Highlights
-
•
The options of treatment for caudate tumors are presented.
-
•
Treatment of caudate tumors by left-sided approach is proposal.
-
•
Better results with left-sided approach are discussed.
-
•
Selection of patients with tumors in caudate lobe for left-sided approach is important.
Keywords: Caudate, Spiegel lobe, Left-sided approach, Caudate lobectomy, Tumor
Abstract
Introduction
The caudate lobe is a distinct liver lobe and surgical resection requires expertise and precise anatomic knowledge. Left-sided approach was described for resection of small tumors originated in the Spiegel lobe but now the procedure has been performed even for tumors more than five centimeters. The aim of this study is to present three cases of tumor of caudate lobe underwent isolated lobectomy by left-sided approach.
Presentation of case
Three patients with metastasis of colorectal cancer, carcinoma hepatocellular and metastasis of neuroendocrine tumor underwent resection. After modified Makuuchi incision, early control of short hepatic e short portal veins before hepatectomy was performed. The operative time was 200, 270 and 230 min respectively. No blood transfusion was used and no postoperative complications were observed. The length of stay was 7, 11 and 5 days respectively.
Discussion
Some approaches have been described to access and resect tumors of the caudate lobe, including the left-sided approach, right-sided approach, combined left- and right-sided approach and the anterior transhepatic approach. For liver resection in patients with malignant disease, parenchymal preservation is important in order to avoid postoperative liver failure or due to the risk of second hepatectomy. In these patients isolated caudate lobectomy is a safe option.
Conclusion
Isolated caudate lobectomy is a feasible procedure. Left-sided approach can be preformed even for tumors larger than 5 cm.
1. Introduction
The caudate lobe is a distinct liver lobe located beneath the confluence of hepatic veins with its vascularization and biliary drainage independent from those of both major lobes. According to Kumon, the caudate lobe consists of three parts: the Spiegel lobe, the paracaval portion and the caudate process. Caudate lobe has the same incidence of developing malignant neoplasms as others segments of the liver. However the rate of caudate lobectomy remains low due to the unique anatomic location and the difficulty of vascular control [1], [2], [3].
Isolated caudate lobectomy is the resection of either part or total caudate lobe. First described by Lerut et al. in 1990, resection of caudate lobe is a challenging procedure and requires expertise in liver resections, precise anatomic knowledge and safe vascular control. Left-sided approach was described for resection of small tumors that originated in the Spiegel lobe [3], [4].
The aims of this study are to present three cases of tumor of caudate lobe underwent isolated lobectomy by left-sided approach and describe the technique for performing the procedure. This work is in line with the SCARE criteria [11].
2. Surgical technique
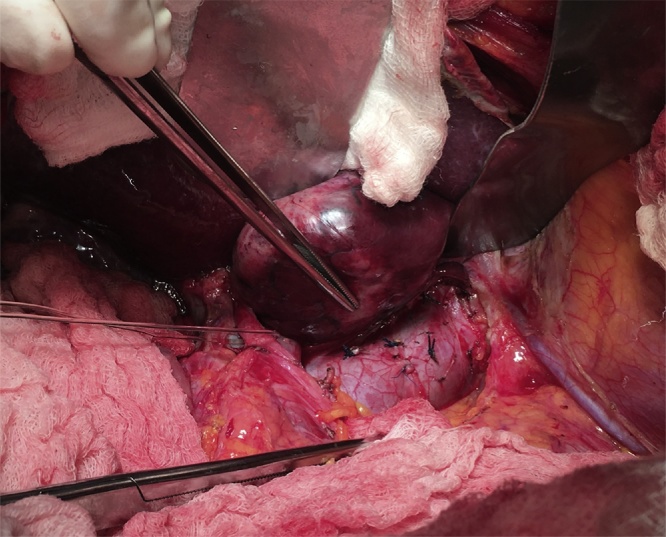
Modified Makuuchi incision is carried out and left lobe is fully mobilized. Intraoperative ultrasonography is performed to exclude others tumors. The round, falciform, left triangular and left coronary ligaments are separated. The venous ligament (Arantius) and vena cava ligaments are ligated and divided to free the upper surface of the caudate lobe (Fig. 1). The roots of the right and middle-left hepatic veins are dissected, exposed and prepared for clamping in case of bleeding from the branches of the hepatic veins at the site of parenchymal dissection. Early control of short hepatic e short portal veins before hepatectomy reduces the technical difficulty of caudate lobectomy (Fig. 2).
Fig. 1.
Vena cava ligaments are ligated and divided.
Fig. 2.
Early control of short hepatic veins.
The hepatoduodenal ligament is isolated and drawing rightward to expose the Spiegel lobe. At the base of the umbilical fissure the blood supply from the portal vein and hepatic artery are dissected and divided. At this time, the caudate hepatic ducts are separated.
The left lateral lobe is rotated rightward and anteriorly. To create a free posterior surface of the caudate lobe, the tumor is elevated from the retrohepatic inferior vena cava to expose all short hepatic veins to direct view and they are ligated and divided. Next, the caudate is drawing downward in order to dissect the upper pole of the tumor from the middle and the left hepatic veins. After the dissection is completed, the caudate lobe with the tumor is transected from the bridge to right lobe (Fig. 3).
Fig. 3.
Specimen with caudate lobe and tumor.
3. Case report
The characteristics of the patients are presented in Table 1.
Table 1.
Characteristics of the patients.
| Characteristics | Patients |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age | 70 | 69 | 54 |
| Sex | Male | Female | Female |
| Diagnosis | Colon Liver Mets | HCC | NET Liver Mets |
| Size (cm) | 5.3 × 5.2 | 4.8 × 4.6 | 4.5 × 4.1 |
| Approach | Left | Left | Left |
| Operative time (min) | 200 | 270 | 230 |
| Transfusion | No | No | No |
| UCI-Time (days) | 1 | 3 | 1 |
| Complications | No | No | No |
| Length of stay (days) | 7 | 11 | 5 |
| Pathology | Well differentiated AC | moderately differentiated HCC | Metastasis of NET |
HCC–Hepatocellular carcinoma; NET–Neuroendocrine tumor; Mets–Metastasis; ICU–Intensive Care Unit.
3.1. Case 1
A 70-year-old male patient with history of left colectomy due to adenocarcinoma of descending colon presented with isolated liver metastasis in caudate lobe, six months after left colectomy.
3.2. Case 2
A 69-year-old female patient with history of cirrhosis due to hepatitis B virus. The liver function was sufficient (Child-Pugh A, MELD 8), and serum α-fetoprotein level was 240 ng/mL. Contrast computed tomography observed a mass of 4.8 × 4.6 cm in the caudate lobe, compatible with hepatocellular carcinoma (Fig. 4). The patient was not indicated for transarterial chemoembolization (TACE) and refused liver transplantation.
Fig. 4.
Computed Tomography showing hepatocellular carcinoma closely attached to the vena cava.
3.3. Case 3
A 54 year-old female underwent left colectomy due to neuroendocrine tumor of rectum four months before. She underwent treatment with octreotide for two months. A computed tomography of the abdomen revealed 4 centimeters metastasis in caudate lobe (Fig. 5).
Fig. 5.
Computed Tomography showing liver metastasis from neuroendocrine tumor in the Spiegel lobe.
All patients had a drain placed on the transection area. Liver function in patient two remained good and postoperative ascites was not observed. The postoperative course of all patients was uneventful, and they were discharged on the 5th, 7th and 11th postoperative days. After six months, contrast computed tomography of the abdomen was performed showing no recurrence.
4. Discussion
Caudate lobectomy is classified as an isolated or combined resection. Isolated caudate lobectomy is a procedure that required knowledge of liver anatomy, experience in liver resection and safe management of vascular structures. It is the most technically difficult and challenge procedure, even when performed by hepatobiliary surgeons. Therefore, it has been considered a dangerous operation. The characteristic of the tumor, location, size and the hepatic functional reserve are factors that should be taken into account when we choose the approach [1], [3], [5].
Some approaches have been described to access and resect tumors of the caudate lobe, including the left-sided approach, right-sided approach, combined left- and right-sided approach and the anterior transhepatic approach. For huge tumors in the caudate lobe, anterior transhepatic approach seems to be better than left approach. However, requires splitting the liver parenchyma, takes time and results in bleeding during the transection. This approach is indicated for huge tumors located especially in paracaval portion. Right approach is recommended for tumors located in the caudate process [3], [6].
Left-sided approach was initially indicated for tumors situated in the Spiegel lobe and less than five centimeters. However, recently even tumors larger than 5 centimeters have been resected [3], [5], [7]. In the present study we resected tumors even more than five centimeters by the left-sided approach and the tumors were located particularly in the Spiegel lobe.
Sufficient mobilization of the tumor from the left to right side of the vena cava is necessary to allow the surgeon to perform parenchymal dissection under direct view. The venous ligament (Arantius) should be divided to free the upper surface of the caudate lobe. An important point regarding the feasibility of the left approach is to rotate the left lobe creating an optimal surgical view before parenchymal dissection [5], [7], [8]. In the present study, the tumor was managed by ligation of short hepatic veins in the area of the vena cava first to avoid spreading neoplastic cells.
Resection of the caudate lobe associated with extended left or right lobectomy is possible but considered to be excessive. However, for patients with liver metastases or dysfunction, preservation of the parenchyma is important and necessary to avoid complications [5], [7], [8]. In this study we have performed this technique in two patients with liver metastases and one patient with hepatocellular carcinoma.
The surgical treatment for patients with hepatocellular carcinoma is liver transplantation or resection. For resection, underlying cirrhosis and preserve adequate postoperative liver function is important while achieving complete tumor clearance. In patients with good liver function reserve and tumor in the caudate lobe, isolated lobectomy is a safe option. The postoperative prognosis in patients with hepatocellular carcinoma underwent anatomic resection has been superior to non-anatomic resection [6], [8]. In the present study we performed anatomic resection, the patient had adequate liver function and the postoperative course was uneventful.
Surgical resection is the best treatment option for patients with hepatic metastases from primary colorectal cancer or neuroendocrine tumors. However, in primary colorectal cancer, the rate of recurrence is as high as 60%. Hepatic recurrence after first hepatectomy can be safely resected, and that second or even third hepatectomy afford a survival benefit. Therefore, during the first resection preserve liver parenchyma is mandatory and the first priority to avoid complications with the future liver remnant [5], [7], [8], [9]. In this study, isolated caudate lobectomy was the best way to preserve liver parenchyma.
Laparoscopic caudate hepatectomy has been reported, but some cases are associated with left hepatectomy. Sufficient mobilization, expertise in advanced laparoscopic surgery and specific devices are necessary. Pure laparoscopic isolated caudate lobectomy is feasible only in selected patients and by hepatobiliary surgeon with experience in laparoscopic liver resection [10].
5. Conclusions
Isolated caudate lobectomy is safe and feasible. Left-sided approach can be preformed even for tumors larger than 5 centimeters, mainly if located in Spiegel lobe.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No financial.
Ethical approval
Ethical approval was not required and patients identifying knowledge were not presented in this report.
Consent
“Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Authors contribution
Orlando Jorge M Torres – Study concept, design, data collection, data analysis, interpretation, written.
Rodrigo Rodrigues Vasques – Data collection, data analysis, interpretation.
Ozimo Pereira Gama-Filho – Data collection, data analysis.
Miguel Eugenio Castelo Branco – Data collection, data analysis.
Camila Cristina S Torres – Design, data collection, data analysis, interpretation, written.
Guarantor
I accept full responsibility for the work and for financial issues.
References
- 1.Chaib E., Ribeiro M.A., Jr., Silva Fde S., Saad W.A., Cecconello I. Caudate lobectomy: tumor location, topographic classification, and technique using right- and left-sided approaches to the liver. Am. J. Surg. 2008;196:245–251. doi: 10.1016/j.amjsurg.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Kumon M. Anatomy of the caudate lobe with special reference to portal vein and bile duct. Acta Hepatol. Jpn. 1985;26:1193–1199. [Google Scholar]
- 3.Peng S.Y., Mou Y.P., Peng C.H., Cai X.J., Jiang X.C., Li J.D. Resection of caudate lobe of liver: report of 26 cases. Chin. J. Surg. 1999;1:12–13. [PubMed] [Google Scholar]
- 4.Lerut J., Gruwez J.A., Blumgart L.H. Resection of the caudate lobe of the liver. Surg. Gynecol. Obstet. 1990;171:160–162. [PubMed] [Google Scholar]
- 5.Sarmiento J.M., Que F.G., Nagomey D.M. Surgical outcomes of isolated caudate lobe resection: a single series of 19 patients. Surgery. 2002;132:697–709. doi: 10.1067/msy.2002.127691. [DOI] [PubMed] [Google Scholar]
- 6.Liu P., Qiu B.A., Bai G., Bai H.W., Xia N.X., Yang Y.X. Choice of approach for hepatectomy for hepatocellular carcinoma located in the caudate lobe: isolated or combined lobectomy. World J. Gastroenterol. 2012;18:3904–3909. doi: 10.3748/wjg.v18.i29.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai W.D., Huang J.S., Hu J.X. Isolated caudate lobe resection for huge hepatocellular carcinoma (10 cm or greater in diameter) Am. Surg. 2014;80:159–165. [PubMed] [Google Scholar]
- 8.Midorikawa Y., Takayama T. Caudate lobectomy (segmentectomy 1) J. Hepatobiliary Pancreat. Sci. 2012;19:48–53. doi: 10.1007/s00534-011-0450-1. [DOI] [PubMed] [Google Scholar]
- 9.Qiu Z.-Q., Tan W.-F., Yan P.-N., Luo X.-J., Zhang B.-H., Wu M.-C. Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy. Hepatobiliary Pancreat. Dis. Int. 2012;11:377–382. doi: 10.1016/s1499-3872(12)60195-7. [DOI] [PubMed] [Google Scholar]
- 10.Cai X., Zhao J., Wang Y., Yu H., Liang X., Jin R. A left-sided, purely laparoscopic approach for anatomic caudate hepatectomy: a single center experience. J. Laparoendosc. Adv. Surg. Tech. A. 2016;26:103–108. doi: 10.1089/lap.2015.0223. [DOI] [PubMed] [Google Scholar]
- 11.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P., for the SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]