Abstract
Background:
Dengue is considered as an important arboviral disease. Safe, low-cost, and effective drugs that possess inhibitory activity against dengue virus (DENV) are mostly needed to try to combat the dengue infection worldwide. Medicinal plants have been considered as an important alternative to manage several diseases, such as dengue. As authors have demonstrated the antiviral effect of medicinal plants against DENV, the aim of this study was to review systematically the published research concerning the use of medicinal plants in the management of dengue using the PubMed database.
Materials and Methods:
Search and selection of publications were made using the PubMed database following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement).
Results:
Six publications met the inclusion criteria and were included in the final selection after thorough analysis.
Conclusion:
It is suggested that medicinal plants’ products could be used as potential anti-DENV agents.
Keywords: Dengue, arbovirus, medicinal plants, PubMed
Introduction
Dengue, chikungunya and zika are mosquito-borne arboviral diseases (Gubler, 2002). Dengue is considered one of the most important of these diseases (Gubler, 2002; Voge et al., 2013 WHO, 2016). Dengue is transmitted by the bite of the Aedes aegypti mosquito infected with one of the four dengue virus (DENV 1 - 4) serotypes (genus Flavivirus, family Flaviviridae) (Voge et al., 2013; Halstead, 2007; Gubler, 2002; Guzman and Isturiz, 2010; WHO, 2016). Infection with one DENV serotype produces only specific antibody against that serotype. When antibody from the first infection is neutralized, secondary infections by other serotypes can cause more serious infection (Leardkamolkarn et al., 2012). Although DENV-2 is known to be more lethal than other serotypes (Goel et al., 2004), some studies have revealed that primary infection with DENV-1 or DENV-3 always results in more dangerous disease than infection with the other serotypes (Guzman and Isturiz, 2010; Tang et al., 2012).
Dengue epidemic has become an important focus of international public health awareness. It is found mainly in tropical and sub-tropical regions of the world, mostly in urban and semi-urban areas (Gubler, 2002; Ahmad et al., 2011). This has made the epidemic difficult to control due to highly populated areas in cities (Abd Kadir et al., 2013).
According to World Health Organization (WHO), dengue is a febrile illness that affects infants, children and adults. The symptoms could appear from 3 up to 14 days after the infective bite. It is not transmitted directly from person-to-person and symptoms range from mild fever, to incapacitating high fever, with severe headache, pain behind the eyes, muscle and joint pain, and rash. There are currently no WHO recommendations for use of a dengue vaccine or any specific medicine to treat dengue. People who have dengue fever should rest, drink plenty of fluids and reduce the fever using paracetamol under medical assistance (WHO, 2016).
Safe, low-cost, and effective drugs that possess inhibitory activity against DENV are mostly needed to try to combat the dengue infection worldwide, especially in the most affected countries, which, in general, have limited resources (Goel et al., 2004; Klawikkan et al., 2011; Rothan et al., 2014). Natural products have been considered as an important alternative to manage several diseases, such as dengue (Rothan et al, 2014; Lee et al., 2013).
A medicinal plant, as a natural product, has been used to traditional healing (Okonkwo, 2012), and as treatment sources for various diseases (Orekhov and Ivanova, 2016; Agnaniet et al., 2016), due to their complex bioactive ingredients and rich source of pharmaceuticals (Hao et al., 2015; Tadtong et al., 2015). Similarly, many extracts and compounds obtained from medicinal plant with antiviral activity have been reported (Kitazato et al., 2007; Rebensburg et al., 2016; Wang et al., 2016). Moreover, in general and in comparison, to the synthetic drugs, natural products are less toxic and inexpensive (Babar et al., 2013).
As authors have demonstrated the antiviral effect of medicinal plants against DENV (Tang et al., 2012; Lee et al., 2013; Rothan et al, 2014), the aim of this study was to review systematically the published research concerning the use of medicinal plants in the management of dengue using the PubMed database. Considering the findings described in the literature, it is hypothesized an antiviral activity against DENV of some medicinal plants.
Materials and Methods
Search Strategy and Selection of the Studies
Systematic review of scientific studies, following guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA statement), was carried out. The PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) was systematically searched for experimental trials. The papers were searched on July 19th, 2016. The search was performed using the keywords (i) chikungunya, (ii) dengue, (iii) zika, (iv) “medicinal plants” and (v) “medicinal plants” and dengue.
Inclusion and exclusion criteria
A systematic selection of the publications was carried based on the following inclusion and exclusion criteria, as it is shown in the Table 1.
Table 1.
Exclusion and Inclusion criteria to select the articles
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Biological activity | Antiviral activity against DENV | Another approach |
| Study design | Experimental trials in vitro and/or in vivo (with medicinal plants) | Ethnobotanical and ethnopharmacological surveys, case reports, expert opinion or consensus statements |
| Language | Articles written in English | Articles written in a language different of English |
Results
Table 2 shows the number of publications found in the PubMed database involving the keywords that were searched. It is possible to verify a strong interest in studies involving “medicinal plants” with almost fifty-nine thousand papers, and about 0.040% of these articles are related to dengue. In addition, dengue has more publications than chikungunya and zika. Although, the number of publications with zika is the lowest in comparison with dengue and chikungunya, it is noted that 987 (87%) of the 1,134 publications with zika are in the last five years.
Table 2.
Number of publications comprising medicinal plants and arboviral diseases transmitted by Aedes aegypti mosquito (based on PubMed database).
| Keyword | Number of publication |
|---|---|
| Chikungunya | 2,914 |
| Dengue | 15,745 |
| “Medicinal plants” | 58,894 |
| “Medicinal plants” AND dengue | 24 |
| Zika | 1,134 |
According to the search strategy involving the keyword “medicinal plants” AND dengue yielded twenty-four articles. Six publications met the inclusion criteria and were select for the final review (Figure 1).
Figure 1.
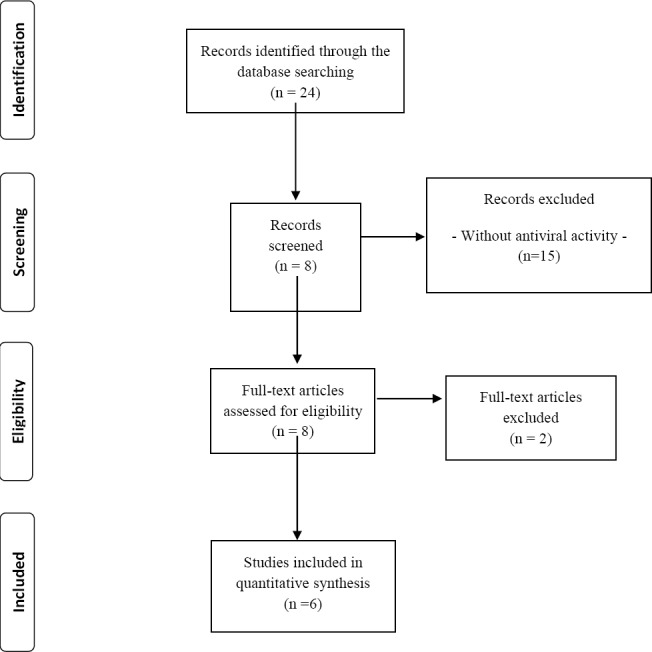
Fluxogram of the search strategy comprising the identification of potentially relevant articles, preliminary screening and final selection of the studies included in this current review (based on PRISMA statements).
It is possible to see in Table 3 information about the species of plants with anti-viral activity, the DENV serotypes and the countries were the studies were performed. There is a predominance of trials with DENV-2 more than with the other serotypes. The species of the medicinal plants used in the studies varied among the selected investigations, and in one of the papers (Gabrielsen et al., 1992), isoquinoline alkaloids were used. A great variety of species Phyllanthus was utilized (Lee et al, 2013). Furthermore, 50% of the studies were performed in Malaysia.
Table 3.
Information about the species of plant, the DENV serotypes and the countries where the selected studies were performed.
| Reference | Species of plant | DENV serotypes | Country |
|---|---|---|---|
| Rothan et al, 2014 | Vernonia cinerea, Hemigraphis reptans, Hedyotis auricularia, Laurentia longiflora, Tridax procumbers and Senna angustifolia. | DENV-2 | Malaysia |
| Lee et al., 2013 | Phyllanthus amarus, Phyllanthus niruri, Phyllanthus urinaria, and Phyllanthus watsonii. | DENV-2 | Malaysia |
| Tang et al., 2012 | Andrographis paniculata, Ocimum sanctum, Pelargonium citrosum, Cymbopogon citratus, Citrus limon and Momordica charantia. | DENV-1 | Malaysia |
| Garcia et al., 2010 | Lepechinia floribunda, Cleome aculeata, Eupatorium arnottianum, Eupatorium catarium, Lantana grisebachii, Trixis divaricata and Lantana camara. | DENV-2 | Argentina |
| Garcia et al., 2003 | Aloysia gratissima, Artemisia douglasiana, Eupatorium patens, Heterotheca latifolia, Hyptis mutabilis, Lippia junelliana, Lippia turbinate and Tessaria absinthioides. | DENV-2 | Argentina |
| Gabrielsen et al., 1992 | 23 Amaryllidaceae isoquinoline alkaloids | DENV-4 | USA |
DENV- dengue virus
In the Table 4 are shown the aim, chemical/compounds related to the medicinal plants, results and conclusion of the findings reported on the selected studies. The aim of the selected papers was mainly identify the anti-DENV effect of medicinal plants. Several chemicals/compounds were used in the investigations, as β-caryophyllene, spathulenol and borneol. Despite the different protocols and procedures, the results indicated an inhibitory action against the DENV due the action of the medicinal plants. Methanolic extracts were used in three (Rothan et al., 2014; Lee et al., 2013; Tang et al., 2012) of the six publications selected. According to the studies, it was concluded that these medicinal plants could be used as a potentially anti-DENV agent.
Table 4.
Aim, chemicals/compounds and results of findings reported on the selected studies
| Reference | Aim | Chemicals/Compounds | Results |
|---|---|---|---|
| Rothan et al, 2014 | To identify anti-dengue activities of medicinal plants extracts that are used in traditional medicine. | General ME | The highest inhibitory activities against dengue NS2B-NS3pro was observed in ethanolic extract of SA leaves, ME of VC leaves and ethanol extract of TP stems. These findings were further verified by in vitro viral inhibition assay. ME of VC leaves, ethanol extract of TP stems and at less extent ethanolic extract of SA leaves were able to maintain the normal morphology of DENV-2-infected Vero cells without causing much CPE. The percentage of viral inhibition of VC and TP extracts were higher than SA extract as measured by plaque formation assay and RT-qPCR. |
| Lee et al., 2013 | To evaluate the possibility of developing a local medicinal plant, Phyllanthus as an anti-dengue agent. | Active compounds including gallic acid, galloylglucopyronside, corilagen, geraniin, rutin, quercetin glucoside, syringing, syringing diamer, digalloylglucopyronside, trigalloylglucopyronside, apigenin rhamnoside, and quercetin rhamnoside have been identified. | The MNTD of both aqueous and ME on Vero cells were 250.0 and 15.63μg/ml respectively. Phyllanthus showed strongest inhibitory activity against DENV-2 with more than 90% of virus reduction in simultaneous treatment. Two-dimensional analysis revealed altered levels of thirteen proteins, which were identified by tandem MS (MS/MS). The altered proteins were involved in biological processes, as viral entry, viral transcription and translation regulations, cytoskeletal assembly, and cellular metabolism. |
| Tang et al., 2012 | To investigate the antiviral effects of standardized ME of AP, CL, CC, MC, OS and PC on DENV-1. | OS contained 88.6% of total flavonoids content, an amount that was the highest among all the six plants tested while the least was detected in MC. | The MNTD of the 6 medicinal plants was determined with ME against Vero E6 cells in vitro. The MNTD was in the decreasing order of MC > CL > PC, OS > AP > CC. Antiviral assay based on CPE denoted by degree of inhibition upon treating DENV-1-infected Vero E6 cells with MNTD of 6 medicinal plants showed that AP has the most antiviral inhibitory effects followed by MC. These results were verified with an in vitro inhibition assay using MTT, in which 113.0% and 98.0% of cell viability were recorded as opposed to 44.6% in DENV-1 infected cells. Although ME of OS and CC showed slight inhibition effect based on CPE, an inhibition was not reflected in MTT assay. ME of CL and PC did not prevent CPE or cell death from DENV-1. |
| Garcia et al., 2010 | To screen for cytotoxicity and in vitro inhibitory activity against HSV-1, DENV-2 and JUNV. | LF (1,8-Cineole, guaiol, β-caryophyllene, camphor, camphene, borneol and aromadendrene). CA (Cadinol-epi-alpha, germacrene-D, spathulenol δ-cadinene, presilphiperfolan-1-ol, α-muurolene, bicyclogermacrene and β–caryophyllene). LG (bicyclogermacrene, germecrene-D, spathulenol, β-caryophyllene, piperitenone, α–copaene). LC (spathulenol, bicyclogermacrene, β-caryophyllene, α-humulene, humulene-epoxide II and phytol). EC (limonene, piperitenone, trans-dihydrocarvone, camphor, cis-dihydrocarvone, β-caryophyllene and bicyclogermacrene). EA (spathulenol, β-caryophyllene, germacrene-D bicyclogermacrene α-humulene, γ-muurolene, α-cadinol, cis-Cadin-4-en-7-ol, and caryophyllene oxide). Trixis divaricata (β–caryophyllene, spathulenol, β-elemene and caryophyllene oxide). | The oils showed a variable virucidal action according to the virus. JUNV was the least susceptible virus in comparison with HSV-1 and DENV-2. The better relationship between cytotoxicity and inhibitory activity was observed for the essential oil of LG against DENV-2 and HSV-1 with IC50 values of 21.1 and 26.1 ppm, respectively. This effect was specific since the selectivity indices (ratio cytotoxicity/virucidal activity) were > 23.7 and > 19.1 for DENV-2 and HSV-1, respectively. The oil from LG was also an effective inhibitor of HSV-2 and acyclovir resistant variants of HSV. |
| Garcia et al., 2003 | To screen for virucidal activity against HSV-1, JUNV and DEN-2. | AG: caryophyllene oxide; cadinol; chrysanthenyl acetate; limonene oxide;β-caryophyllene. AD: α-thujone; β-thujone; borneol; p-cymene; 1.8-cineole; isocaryophylene-epoxide. EP: D-germacrene; β-caryophyllene; bicyclogermacrene;α-pinene; caryophyllene oxide. HL: borneol; camphor; limonene; β-pinene. HM: β-caryophyllene; germacrene D; curzerene; bicyclogermacrene. LJ: piperitenone oxide; limonene; camphor; spathulenol. LT: limonene; piperitenone oxide; β-caryophyllene. TA: caryophyllene oxide; (E)-β-damacenone; γ-eudesmol; α-gurjunene; terpinen-4ol. | The most potent inhibition was observed with the essential oil of LJ and LT against JUNV with VC50 values in the range 14–20 ppm, whereas AG, HL and TA inhibited JUNV in the range 52–90 ppm. Virucidal activity was time-and-temperature-dependent. Essential oils of AG, AD, EP and TA inactivated HSV-1 at 65–125 ppm. Only AD and EP had any discernible effect on DENV-2 infectivity with VC50 values of 60 and 150 ppm, respectively. |
| Gabrielsen et al., 1992 | To evaluate the effect of 23 Amaryllidaceae isoquinoline alkaloids and related synthetic analogues isolated or synthesized and subsequently in cell culture against the RNA-containing flaviviruses. | Amaryllidaceae isoquinoline alkaloids and related synthetic analogues isolated or synthesized | Activity against SF virus was only observed with 7deoxy analogues. In most cases, selectivity of the active compounds was low, with toxicity in uninfected cells (TC50) within 10- fold that of the viral IC50. No activity was observed against HIV-1, VEEV, or vaccinia viruses. PN and its 7deoxy analogue were evaluated in JE mouse models (differing in viral dose challenge, among other factors). In experiments (low LD, viral challenge, variant I), prophylactic administration of PN at 4 and 6 mg/kg/ day (2% EtOH/saline, sc, once daily for 7 days) increased survival of JE-virus-infected mice. Prophylactic administration of 5 at 40 mg/kg/day in hydroxypropylcellulose (sc, once daily for 7 days) increased survival of JE-virus-infected mice. With high LD, viral challenge, administration of 4 at 6 mg/kg/day (ip, twice daily for 9 days) resulted in a 50% survival rate. PN and 7deoxy-PN demonstrated activity in mice infected with JE virus at near toxic concentrations. |
PN-Pancratistatin, ME-methanolic extracts, SA-Senna angustifolia, VC-Vernonia cinerea, TP-Tridax procumbers, AP-Andrographis paniculata, CL- Citrus limon, CC-Cymbopogon citrates, MC - Momordica charantia, OS- Ocimum sanctum, PC- Pelargonium citrosum, LF-Lepechinia floribunda, CA-Cleome aculeata, LG-Lantana grisebachii, LC-Lantana camara, EC-Eupatorium catarium, EA-Eupatorium arnottianum, AG-Aloysia gratissima, AD-Artemisia douglasiana, EP-Eupatorium patens, HL-Heterotheca latifolia, HM-Hyptis mutabilis, LJ-Lippia junelliana, LT-Lippia turbinate, TA-Tessaria absinthioides DENV – dengue virus; CPE - cytopathic effects, RTq-PCR - real time quantitative PCR, MNTD - maximum non-toxic dose, VC50 - virucidal concentration 50%, JUNV – junin virus; HSV – herpes simplex virus, MS- mass spectrometry, IC50 - inhibitory concentration 50%, HIV- human immunodeficiency virus, JE - japanese encephalitis, LD –letal dose, YF - yellow fever; PT - punta toro; SF - sandfly fever-Sicilian; RVF - rift valley fever, VEEV - Venezuelan equine encephalomyelitis virus
Discussion
Investigations about dengue have relevance because it is the fastest spreading vector-borne viral disease, and it is endemic in over 100 countries. It is reported that 40% of the world’s population live in areas at risk for dengue (WHO, 2016). These reasons justify the increase of publications in PubMed with the arboviral diseases (dengue, chikungunya and zika virus) transmitted by Aedes aegypti mosquito (Table 2), mainly in the last years (2012 up to 2016).
Development of a safe, low-cost and effective dengue vaccine is a high priority, especially in the most affected countries (Goel et al., 2006; Klawikkan et al., 2011; Rothan et al., 2014). Six studies have reached the inclusion criteria to be analyzed (Figure 1). The relevance of the medicinal plants in the management of diseases reveals the importance of the investigations about their effects in DENV. There are a variety of medicinal plants utilized in the treatment of dengue (Table 3), however, only 0.040% of the articles with the keyword “medicinal plants” are related to dengue (Table 2). An interesting finding is related to the country where the investigations were performed. Fifty percent of the studies was carried out in Malaysia. This fact could be associated with the fact that all the 4 DENV genotypes are thought to have independently evolved from a sylvatic ancestral lineage, perhaps in Malaysia (Wang et al., 2000). Moreover, Teoh et al., 2010 reported that an ancestral sylvatic DENV-1, which was isolated from a monkey in 1972, it was present in a patient with dengue in Malaysia.
As shown in Table 3, serotype DENV-2 is most studied, perhaps because is known to be more lethal than other serotypes (Goel et al., 2004). No study in the PubMed database involving medicinal plants and dengue about the DENV-3 was found, although some authors consider that it with the DENV-1 in primary infection always results in more dangerous disease than infection with the others serotypes (Guzman and Isturiz, 2010; Tang et al., 2012).
In all these investigations, an antiviral effect against DENV was detected (table 3). Rothan et al, 2014 have verified that methanolic extract of Vernonia cinerea leaves and ethanol extract of Tridax procumbers stems possessed high inhibitory activates against DENV. Lee et al., 2013 have described that Phyllanthus could be potentially developed as an anti-DENV agent. Jung et al., 2015 also reported that Phyllanthus urinaria extract inhibited hepatitis B virus (HBV) DNA synthesis and surface antigen (HBsAg) and core antigen (HBcAg) secretion by replicating cells harboring HBV wild-type and lamivudine (LMV)-resistant mutants, likely by inducing the expression of interferon-beta (IFN-β), cyclooxygenase-2 (COX-2), and interleukin-6 (IL-6). Tang et al., 2012 have verified that the methanol extracts of Andrographis paniculata and Momordica charantia possess the ability of inhibiting the activity of DENV-1 in in vitro assays. Cai et al, 2015 have also described that Andrographis paniculata, exerted potent anti-influenza A virus activity against A/chicken/Hubei/327/2004 (H5N1), A/duck/Hubei/XN/2007 (H5N1), A/PR/8/34 (H1N1), A/NanChang/08/2010 (H1N1) and A/HuNan/01/2014 (H3N2) in vitro. Garcia et al., 2010 demonstrate the effective and selective inhibitory activity of the essential oil from Lantana grisebachii against HSV and DENV by direct virus inactivation. Garcia et al., 2003 support the potential use of essential oils in toto from medicinal plants as agents against viral infections. Gabrielsen et al., 1992 reported that Amaryllidaceae alkaloids related to narciclasine and lycorine possess antiviral activity with accompanying low selectivity in vitro against three flaviviruses, JE, YF, and DENV; against the bunyaviruses, PT and RVF viruses; and, to a lesser extent, against SF virus.
The current study has several limitations that must be considered in the interpretation of the findings in this review. It is suggested caution in generalizing these findings due to the final select publications have methodological variations concerning to the experimental procedure and study design utilized. Furthermore, although we have tried to retrieve the articles following the selected keywords, it was not retrieved all the papers identified for inclusion, including articles that were not published in English, as well, those that are not indexed in the PubMed database.
Considering the findings described in this study, it is possible to conclude that medicinal plants’ products could be used for the development of potential anti-DENV agents. However, it is important to consider the small number of publications available in the PubMed database involving searches evaluating the anti-DENV effect of medicinal plants.
References
- Abd Kadir S. L, Yaakob H, Mohamed Zulkifli R. Potential anti dengue medicinal plants:a review. J. Nat Med. 2013;67(4):677–689. doi: 10.1007/s11418-013-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnaniet H, Mbot E.J, Keita O, Fehrentz J.A, Ankli A, Gallud A, Garcia M, Gary-Bobo M, Lebibi J, Cresteil T, Menut C. Antidiabetic potential of two medicinal plants used in Gabonese folk medicine. BMC Complement. Altern Med. 2016;16:71. doi: 10.1186/s12906-016-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Fazal H, Ayaz M, Abbasi B. H, Mohammad I, Fazal L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac. J. Trop Biomed. 2011;1(4):330–333. doi: 10.1016/S2221-1691(11)60055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babar M, Najam-us-Sahar SZ, Ashraf M, Kazi AG. Antiviral drug therapy- exploiting medicinal plants. J Antivir Antiretrovir. 2013;5:028–036. [Google Scholar]
- Cai W, Li Y, Chen S, Wang M, Zhang A, Zhou H, Chen H, Jin M. 14-Deoxy-11,12-dehydroandrographolide exerts anti-influenza A virus activity and inhibits replication of H5N1 virus by restraining nuclear export of viral ribonucleoprotein complexes. Antiviral Res. 2015;118:82–92. doi: 10.1016/j.antiviral.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Gabrielsen B, Monath T. P, Huggins J. W, Kefauver D. F, Pettit G. R, Groszek G, Hollingshead M, Kirsi J. J, Shannon W. M, Schubert E. M, et al. Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J. Nat Prod. 1992;55(11):1569–1581. doi: 10.1021/np50089a003. [DOI] [PubMed] [Google Scholar]
- Garcia C. C, Acosta E. G, Carro A. C, Fernandez Belmonte M. C, Bomben R, Duschatzky C. B, Perotti M, Schuff C, Damonte E. B. Virucidal activity and chemical composition of essential oils from aromatic plants of central west Argentina. Nat. Prod. Commun. 2010;5(8):1307–1310. [PubMed] [Google Scholar]
- Garcia C. C, Talarico L, Almeida N, Colombres S, Duschatzky C, Damonte E. B. Virucidal activity of essential oils from aromatic plants of San Luis, Argentina. Phytother Res. 2003;17(9):1073–1075. doi: 10.1002/ptr.1305. [DOI] [PubMed] [Google Scholar]
- Goel A, Patel D.N, Lakhani K.K, Agarwal S.B, Agarwal A, Singla S, Agarwal R. Dengue fever—a dangerous foe. J. Indian Acad. Clin Med. 2004;5:247–258. [Google Scholar]
- Gubler D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33(4):330–42. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Guzman A, Isturiz R. E. Update on the global spread of dengue. Int. J. Antimicrob Agents. 2010;36(Suppl 1):S40–42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Halstead S. B. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- Hao D. C, Xiao P. G, Ma H. Y, Peng Y, He C. N. Mining chemodiversity from biodiversity:pharmacophylogeny of medicinal plants of Ranunculaceae. Chin. J. Nat Med. 2015;13(7):507–520. doi: 10.1016/S1875-5364(15)30045-5. [DOI] [PubMed] [Google Scholar]
- Jung J, Kim N. K, Park S, Shin H. J, Hwang S. G, Kim K. Inhibitory effect of Phyllanthus urinaria L. extract on the replication of lamivudine-resistant hepatitis B virus in vitro. BMC Complement. Altern Med. 2015;15:255. doi: 10.1186/s12906-015-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazato K, Wang Y, Kobayashi N. Viral infectious disease and natural products with antiviral activity. Drug Discov Ther. 2007;1(1):14–22. [PubMed] [Google Scholar]
- Klawikkan N, Nukoolkarn V, Jirakanjanakir N, Yoksan S, Wiwat C, Thirapanmethee K. Effect of Thai medicinal plant extracts against Dengue virus in vitro. MU J Pharm. 2011;38:13–18. [Google Scholar]
- Leardkamolkarn V, Srigulpanit W, Phurimsak C, Kumkate S, Himakoun L, Sripanidkulchai B. The inhibitory actions of Houttuynia cordata aqueous extract on Dengue virus and Dengue-infected cells. J Food Biochem. 2012;26:86–92. [Google Scholar]
- Lee S. H, Tang Y. Q, Rathkrishnan A, Wang S. M, Ong K. C, Manikam R, Payne B. J, Jaganath I. B, Sekaran S. D. Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement. Altern Med. 2013;13:192. doi: 10.1186/1472-6882-13-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo E.E. Traditional Healing Systems Among Nsukka Igbo. J Tourism Heritage Stud. 2012;1:69–81. [Google Scholar]
- Orekhov A.N, Ivanova E.A. Cellular models of atherosclerosis and their implication for testing natural substances with anti-atherosclerotic potential. Phytomedicine. 2016 doi: 10.1016/j.phymed.2016.01.003. pii S0944-7113(16)00019-2. [DOI] [PubMed] [Google Scholar]
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses [homepage on the Internet] [Cited 2016 Jan 15];PRISMA. Available from: http://www.prisma-statement.org .
- Rebensburg S, Helfer M, Schneider M, Koppensteiner H, Eberle J, Schindler M, Gurtler L, Brack-Werner R. Potent in vitro antiviral activity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Sci Rep. 2016;6:20394. doi: 10.1038/srep20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A, Zulqarnain M, Ammar Y.A, Tan E.C, Rahman N.A, Yusof R. Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay 2014. Trop Biomed. 31(2):286–96. [PubMed] [Google Scholar]
- Tadtong S, Kamkaen N, Watthanachaiyingcharoen R, Ruangrungsi N. Chemical Components of Four Essential Oils in Aromatherapy Recipe. Nat. Prod Commun. 2015;10(6):1091–1092. [PubMed] [Google Scholar]
- Tang L. I, Ling A. P, Koh R. Y, Chye S. M, Voon K. G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement. Altern Med. 2012;12:3. doi: 10.1186/1472-6882-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh B. T, Sam S. S, Abd-Jamil J, AbuBakar S. Isolation of ancestral sylvatic dengue virus type 1, Malaysia. Emerg. Infect Dis. 2010;16(11):1783–1785. doi: 10.3201/eid1611.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voge N.V, Sánchez-Vargas I, Blair C.D, Eisen L, Beaty B.J. Detection of dengue virus NS1 antigen in infected Aedes aegypti using a commercially available kit. Am J Trop Med Hyg. 2013;88(2):260–6. doi: 10.4269/ajtmh.2012.12-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Ni H, Xu R, Barrett A. D, Watowich S. J, Gubler D. J, Weaver S. C. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J Virol. 2000;74(7):3227–3234. doi: 10.1128/jvi.74.7.3227-3234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tao L, Xu H. Chinese herbal medicines as a source of molecules with anti-enterovirus 71 activity. Chin. Med. 2016;11:2. doi: 10.1186/s13020-016-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization- WHO [homepage on the Internet] [Cited 2016 July 19]; Available from: http://www.who.int/topics/dengue/en/


