districts of northeast China. Int J Environ Health Res. 2011;21:173–88. doi: 10.1080/09603123.2010.515673. [DOI] [PubMed] [Google Scholar]
- 8.Carlsten C, Brauer M, Dimich-Ward H, Dybuncio A, Becker AB, Chan-Yeung M. Combined exposure to dog and indoor pollution: incident asthma in a high-risk birth cohort. Eur Respir J. 2011;37:324–30. doi: 10.1183/09031936.00187609. [DOI] [PubMed] [Google Scholar]
- 9.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Sly PD. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol. 2011;11:24–8. doi: 10.1097/ACI.0b013e328342309d. [DOI] [PubMed] [Google Scholar]
- 11.Ryan PH, LeMasters G, Biagini J, et al. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol. 2005;116:279–84. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Huang Y, Lin X, et al. Influence of degree of specific allergic sensitivity on severity of rhinitis and asthma in Chinese allergic patients. Respir Res. 2011;12:95. doi: 10.1186/1465-9921-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan PH, Lemasters GK, Biswas P, et al. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect. 2007;115:278–84. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan PH, Lemasters GK, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404:139–47. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 16.Langone JJ, Gjika HB, Van Vunakis H. Nicotine and its metabolites. Radio-immunoassays for nicotine and cotinine. Biochemistry. 1973;12:5025–30. doi: 10.1021/bi00748a032. [DOI] [PubMed] [Google Scholar]
- 17.Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- 18.Illi S, von ME, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 19.Singh SP, Mishra NC, Rir-Sima-Ah J, et al. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol. 2009;183:2115–21. doi: 10.4049/jimmunol.0900826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusunoki T, Hosoi S, Asai K, et al. Relationships between atopy and lung function: results from a sample of one hundred medical students in Japan. Ann Allergy Asthma Immunol. 1999;83:343–7. doi: 10.1016/S1081-1206(10)62676-2. [DOI] [PubMed] [Google Scholar]
- 21.Holt PG, Jones CA. The development of the immune system during pregnancy and earl life. Allergy. 2000;55:688–97. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 22.de Jong AB, Dikkeschei LD, Brand PL. Sensitization patterns to food and inhalant allergens in childhood: a comparison of non-sensitized, monosensitized, and polysensitized children. Pediatr Allergy Immunol. 2011;22:166–71. doi: 10.1111/j.1399-3038.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Rennie DC, Lockinger LA, Dosman JA. Gender, environmental tobacco smoke, and pulmonary function in rural children and adolescents: the Humboldt study. J Agric Saf Health. 2005;11:167–73. doi: 10.13031/2013.18183. [DOI] [PubMed] [Google Scholar]
- 24.Demissie K, Ernst P, Joseph L, Becklake MR. The role of domestic factors and day-care attendance on lung function of primary school children. Respir Med. 1998;92:928–35. doi: 10.1016/s0954-6111(98)90192-5. [DOI] [PubMed] [Google Scholar]
- 25.Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335:931–7. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- 26.Gray SH, Ebe LK, Feldman HA, et al. Salivary progesterone levels before menarche: a prospective study of adolescent girls. J Clin Endocrinol Metab. 2010;95:3507–11. doi: 10.1210/jc.2010-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Windham GC, Bottomley C, Birner C, Fenster L. Age at menarche in relation to maternal use of tobacco, alcohol, coffee, and tea during pregnancy. Am J Epidemiol. 2004;159:862–71. doi: 10.1093/aje/kwh117. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell VL, Gershwin LJ. Progesterone and environmental tobacco smoke act synergistically to exacerbate the development of allergic asthma in a mouse model. Clin Exp Allergy. 2007;37:276–86. doi: 10.1111/j.1365-2222.2007.02658.x. [DOI] [PubMed] [Google Scholar]
- 29.Marseglia GL, Cirillo I, Vizzaccaro A, et al. Role of forced expiratory flow at 25–75% as an early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007;28:74–8. doi: 10.2500/aap.2007.28.2920. [DOI] [PubMed] [Google Scholar]
- 30.Simon MR, Chinchilli VM, Phillips BR, et al. Forced expiratory flow between 25% and 75% of vital capacity and FEV1/ forced vital capacity ratio in relation to clinical and physiological parameters in asthmatic children with normal FEV1 values. J Allergy Clin Immunol. 2010;126:527–34. doi: 10.1016/j.jaci.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]