Abstract
Background:
Exposure to whole body vibration exercises (WBVE), besides some biological effects, causes alterations in the concentration of some blood biomarkers. The aim of this study is to evaluate the action of vibration (10 Hz) of WBVE on the concentration of blood biomarkers in Wistar rats.
Materials and Methods:
Wistar rats were divided in 2 groups. The experimental group (EG) was subjected to vibrations of 10Hz (one min per day, one week, total time of seven min), while the control group (CG) has not experienced vibration. Samples of whole blood were drawn for biochemical analysis of the concentration of total cholesterol, triglycerides, HDL, LDL, VLDL, glucose, CPK, albumin, alkaline phosphates, TGP, TGO, γGT, lipase, amylase, urea and creatinine.
Results:
White blood cell count and a platelet-hemogram were also performed. Significant (p<0.05) increase in TGP, TGO and white blood cells and decrease in LDL concentration was found after exposure of 10Hz mechanical vibration.
Conclusion:
Although these findings were obtained with rats, they might contribute to try to understand better these mechanisms that occur following exposure to a frequency of 10Hz.
Keywords: whole-body vibration, biomarkers, Wistar rats, oscillating/vibratory platform, mechanical vibration
Introduction
Exposure to vibration generated in oscillating/vibratory platforms (OVP) promotes whole body vibration (WBV) exercises in a subject that is in direct contact with the base of the platform (Rittweger, 2010).
The interest in the use of these WBV generated by OVP is growing in various fields of the Health Sciences, as a form of a non-pharmacological intervention to achieve therapeutic, preventative and/or physical performance goals. During the WBV exercises, in general, a subject normally stands on the base of the platform in a static position or performing dynamic exercise (Cochrane, 2011; Prisby et al. 2008; Rauch et al. 2010; Rittweger, 2010).
Investigations have shown that the treatment with WBV increases leg muscle force, power, rate of force development and movement velocity (Bosco et al. 2000; Cochrane et al. 2010). Moreover, provide significant improvements in the functional capacity in severe chronic obstructive pulmonary disease (Gloeckl et al. 2015; Pleguezuelos et al. 2013). WBV has also been associated with improvements in physical/functional parameters in various populations including healthy individuals and persons with neuromuscular conditions, as in Parkinson’s disease, stroke, multiple Sclerosis and cerebral palsy (Prisby et al. 2008; Rittweger, 2010; Sá-Caputo et al. 2016; Santos-Filho et al. 2012; Saquetto et al. 2015) and in patients with osteogenesis imperfecta (Semler et al. 2008). Significant increase in bone mineral density has been also reported in several clinical situations (Prisby et al. 2008; Rittweger, 2010; Sá-Caputo et al. 2016; Totosy et al. 2009). Pinto et al. (2011) have shown that WBV to be effective in reducing delayed onset muscle soreness and stiffness following running in an untrained athlete; and in patients with neurological disorders, a temporary decrease in spasticity has also been reported (Ahlborg et al. 2006; Haas et al. 2006; Sharififar et al. 2014).
Despite the positive effects of WBV, undesirable side effects of these exercises can occur and have been reported. Crewther et al. (2004) observed in untrained participants exposed to acute vibration, hot feet, itching of the lower limbs, vertigo and severe hip discomfort. Cronin et al. (2004) reported also in untrained participants, pain of jaw, neck and lower limbs from an acute intermittent WBV. Monteleone et al. (2007) reported a case of significant morbidity following one session of WBV in a patient with asymptomatic nephrolithiasis. Franchignoni et al. (2013) have reported that a healthy elite athlete suffered two episodes of hematuria after WBV training.
The desirable and undesirable effects of WBV have stimulated investigations with animals. In some experimental models, Naghii et al. (2012) have studied the effect of consumption of fatty acids and selected nutrients, along with regular WBV (10-50Hz), on cardiovascular disease (CVD) risk factors. The findings show that WBV is effective in improving health status by influencing CVD risk factors. Frederico et al. (2014) have verified, significantly (p<0.05), that WBV with mechanical vibration with 12 Hz induced in rats (i) an increase in the uptake of the radiopharmaceutical 99mTc-sodium pertechnetate in the spleen, (ii) a reduction in the plasma concentration of cholesterol, triglyceride, bilirubin and (iiii) an increase of the plasma concentration of alkaline phosphatase and creatine kinase (CK). Moreover, Pereira et al. (2013) have shown that, in rats, the exposure to mechanical vibration (20 Hz) can alter the uptake of the 99mTc-methylenediphosphonate in stomach, bowel, kidneys, urinary bladder and prostate. It was also demonstrated that WBV applied at increasing accelerations enhanced trabecular bone volume in a non-dose-dependent fashion as assessed by histo-morphometry in the proximal tibia of adult mice (Christiansen et al. 2006). Rubin et al. (2001) observed a relevant increase in femoral trabecular bone mass in adult ewes following 1 year of vibration as compared to controls. Monteiro et al. (2011) have shown that the vibration generated by oscillating/vibratory platform is capable of interfering with the osmotic fragility of samples of whole blood that were isolated from rats.
Studies about the effect of mechanical vibrations in the concentrations of some biomarkers in human beings have been reviewed. Important papers have been published, as an increase in testosterone and growth hormone and a decrease in cortisol were found (Bosco et al. 2000; Kvorning et al. 2006; Cardinale et al. 2010; Santos-Filho et al. 2011) and changes in the fatty acid concentrations (Goto et al. 2005), as well as change in blood glucose levels (Behboudi et al. 2011). An increase of epinephrine and norepinephrine was also reported (Di Loreto et al., 2004). Theodorou et al. 2015 have performed, in human, blood chemistry measurements (hematology, CK, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, C-reactive protein, glucose, insulin, triacylglycerols, total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), apolipoprotein A1, apolipoprotein B and lipoprotein, thiobarbituric-acid reactive substances, protein carbonyls, total antioxidant capacity, uric acid, albumin and bilirubin) that were assessed pre-exercise and post-exercise at the first and eighth week of WBV exercise with 20-25 Hz in both control and vibration groups. Naghii et al. (2011) have verified in male rats submitted to vibrations with frequencies of 10-50 Hz significant differences in plasma levels of CK, estradiol-2, and interleukin (IL)-6 between the vibration and control group. The mean of vitamin D level was 15% higher IL-6 level was 32% higher in vibration group. Naghii and Hedayat, 2013 have reported a significant difference (P<0.05) only in plasma levels of xanthine oxidase between the vibration and control group. Nevertheless, Pawlak et al. (2013 have submitted Wistar rats for three (3 months) and six months (6 months) to four bouts lasting of 30 s and resting for 1 min. Blood was collected and red and white blood cells, lymphocytes, monocytes, granulocytes, hemoglobin, and hematocrit, as well as IL-1b, IL-10, IL-6, and vascular endothelial growth factor levels were determined. No significant differences between 3 months, months, and the control groups in complete blood counts or in immunological parameters were found. Monteiro et al., 2015 have demonstrated that the rats exposed to short period of WBV of 20 Hz can reduce the plasma level of gamma glutamyl transpetidase and of very low-density protein (VLDL).
Rauch et al. (2010) have pointed out that several parameters can be controlled in the investigations about the effect of the WBV exercise, as the frequency and the peak to peak displacement of the mechanical vibration. Miyazaki (2000) has evaluated the electrogastrography (EGG) in healthy male volunteers’ exposure to vibration of 4, 8 and 16 Hz. This author has observed that only the vibrations of 4 and 8 Hz have decreased the amplitude of the EGG.
Putting together all the information cited, the aim of this investigation is to determine the effect of 10Hz vibration generated by an oscillating/vibratory platform on various biochemical and blood markers in Wistar rats.
Materials and Methods
Ethical approval
All the experimental procedures have followed the Ethical Guidelines of the Instituto de Biologia Roberto Alcantara Gomes, Universidade do Estado do Rio de Janeiro, Brazil, with the protocol number CEA/024/2009. A Veterinary Physician was always present during the experiment.
Characteristics of the oscillating/vibratory platform
The platform used in the experiment was an oscillating system (Novaplate fitness evolution, DAF, Produtos Hospitalares Ltda, São Paulo) with reciprocating vertical displacements on the left and right side of a fulcrum. It is a side-alternating vibration device working as a teeterboard (28 cm × 58 cm) with amplitude of 0 mm in the center of the platform up to the maximum in the edge that was 7.07 mm.
Study design
As reported by several authors (Behboudi et al. 2011; Bosco et al. 2000; Cardinale et al. 2010; Goto et al. 2005; Kvorning et al. 2006; Sá-Caputo et al. 2015; Santos-Filho et al. 2011), the WBV can have influence in various pathways of the metabolism of various organs. The selection of the biomarkers in this investigation has followed this consideration. The biomarkers evaluated in the control group (CG) and in the experimental group (EG) were: total cholesterol, triglycerides, HDL, LDL, VLDL (very low density lipoprotein), glucose, CK, albumin, alkaline phosphatase, GPT (glutamic pyruvic transaminase), GOT (glutamic oxaloacetic transaminase), γGT (gamma glutamyl transpeptidase), lipase, amylase, urea, creatinine. The effect of vibration on the number of white blood cells and platelets, and in the percentage of neutrophils, lymphocytes, monocytes, eosinophils and basophils were also investigated.
Experimental procedure
Eight Wistar rats, all male, 3-4 months of age, weighing between 260-370g were used in this study. The rats were kept under the same environmental conditions (25±2°C, 12h of light/dark cycle), given water ad libitum and fed a normal diet. The rats were sedated (sodium thiopental, 50mg/kg/weight) and divided in two groups. They were positioned one on each side of the platform and had its head fixed with tape resting on a makeshift gauze pillow, and there was a small difference in the peak-to-peak displacements that were 1.5 and 3.8 mm considering the center line of the rats (Monteiro et al., 2015). The animals of the EG were submitted to 10 Hz for one min per day for one week (seven sessions) with a total of seven minutes of WBV. The frequency of 10 Hz has been used in some publications (Naghii et al. 2012; Naghii et al. 2011). The duration of the WBV sessions is highly among the various publications using rats (Frederico et al. 2014; Pawlak et al. 2013; Naghii et al. 2012; Naghii et al. 2011). Four animals were designated to be in the CG and they were not submitted to the vibration, however, they were closed of the platform. In consequence, they were submitted to the same stress conditions. In this investigation was followed the protocol previously published by Monteiro et al. (2015).
Blood Samples
As all the rats were under controlled conditions since they were born, no baseline evaluation was performed. Just after completing the one week intervention protocol, heparinized (heparin 4%) whole blood from the animals of both groups was drawn (about 4ml/animal) by cardiac puncture (under anesthesia). The concentration of selected biomarkers was then determined in a clinical laboratory of the Universidade do Estado do Rio de Janeiro. The measurements were performed in automated equipment (COBAS INTEGRA 400 plus, Roche, Basel, Switzerland).
Statistical analysis
Although statistical analysis was used to verify possible differences between of the two groups of rats (control and experimental groups), as an option, ANOVA variance analysis test following by Bonferroni test was performed to determine possible differences between the CG and EG. As these rats are a homogenous group, bred and exposed to the same environmental and stress conditions the post-test measurements allow for the assumption that the change in concentrations seen in the blood can be attributed to the effect of the intervention. Data are presented as mean ± standard deviation (±SD). Statistical significance was accepted at p<0.05. Absolute effect sizes, d, were analyzed to determine the magnitude of an effect independent on sample size. Effect sizes were analyzed to determine the magnitude of an effect independent of sample size. Cohen´s effect sizes (d) were measured by the formula.
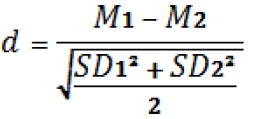
SD are the standard deviations. M are the values of the media and 1 represents the biggest value and 2 the smallest value.
Small effect sizes are considered d ≤ 0.2, moderate effect sizes are 0.2< d <0.8, and large effects sizes are d ≥ 0.8 (Cohen, 1988).
Results
The exposition of rats to mechanical vibration produced in oscillating/vibratory platform promoted a variety of outcome on the plasma concentration of biochemical and biomarkers. The effects of 10 Hz vibration exposure are summarized in Table 1 for biomarkers and some of them are related to the liver function. The GPT and GOT concentrations were significantly increased (p<0.05) and the CK concentration was significantly decreased (p<0.05) in the EG compared to the CG with a large effect size. Glucose, albumin, γGT, and alkaline phosphatase concentrations were not significantly different between the studied groups.
Table 1.
Effects of vibration of 10 Hz on the level of some biomarkers (some related to the liver function) in the blood of rats
| Biomarker | CG | EG | p | Effect size |
|---|---|---|---|---|
| Glucose (mmol/L) | 8.00±1.72 | 7.94±1.57 | 0.9573 | 0.03 |
| CK (UI/h) | 617.2±196.26 | 337.00±42.42 | 0.0315 | 1.97 |
| Albumin (g/dl) | 3.30±0.20 | 3.70±0.89 | 0.4142 | 0.62 |
| Alkaline phosphatase (U/ml) | 120.1±20.1 | 140.3±45.62 | 0.4486 | 0.57 |
| TGP (UI/I) | 58.8±21.04 | 88.5±2.12 | 0.0308 | 1.98 |
| TGO (UI/I) | 124.8±45.9 | 458.0±53.74 | 0.0001 | 6.66 |
| γGT (UI/I) | 6.7±2.5 | 6.3±1.09 | 0.7791 | 0.20 |
CG - control group, EG - group submitted to vibration
Table 2 shows the effects of 10 Hz vibration exposure on the levels of biomarkers related to lipid metabolism. The LDL concentrations decreased significantly (p<0.05) in the EG with a large effect size. No significant differences were found between the two groups for total cholesterol, triglycerides, HDL and VLDL concentrations.
Table 2.
Effects of vibration of 10 Hz on the values of some plasma biomarkers related to the lipid metabolism in the blood of rats
| Biomarker | CG | EG | p | Effect size |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 43.16±11.30 | 46.67±6.81 | 0.6138 | 0.36 |
| Triglycerides (mg/dl) | 21.33±13.96 | 24.00±14.14 | 0.7971 | 0.19 |
| HDL (mg/dl) | 40.83±9.98 | 40.67±4.51 | 0.9776 | 0.02 |
| LDL (mg/dl) | 1.50±0.98 | 0.20±0.001 | 0.0379 | 1.87 |
| VLDL (mg/dl) | 4.46±1.15 | 4.80±2.82 | 0.8307 | 0.157 |
CG - control group, EG - group submitted to vibration
As a result of the exposure to 10 Hz mechanical vibrations, for measures of biomarkers related to kidney and pancreas functions no significant (p>0.5) differences between the EG and CG were found (Table 3). Nevertheless, a large effect size was observed to the finding with lipase.
Table 3.
Effects of the vibration of 10 Hz on the biochemical values of some biomarkers related to kidney and pancreas functions
| Biomarker | CG | EG | P | Effect size |
|---|---|---|---|---|
| Lipase (µg/ml) | 4.95±0.75 | 6.40±2.19 | 0.2569 | 0.88 |
| Amylase (µg/l) | 2360.16±265.21 | 2798.33±740.81 | 0.3080 | 0.78 |
| Urea (mg/ml) | 54.83±9.02 | 54.00±10.00 | 0.9059 | 0.08 |
| Creatinine (mg/ml) | 0.36±0.08 | 0.40±0.10 | 0.5552 | 0.44 |
CG - control group, EG - group submitted to vibration
Table 4 shows the effect of 10Hz vibration exposure on the concentration of some blood cells and platelets in rats. The concentration of white blood cells increased significantly in the EG (p<0.05) with a strong large effect size. However, no alterations were found in the concentrations of the other evaluated cellular structures of the blood, and a large effect size was found to the concentrations of basophils and platelets.
Table 4.
Effects of vibrations of 10 Hz on the number of white blood cells and platelets in the blood of rats
| Cell type | CG | EG | P | Effect size |
|---|---|---|---|---|
| WBC (109/L) | 4353±359 | 8000±127 | 0.0001 | 13.54 |
| Neutrophils (%) | 2.60±0.91 | 2.89±0.36 | 0.6969 | 0.41 |
| Lymphocytes (%) | 5.00±2.38 | 5.02±0.30 | 0.9872 | 0.01 |
| Monocytes (%) | 0.032±0.014 | 0.047±0.037 | 0.4770 | 0.53 |
| Eosinophils (%) | 0.037±0.019 | 0.035±0.009 | 0.8554 | 0.13 |
| Basophils (%) | 0.071±0.04 | 0.041±0.028 | 0.2651 | 0.83 |
| Platelets (103cells/ µl) | 922±152 | 1074±86 | 0.1324 | 1.23 |
CG - control group, EG - group submitted to vibration
Discussion
The findings of this investigation indicate that the exposition to mechanical vibration generated in oscillating/vibratory platform at 10 Hz promoted physiological disturbances. In consequence, the concentrations of biochemical and other biomarkers in the blood of rats exposed to the mechanical vibration for 1 min/day for seven days. This discussion focuses these effects in physiological alterations due to influence of the frequency that is a biomechanical parameter.
Clark et al. (2003) and Goyal et al. (2014) have suggested that elevated activities of the two serum transaminases, GPT and GOT are commonly associated with liver disease. Pettersson et al., (2008) showed that heavy weight training in healthy men affected liver functions. Monteiro et al. (2015) in a similar investigation with mechanical vibration with 20 Hz did not find alteration in these enzymes, but they found alteration in another enzyme related with the liver function, the gamma glutamyl transpeptidase. The increase of the concentrations of GPT and GOT found in this study (Table I) suggests that the 10Hz mechanical vibration may be effective in promoting effects associated with physiological disturbances in the liver.
High levels of serum CK have been associated with swelling and muscle soreness experienced in (untrained) athletes (Baird et al. 2012). Similar findings were reported by Gojanovic et al. (2011) who found that five participants (25%) who took part in a training program also showed a significant increase in post-exercise CK levels (> double baseline concentrations). Naghii et al. (2011) have reported in male rats submitted to vibrations with frequencies of 10-50 Hz, the plasma levels of CK were significantly higher in the animals of the vibration in comparison with the controls. Blood measurement of CK in subjects that have participated in an investigation of acute and chronic WBV with 20-25 Hz was not altered (Theodorou et al., 2015). Our findings (Table 1) showed that vibration of 10 Hz decreased the plasma CK levels. Similarly, Lin et al. (2015), in mice, have reported that WBV decreased the plasma level of CK due to vibration (5.6 and 13 Hz) after swimming test. It could be hypothesized that lower frequencies (in this case 10 Hz for one week) can assist in maintaining, at least in part, the integrity of the functions of the organs or tissues related to CK.
Behboudi et al. (2011) have reported that the WBV exercises could reduce the glucose levels in patients with diabetes mellitus type 2. Our findings with rats indicate (Table I) a no significant alteration in the concentration of this biomarker in the blood of rats; and they are in agreement with Di Loreto et al. (2004) that reported that vibration at 30 Hz of frequency resulted in no significant reduction in blood glucose levels in patients with DM-2. Theodorou et al. (2015) have also not found alteration in the level of glucose after WBV exercise with 20-25 Hz. Lin et al. (2015), in mice, have reported that WBV did not alter the plasma level of glucose due to vibration (5.6 and 13 Hz).
The concentration of the alkaline phosphatase was not altered with vibration with 10 Hz (Table 1). Naghii et al. (2011) have reported the same finding with vibration of 10-50Hz in animals, as well as, Monteiro et al. (2015) in a study with 20 Hz mechanical vibration.
The effects of 10 Hz vibration on the levels of some biomarkers of lipid metabolism in the blood were varied (Table 2). While the concentration of the LDL decreased significantly, triglycerides, total cholesterol, VLDL and HDL concentrations were not affected by the vibration exposure. Naghii et al. (2011) have determined the plasma lipid concentrations (total cholesterol, LDL and HDL) in rats submitted to vibrations in the frequencies of 10-50 Hz and they did not found alteration in the concentrations of these biomarkers. However, Monteiro et al. (2015) have observed a reduction in the VLDL cholesterol, while the other biomarkers of lipid metabolism were not altered in rats submitted to 20 Hz. The consideration of these finding of the current investigation might be important mainly in an obese population, due to the importance of the VLDL plasma level.
No significant differences were found regarding the urea and creatinine concentrations following exposure to 10Hz vibration (Table 3) was found. This same result was reported by Monteiro et al. (2015) to these biomarkers with 20 Hz mechanical vibration. Naghii and Hedayati (2013) have also reported that the concentration of uric acid was not altered in rats exposure to vibrations with 10-50 Hz. At least, considering these biochemical markers (Gowda et al. 2010), the vibrations in these frequencies are not capable in interfering in the renal function.
Concerning to the concentrations of blood elements, vibration of 10 Hz significantly did not have effect to neuthrophilis, eosinophils, basophils, monocytes and platelets. However, significant increase in the concentration of white blood cells was found (Table 4). Previously, Monteiro et al., 2015 have reported that in the blood, after exposure at 20 Hz vibration for one week, the number of leukocytes increased significantly (Table 4). Pawlak et al. (2013) in an investigation with rats exposed to mechanical vibration of 50 Hz did not find alterations in the concentrations of leucocytes, lymphocytes, monocytes, granulocytes, hemoglobin, and hematocrit, as well as IL-1b, IL-10, IL-6, and vascular endothelial growth factor levels in comparison to control group. As the frequencies used in our investigations (10 Hz) and in the study of Pawlak et al. (2013) were different, further investigation into optimal frequencies is warranted. This notion is also supported by Garman et al., 2007 who suggested that the enhancements in BFR/BS and MS/BS reported in their study were apparently acceleration-dependent. In addition, the findings reported by Tossige-Gomes et al. (2012) in which is described that the proliferative response of TCD4+ cells showed a significant decrease in the WBV group compared to the control group, while there was no difference between groups regarding the proliferative response of TCD8+ cells.
The findings reported in this investigation could be suitable to bring proper conditions to aid to lead to recommendations to protocols, at least concerning the length-in-time treatment and the used frequencies.
A limitation of this study is due to the absence of a baseline to the values of the concentrations of the biomarkers. However, all the rats used were a homogenous group, bred and exposed to the same environmental and stress conditions. Another limitation was the small number of animals in each group, however, it was followed the criteria described by de Boo and Hendriksen (2005). The action mechanism associated with the effects of the vibrations generated in oscillating/vibratory is highly complex and seem to be associated with several parameters, and the frequency of the vibration would be important in generating biological effects (Prisby et al. 2008; Rauch et al. 2010; Rittweger, 2010; Totosy et al. 2009). A comparison of results presented in this study with the findings reported by other authors has revealed what is necessary in further investigations. It would be important to consider in the investigations various controlled conditions to try to better understand the physiological mechanisms and to establish a relationship with several parameters, including frequency, length of the study and peak-to-peak displacement. In addition, it is clear that the protocols used in the investigations with animals were different (Frederico et al. 2014; Pawlak et al. 2013; Naghii et al. 201; Naghii et al. 2011).
In conclusion, when compared to other findings in the literature, it becomes evident that determination of the concentration of the studied biomarkers may be an important aspect to be considered when investigating biological effects of the vibrations generated in the oscillating/vibratory platform. Although it is an investigation with rats, the data are important and the findings are of potential relevance to the clinic. These results might contribute to understand better these mechanisms that occur following exposure to a frequency of 10Hz. Further studies with various protocols, including the same type of platform, frequency of the vibration, number of bouts, time of the bouts, are ongoing to better understand these physiological effects related to the effect these vibrations generated in the oscillating/vibratory platform.
Acknowledgments.
The authors thank the Conselho Nacional de Desenvolvimento e Pesquisa (CNPq), the Fundação Carlos Chagas de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Universidade do Estado do Rio de Janeiro (UERJ) for the financial support.
The authors also thank the PhD student Adriano Arnóbio for the statistical analysis.
References
- Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training:effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J. Rehabil Med. 2006;38:302–308. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- Baird M.F, Graham S.M, Baker J.S, Bickerstaff G.F. Creatine-Kinase - and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012;2012:960363. doi: 10.1155/2012/960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboudi L, Azarbayjani M.A, Aghaalinejad H, Salavati M. Effects of aerobic exercise and whole body vibration on glycaemia control in type 2 diabetic males. Asian J Sports Med. 2011;2:83–90. doi: 10.5812/asjsm.34789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco C, Iacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, Viru M, De Lorenzo A, Viru A. Hormonal responses to whole-body vibration in men. Eur. J. Appl Physiol. 2000;81:449–454. doi: 10.1007/s004210050067. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Soiza R.L, Leiper J.B, Gibson A, Primrose W.R. Hormonal responses to a single session of whole body vibration exercise in older individuals. Br. J. Sports Med. 2010;44:284–288. doi: 10.1136/bjsm.2007.043232. [DOI] [PubMed] [Google Scholar]
- Christiansen B.A, Silva M.J. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann. Biomed Eng. 2006;34:1149–1156. doi: 10.1007/s10439-006-9133-5. [DOI] [PubMed] [Google Scholar]
- Clark J.M, Brancati F.L, Diehl A.M. The prevalence and etiology of elevated aminotransferase levels in the United States. Am. J. Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- Cochrane D.J. Vibration exercise:the potential benefits. Int. J. Sports Med. 2011;32:75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- Cochrane D.J, Stannard S.R, Firth E.C, Rittweger J. Acute whole-body vibration elicits post-activation potentiation. Eur. J. Appl Physiol. 2010;108:311–319. doi: 10.1007/s00421-009-1215-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, London: Lawrence Earlbaum Associates Publisher; 1988. [Google Scholar]
- Crewther B, Cronin J, Keogh J. Gravitational forces and whole body vibration:implications for prescription of vibratory stimulation. Phys. Ther. Sport. 2004;5:37–43. [Google Scholar]
- Cronin J.B, Oliver M, McNair P.J. Muscle stiffness and injury effects of whole body vibration. Phys. Ther. Sport. 2004;5:68–74. [Google Scholar]
- de Boo J, Hendriksen C. Reduction strategies in animal research:a review of scientific approaches at the intra-experimental, supra experimental and extra-experimental levels. Altern. Lab. Anim. 2005;33:369–377. doi: 10.1177/026119290503300404. [DOI] [PubMed] [Google Scholar]
- Di Loreto C, Ranchelli A, Lucidi P, Murdolo G, Parlanti N, De Cicco A, Tsarpela O, Annino G, Bosco C, Santeusanio F, Bolli G.B, De Feo P. Effects of whole-body vibration exercise on the endocrine system of healthy men. J. Endocrinol Invest. 2004;27:323–327. doi: 10.1007/BF03351056. [DOI] [PubMed] [Google Scholar]
- Franchignoni F, Vercelli S, Ozçakar L. Hematuria in a runner after treatment with whole body vibration:a case report. Scand. J. Med. Sci. Sports. 2013;23:383–385. doi: 10.1111/j.1600-0838.2012.01478.x. [DOI] [PubMed] [Google Scholar]
- Frederico E.H.F.F, Carmo F.S, Arnóbio A, Guedes S.S.V, Sá-Caputo D.C, Bernardo L.C, Guimarães C.A.S, Asad N.R, Bernardo-Filho M. Does the whole body vibration alter the effect of a coriandrum sativum extract on the biodistribution of the radiopharmaceutical technetium-99m sodium pertechnetate and some biomarkers in wistar rats? Inter. J. Pharm. Sci. Res. 2014;5:3529–3535. [Google Scholar]
- Garman R, Gaudette G, Donahue L.R, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- Gloeckl R, Heinzelmann I, Kenn K. Whole body vibration training in patients with COPD:A systematic review. Chron. Respir Dis. 2015;12:212–221. doi: 10.1177/1479972315583049. [DOI] [PubMed] [Google Scholar]
- Gojanovic B, Feihl F, Liaudet L, Gremion G, Waeber B. Whole-body vibration training elevates creatine kinase levels in sedentary subjects. Swiss. Med Wkly. 2011;141:w13222. doi: 10.4414/smw.2011.13222. [DOI] [PubMed] [Google Scholar]
- Goto K, Takamatsu K. Hormone and Lipolytic responses to whole body vibration in young men. Jpn. J. Physiol. 2005;55:279–284. doi: 10.2170/jjphysiol.RP000305. [DOI] [PubMed] [Google Scholar]
- Gowda S, Desai P.B, Kulkarni S.S, Hull V.V, Math A.A, Vernekar S.N. Markers of renal function tests American. N. Am. J. Med Sci. 2010;2:170–173. [PMC free article] [PubMed] [Google Scholar]
- Goyal V, Chugh K, Agrawal Y. Association of serum glutamic pyruvic transaminase and non-alcoholic fatty liver disease in controlled and uncontrolled diabetes. J Heath Specialities. 2014;2:169–173. [Google Scholar]
- Haas C.T, Turbanski S, Kessler K, Schmidtbleicher D. The effects of random whole-body-vibration on motor symptoms in Parkinson’s disease. NeuroRehabilitation. 2006;21:29–36. [PubMed] [Google Scholar]
- Kvorning T, Bagger M, Caserotti P, Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur. J. Appl. Physiol. 2006;96:615–625. doi: 10.1007/s00421-006-0139-3. [DOI] [PubMed] [Google Scholar]
- Lin C.I, Huang W.C, Chen W.C, Kan N.W, Wei L, Chiu Y.S, Huang C.C. Effect of whole-body vibration training on body composition, exercise performance and biochemical responses in middle-aged mice. Metabolism. 2015;64:1146–1156. doi: 10.1016/j.metabol.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y. Adverse effects of whole-body vibration on gastric motility. Kurume. Med J. 2000;47:79–86. doi: 10.2739/kurumemedj.47.79. [DOI] [PubMed] [Google Scholar]
- Monteiro M.O, Sá-Caputo D.C, Carmo F.S, Bernardo R.M, Pacheco R, Arnóbio A, Guimarães C.A, Bernardo L.C, Santos-Filho S.D, Asad N.R, Unger M, Marin P.J, Bernardo-Filho M. Effects of short-period Whole-Body Vibration of 20 Hz on Selected Blood Biomarkers in Wistar Rats. Chin. J Physiol. 2015;58:211–218. doi: 10.4077/CJP.2015.BAD303. [DOI] [PubMed] [Google Scholar]
- Monteiro M.O.B, Pinto N.S, Marin P.J, Santos-Filho S.D, Bernardo-Filho M. Effect on osmotic fragility of red blood cells of whole blood submitted to vibrations in an oscillating platform. African J Biotechnol. 2011;10:14197–14202. [Google Scholar]
- Monteleone G, De Lorenzo A, Sgroi M, De Angelis S, Di Renzo L. Contraindications for whole body vibration training:a case of nephrolitiasis. J. sports med. Phys. fitness. 2007;47:443–445. [PubMed] [Google Scholar]
- Naghii M.R, Darvishi P, Ebrahimpour Y, Ghanizadeh G, Mofid M, Hedayati M, Asgari A.R. Effect of combination therapy of fatty acids, calcium, vitamin D and boron with regular physical activity on cardiovascular risk factors in rat. J. Oleo Sci. 2012;61:103–111. doi: 10.5650/jos.61.103. [DOI] [PubMed] [Google Scholar]
- Naghii M.R, Ghanizadeh G, Darvishi P, Ebrahimpour Y, Mofid M, Torkaman G, Asgari A.R, Hedayati M. Whole body vibration is a safe exercise training method and induces no impaired alterations on rat plasma parameters. Acta. Physiol Hung. 2011;98:442–448. doi: 10.1556/APhysiol.98.2011.4.7. [DOI] [PubMed] [Google Scholar]
- Naghii M.R, Hedayati M. Whole body vibration as a safe exercise training method induces no impaired alterations on rat plasma antioxidant biomarkers. Acta. Physiol Hung. 2013;100:321–328. doi: 10.1556/APhysiol.100.2013.009. [DOI] [PubMed] [Google Scholar]
- Pawlak M, Kaczmarek D, Nowak A, Krutki P. Low-volume whole-body vibration lasting 3 or 6 month does not affect biomarkers in blood serum of rats. Acta. Physiol Hung. 2013;100:48–53. doi: 10.1556/APhysiol.99.2012.003. [DOI] [PubMed] [Google Scholar]
- Pereira M.O, Pinto N.S, Monteiro M.O, Santos-Filho S.D, Carmo F.S, Diniz C.L, Marin P.J, Bernardo-Filho M. Influence of whole-body vibration on biodistribution of the radiopharmaceutical [99mTc] methylenediphosphonate in Wistar rats. Int. J. Radiat Biol. 2013;89:668–672. doi: 10.3109/09553002.2012.715790. [DOI] [PubMed] [Google Scholar]
- Pettersson J, Hindorf U, Persson P, Bengtsson T, Malmqvist U, Werkström V, Ekelund M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin Pharmacol. 2008;65:253–259. doi: 10.1111/j.1365-2125.2007.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto N.S, Monteiro M.B, Arthur A.P, Paiva D.N, Meyer P.F, Santos-Filho S.D, Marín P.J, Bernardo-Filho M. Effectiveness of a protocol involving acute whole-body vibration exercises in an adult and health individual with delayed-onset muscle soreness observed after running:a case report. J. Med. Med Sci. 2011;2:612–617. [Google Scholar]
- Pleguezuelos E, Pérez M.E, Guirao L, Samitier B, Costea M, Ortega P, González M.V, Del Carmen V.A, Ovejero L, Moreno E, Miravitlles M. Effects of whole body vibration training in patients with severe chronic obstructive pulmonary disease. Respirology. 2013;18:1028–1034. doi: 10.1111/resp.12122. [DOI] [PubMed] [Google Scholar]
- Prisby R.D, Lafage-Proust M.H, Malaval L, Belli A, Vico L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models:what we know and what we need to know. Ageing. Res Rev. 2008;7:319–329. doi: 10.1016/j.arr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, Roth J, Schoenau E, Verschueren S, Rittweger J. International Society of Musculoskeletal and Neuronal Interactions. Reporting whole-body vibration intervention studies:recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J. Musculoskelet Neuronal Interact. 2010;10:193–198. [PubMed] [Google Scholar]
- Rittweger J. Vibration as an exercise modality:how it may work, and what its potential might be. Eur. J. Appl Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- Rubin C.T, Sommerfeldt D.W, Judex S, Qin Y. Inhibition of osteopenia by low magnitude, high frequency mechanical stimuli. Drug. Discov Today. 2001;6:848–858. doi: 10.1016/s1359-6446(01)01872-4. [DOI] [PubMed] [Google Scholar]
- Sá-Caputo D.C, Costa-Cavalcanti R, Carvalho-Lima R.P, Arnóbio A, Bernardo R.M, Ronikeile-Costa P, Kutter C, Giehl P.M, Asad N.R, Paiva D.N, Pereira H.V, Unger M, Marin P.J, Bernardo-Filho M. Systematic review of whole body vibration exercises in the treatment of cerebral palsy. Brief report. Dev Neurorehabil. 2016;19:327–333. doi: 10.3109/17518423.2014.994713. [DOI] [PubMed] [Google Scholar]
- Sá-Caputo D.C, Marconi E.M, Cavalcanti R.G.C, Domingos L.L, Giehl P.M, Paiva D.N, Asad N.R, Marin P.J, Bernardo-Filho M. Alterations on the plasma concentration of hormonal and non hormonal biomarkers in human beings submitted to whole body vibration exercises. Sci. Res and Essays. 2015;10:287–297. [Google Scholar]
- Santos-Filho S.D, Cameron M.H, Bernardo-Filho M. Benefits of whole-body vibration with an oscillating platform for people with multiple sclerosis:a systematic review. Mult. Scler Int. 2012;2012:274728. doi: 10.1155/2012/274728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Filho S.D, Pinto N.S, Monteiro M.B, Arthur A.P, Misssailidis S, Marín P.J, Bernardo-Filho M. The Ageing, the decline of hormones and the whole-body vibration exercises in vibratory platforms:a review and a case report. Int. J. Med Sci. 2011;2:925–931. [Google Scholar]
- Saquetto M, Carvalho V, Silva C, Conceição C, Gomes-Neto M. The effects of whole body vibration on mobility and balance in children with cerebral palsy:a systematic review with meta-analysis. J. Musculoskelet Neuronal Interact. 2015;15:137–144. [PMC free article] [PubMed] [Google Scholar]
- Semler O, Fricke O, Vezyroglou K, Stark C, Stabrey A, Schoenau E. Results of a prospective pilot trial on mobility after whole body vibration in children and adolescents with osteogenesis imperfecta. Clin Rehabil. 2008;22:387–394. doi: 10.1177/0269215507080763. [DOI] [PubMed] [Google Scholar]
- Sharififar S, Coronado R.A, Romero S, Azari H, Thigpen M. The effects of whole body vibration on mobility and balance in Parkinson disease:a systematic review. Iran J. Med Sci. 2014;39:318–326. [PMC free article] [PubMed] [Google Scholar]
- Theodorou A.A, Gerodimos V, Karatrantou K, Paschalis V, Chanou K, Jamurtas A.Z, Nikolaidis M.G. Acute and Chronic Whole-Body Vibration Exercise does not Induce Health-Promoting Effects on The Blood Profile. J. Hum Kinet. 2015;46:107–118. doi: 10.1515/hukin-2015-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossige-Gomes R, Avelar N.C, Simão A.P, Neves C.D, Brito-Melo G.E, Coimbra C.C, Rocha-Vieira E, Lacerda A.C. Whole-body vibration decreases the proliferative response of TCD4(+) cells in elderly individuals with knee osteoarthritis. Braz. J. Med. Biol Res. 2012;45:1262–1268. doi: 10.1590/S0100-879X2012007500139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totosy de Zepetnek J.O, Giangregorio L.M, Craven B.C. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis:a review. J. Rehabil. Res Dev. 2009;46:529–542. doi: 10.1682/jrrd.2008.09.0136. [DOI] [PubMed] [Google Scholar]


