Abstract
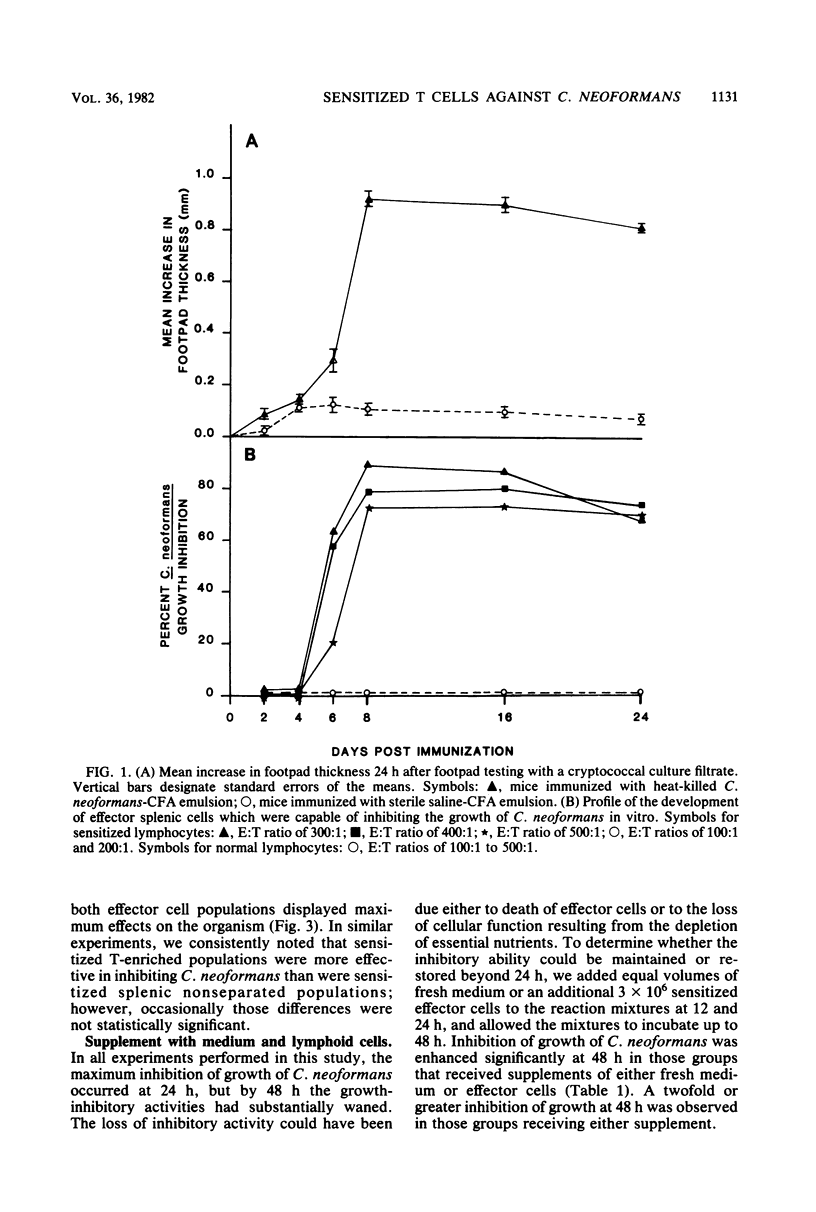
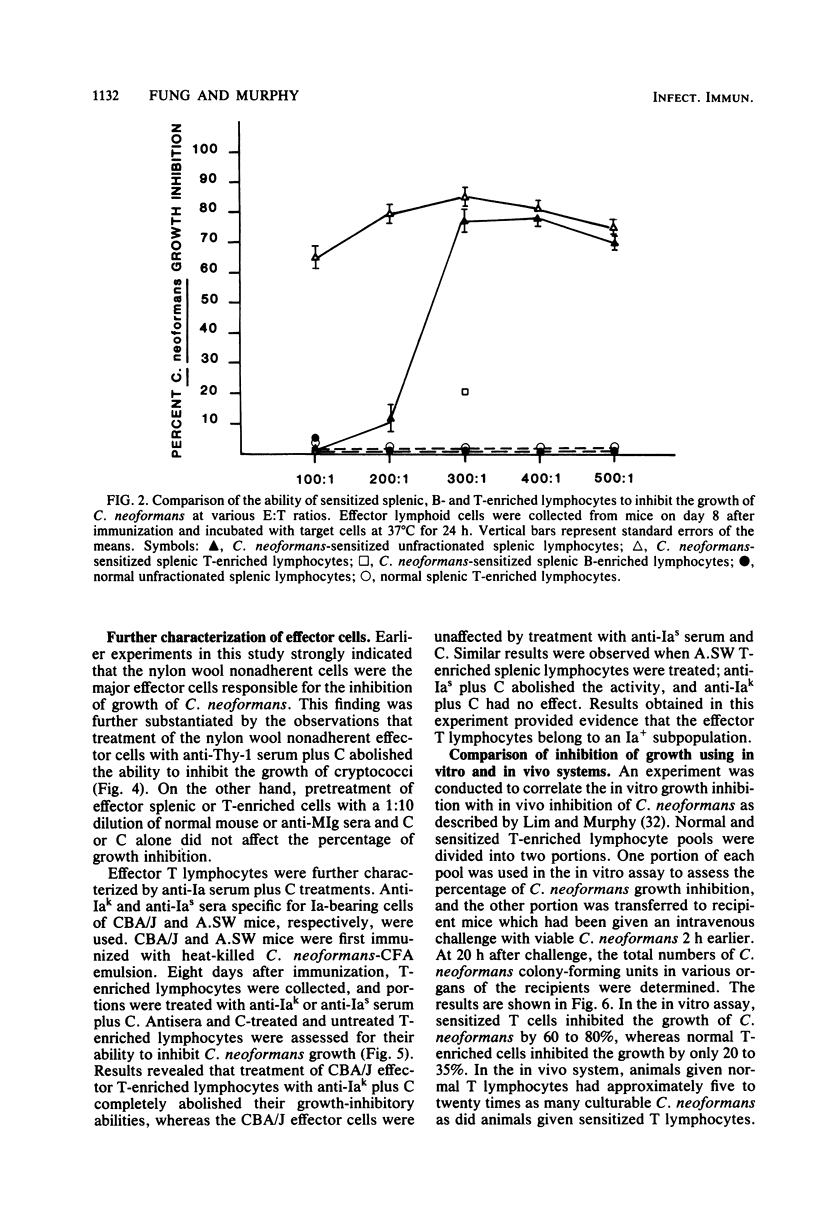
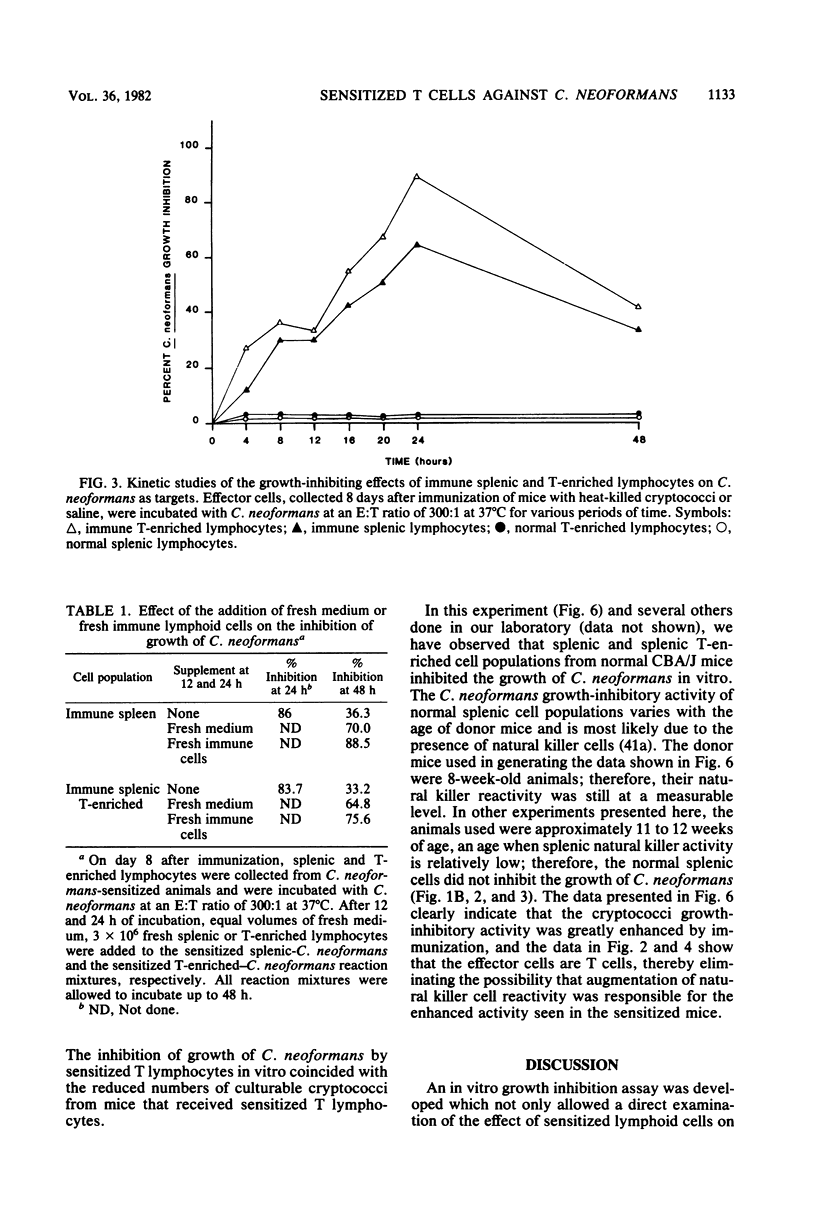
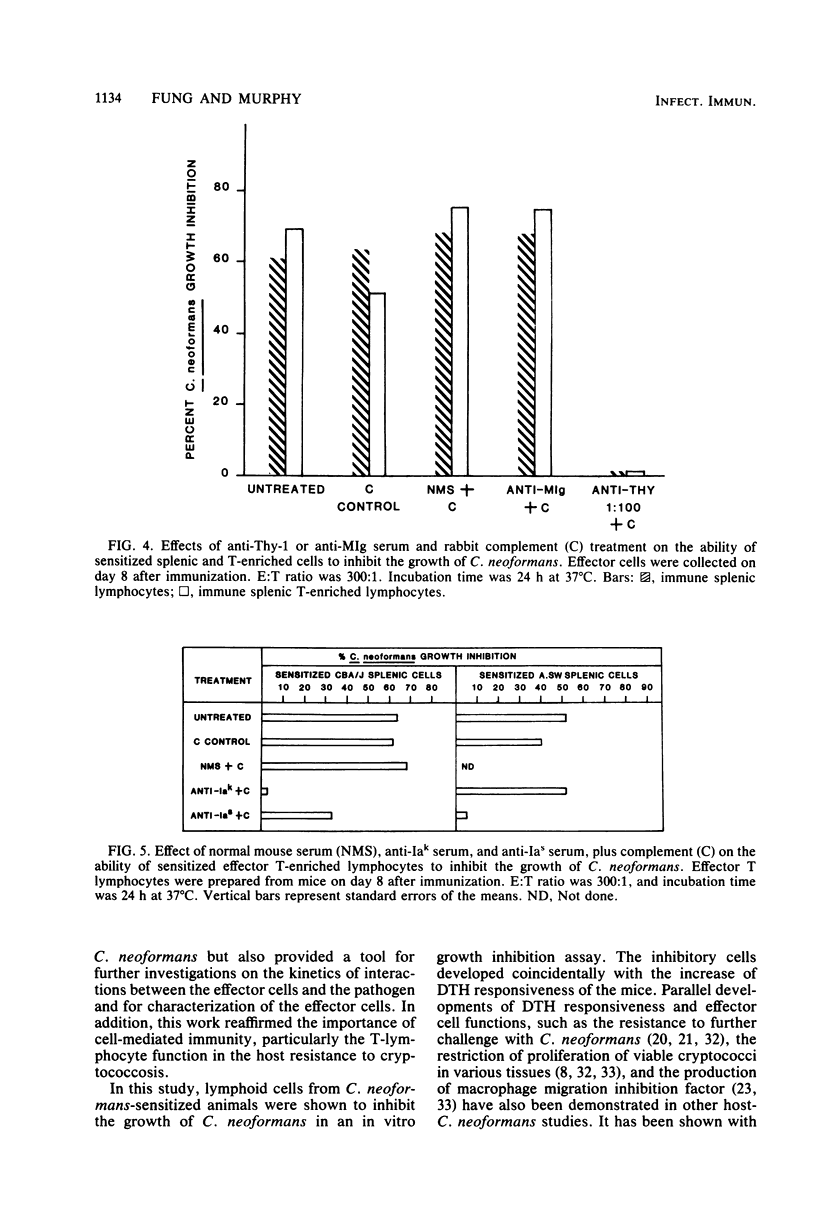
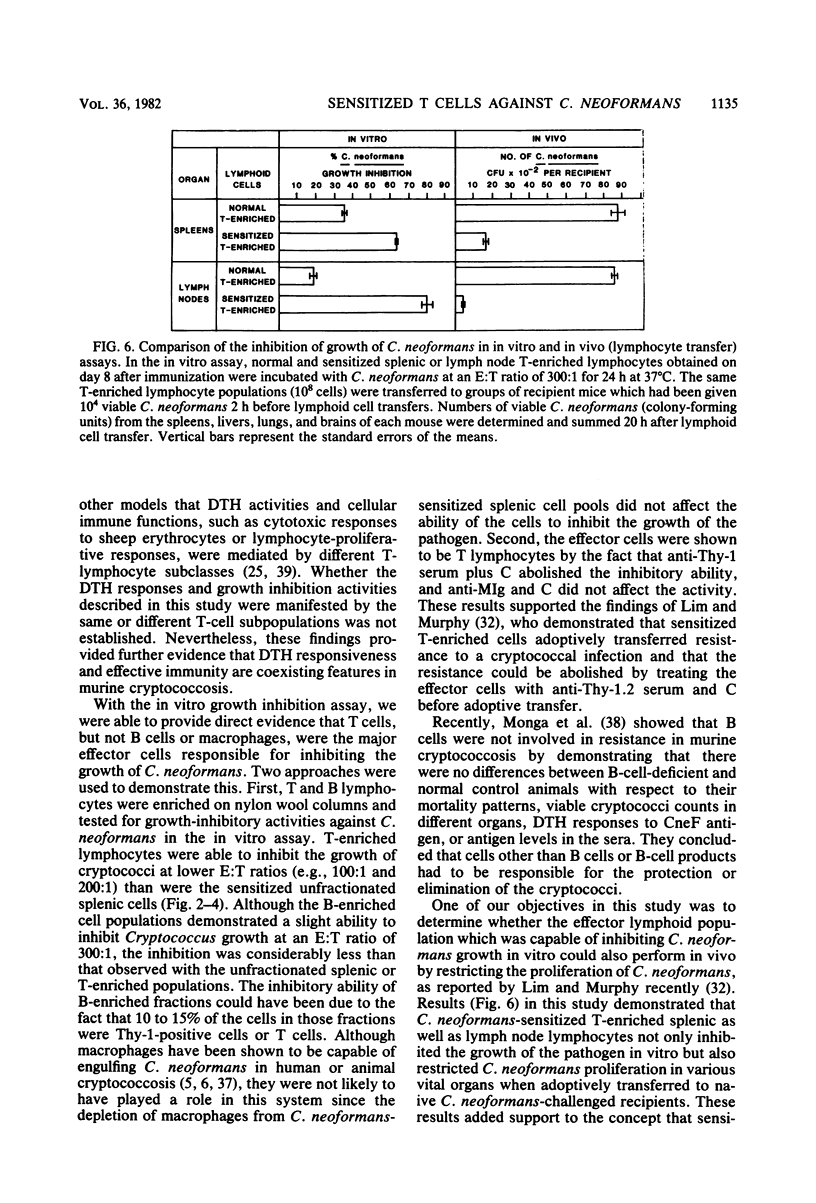
CBA/J mice immunized subcutaneously with emulsions of heat-killed Cryptococcus neoformans in complete Freund adjuvant displayed delayed-type hypersensitivity to cryptococcal culture filtrate antigen and developed sensitized splenic lymphoid cells which inhibited the growth of C. neoformans in vitro. The in vitro assay of growth inhibition served to investigate further the kinetics of the effect of sensitized lymphoid cells on the pathogen. There was a close correlation between the delayed-type hypersensitivity response in mice and inhibition of growth of C. neoformans by lymphoid cells. Sensitized splenic lymphocytes capable of inhibiting the growth of the cryptococci were detected at day 6 after immunization and reached maximum levels by days 8 through 16. Inhibition of growth was highest with effector-to-target cell ratios of 300:1 or greater. Inhibition of growth of C. neoformans by sensitized lymphoid cells was detectable as early as 4 h after effector and target cells were mixed and increased gradually, reaching a maximum at 24 h, but dropped significantly by 48 h. By supplementing the reaction mixtures with fresh medium or additional sensitized effector cells during incubation, the inhibition of growth of C. neoformans could be maintained through 48 h. C. neoformans-sensitized effector lymphoid populations not only inhibited the growth of the pathogen in vitro but also restricted C. neoformans proliferation in various vital organs upon transfer to naive recipient animals, indicating that the in vitro growth inhibition assay may be a means of assessing the resistance of animals to C. neoformans. The effector cells from sensitized animals were nylon wool-nonadherent Thy-1+ and Ia+ lymphocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson D. M., Cozad G. C. Effect of antilymphocyte serum on animals experimentally infected with Histoplasma capsulatum or Cryptococcus neoformans. J Bacteriol. 1969 Dec;100(3):1271–1276. doi: 10.1128/jb.100.3.1271-1276.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Pappagianis D., Benjamini E. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun. 1977 Sep;17(3):580–585. doi: 10.1128/iai.17.3.580-585.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschadler D. D., Bennett J. E. Serology of human cryptococcosis. Ann Intern Med. 1968 Jul;69(1):45–52. doi: 10.7326/0003-4819-69-1-45. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. II. Phagocytosis by human leukocytes. J Bacteriol. 1967 Nov;94(5):1480–1483. doi: 10.1128/jb.94.5.1480-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Tacker J. R. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect Immun. 1975 Jan;11(1):73–79. doi: 10.1128/iai.11.1.73-79.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Suppression of delayed hypersensitivity in vitro by inhibition of protein synthesis. J Exp Med. 1965 Dec 1;122(6):1125–1134. doi: 10.1084/jem.122.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973 Jun;127(6):694–697. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- Diamond R. D. Effects of stimulation and suppression of cell-mediated immunity on experimental cryptococcosis. Infect Immun. 1977 Jul;17(1):187–194. doi: 10.1128/iai.17.1.187-194.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M., Frank M. M., Bennett J. E. The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):312–315. doi: 10.3181/00379727-144-37580. [DOI] [PubMed] [Google Scholar]
- Dykstra M. A., Friedman L. Pathogenesis, lethality, and immunizing effect of experimental cutaneous cryptococcosis. Infect Immun. 1978 May;20(2):446–455. doi: 10.1128/iai.20.2.446-455.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Mitchell L., Drutz D. J. Host defense in cryptococcosis. III. Protection of nude mice by thymus transplantation. J Infect Dis. 1979 Oct;140(4):546–552. doi: 10.1093/infdis/140.4.546. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Taylor R. L. Host defense in cryptococcosis. I. An in vivo model for evaluating immune response. Int Arch Allergy Appl Immunol. 1978;57(2):101–113. [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Hay R. J., Reiss E. Delayed-type hypersensitivity responses in infected mice elicited by cytoplasmic fractions of Cryptococcus neoformans. Infect Immun. 1978 Oct;22(1):72–79. doi: 10.1128/iai.22.1.72-79.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A. T., Bakerspigel A. Factors affecting serum inhibited growth of Candida albicans and Cryptococcus neoformans. Sabouraudia. 1969 Oct;7(3):219–229. [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igel H. J., Bolande R. P. Humoral defense mechanisms in cryptococcosis: substances in normal human serum, saliva, and cerebrospinal fluid affecting the growth of Cryptococcus neoformans. J Infect Dis. 1966 Feb;116(1):75–83. doi: 10.1093/infdis/116.1.75. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia. 1975 Nov-Dec;67(6):1197–1200. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Letarte M., Teh H. S., Meghji G. Increased expression of Ia and Thy-1 antigens on mitogen-activated murine spleen lymphocytes. J Immunol. 1980 Jul;125(1):370–377. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga D. P., Kumar R., Mohapatra L. N., Malaviya A. N. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect Immun. 1979 Oct;26(1):1–3. doi: 10.1128/iai.26.1.1-3.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga D. P. Role of macrophages in resistance of mice to experimental cryptococcosis. Infect Immun. 1981 Jun;32(3):975–978. doi: 10.1128/iai.32.3.975-978.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Moorhead J. W., Walters C. S., Claman H. N. Immunologic reactions to haptens on autologous carriers. I. Participation of both thymus-derived and bone marrow-derived cells in the secondary in vitro response. J Exp Med. 1973 Feb 1;137(2):411–423. doi: 10.1084/jem.137.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Sundsmo J. S., Weiser R. S. Lymphokine toxicity for yeast cells. J Immunol. 1973 May;110(5):1444–1446. [PubMed] [Google Scholar]
- Staib F., Grave B., Altmann L., Mishra S. K., Abel T., Blisse A. Epidemiology of Cryptococcus neoformans. Mycopathologia. 1978 Dec 18;65(1-3):73–76. doi: 10.1007/BF00447178. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., McKenzie I. F., Chism S. E., Shen F. W., Boyse E. A., Gamble J. R., Whitelaw A. M. Ly and Ia antigen phenotypes of T cells involved in delayed-type hypersensitivity and in suppression. J Exp Med. 1976 Jul 1;144(1):10–19. doi: 10.1084/jem.144.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Graybill J. R., Drutz D. J. Histoplasma capsulatum infection in nude mice. Infect Immun. 1978 Sep;21(3):973–977. doi: 10.1128/iai.21.3.973-977.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN L. E., RAPPAPORT H. Occurrence of Cryptococcosis in patients with malignant disease of reticuloendothelial system. Am J Clin Pathol. 1954 Sep;24(9):1050–1072. doi: 10.1093/ajcp/24.9.1050. [DOI] [PubMed] [Google Scholar]
- Zwerner R. K., Barstad P. A., Acton R. T. Isolation and characterizaiton of murine cell surface components. I. Purification of milligram quantities of Thy-1.1. J Exp Med. 1977 Oct 1;146(4):986–1000. doi: 10.1084/jem.146.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]


