Abstract
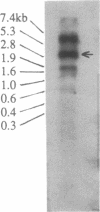
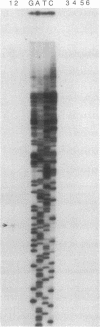
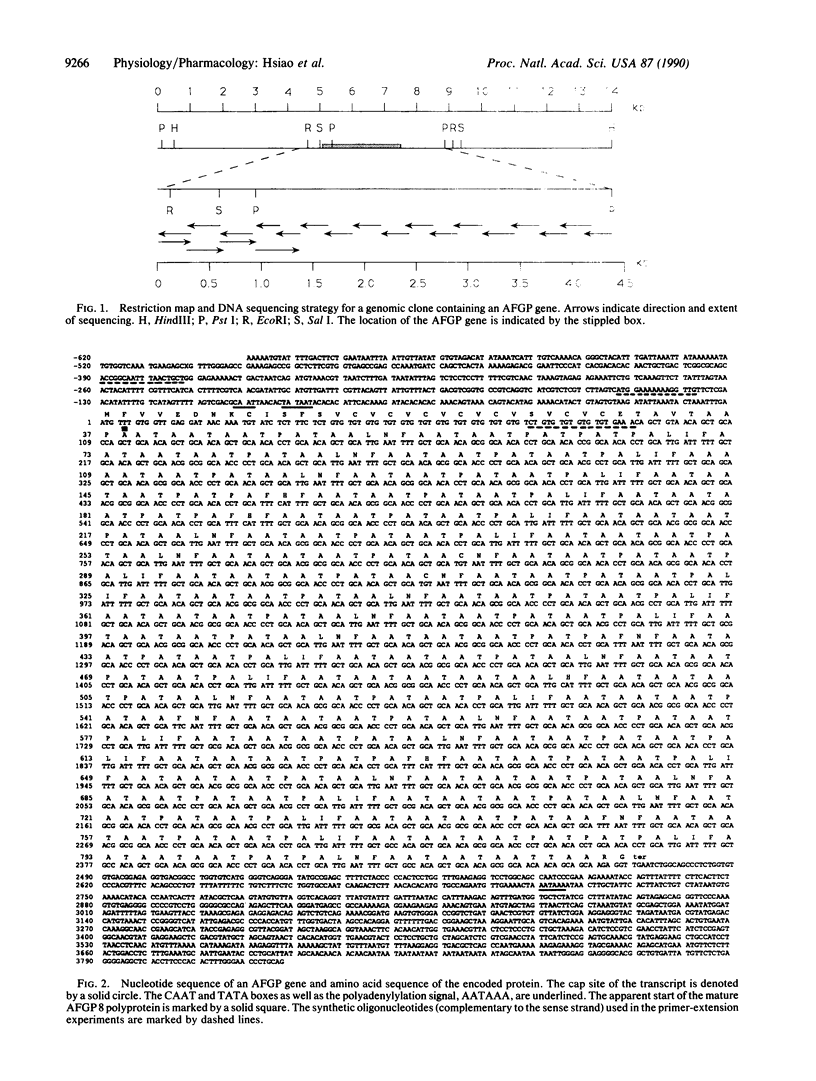
The antarctic fish Notothenia coriiceps neglecta synthesizes eight antifreeze glycopeptides (AFGP 1-8; Mr 2600-34,000) to avoid freezing in its ice-laden freezing habitat. We report here the sequence of one of its AFGP genes. The structural gene contains 46 tandemly repeated segments, each encoding one AFGP peptide plus a 3-amino acid spacer. Most of the repeats (44/46) code for peptides of AFGP 8; the remaining 2 code for peptides of AFGP 7. At least 2 of the 3 amino acids in the spacers could act as substrate for chymotrypsin-like proteases. The nucleotide sequence between the translation initiation codon (ATG) and the first AFGP-coding segment is G + T-rich and encodes a presumptive 37-residue signal peptide of unusual sequence. Primer extension establishes the transcription start site at nucleotide 43 upstream from ATG. CAAT and TATA boxes begin at nucleotides 53 and 49, respectively, upstream from the transcription start site. The polyadenylylation signal, AATAAA, is located approximately 240 nucleotides downstream from the termination codon. A mRNA (approximately 3 kilobases) was found that matches the size of this AFGP gene. Thus, this AFGP gene encodes a secreted, high-copy-number polyprotein that is processed posttranslationally to produce active AFGPs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlgren J. A., Cheng C. C., Schrag J. D., DeVries A. L. Freezing avoidance and the distribution of antifreeze glycopeptides in body fluids and tissues of Antarctic fish. J Exp Biol. 1988 Jul;137:549–563. doi: 10.1242/jeb.137.1.549. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cheng C. H., DeVries A. L. Structures of antifreeze peptides from the antarctic eel pout, Austrolycicthys brachycephalus. Biochim Biophys Acta. 1989 Jul 27;997(1-2):55–64. doi: 10.1016/0167-4838(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- DeVries A. L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science. 1971 Jun 11;172(3988):1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- Devries A. L., Lin Y. Structure of a peptide antifreeze and mechanism of adsorption to ice. Biochim Biophys Acta. 1977 Dec 20;495(2):388–392. doi: 10.1016/0005-2795(77)90395-6. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gourlie B., Lin Y., Price J., DeVries A. L., Powers D., Huang R. C. Winter flounder antifreeze proteins: a multigene family. J Biol Chem. 1984 Dec 10;259(23):14960–14965. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Joshi S., Wang N. C., Kao M. H., Ananthanarayanan V. S. Structures of shorthorn sculpin antifreeze polypeptides. Eur J Biochem. 1985 Aug 15;151(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Wang N. C., Joshi S., Fletcher G. L., Scott G. K., Hayes P. H., Buettner B., Davies P. L. Multiple genes provide the basis for antifreeze protein diversity and dosage in the ocean pout, Macrozoarces americanus. J Biol Chem. 1988 Aug 25;263(24):12049–12055. [PubMed] [Google Scholar]
- Hoffmann W., Bach T. C., Seliger H., Kreil G. Biosynthesis of caerulein in the skin of Xenopus laevis: partial sequences of precursors as deduced from cDNA clones. EMBO J. 1983;2(1):111–114. doi: 10.1002/j.1460-2075.1983.tb01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S., DeVries A. L., Feeney R. E. Studies of the structure of freezing point-depressing glycoproteins from an Antarctic fish. J Biol Chem. 1970 Jun 10;245(11):2909–2913. [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Li X. M., Trinh K. Y., Hew C. L., Buettner B., Baenziger J., Davies P. L. Structure of an antifreeze polypeptide and its precursor from the ocean pout, Macrozoarces americanus. J Biol Chem. 1985 Oct 25;260(24):12904–12909. [PubMed] [Google Scholar]
- Lin Y., Duman J. G., DeVries A. L. Studies on the structure and activity of low molecular weight glycoproteins from an antarctic fish. Biochem Biophys Res Commun. 1972 Jan 14;46(1):87–92. doi: 10.1016/0006-291x(72)90633-x. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Thompson M. R., Osuga D. T., Ahmed A. I., Chan S. M., Vandenheede J. R., Feeney R. E. Antifreeze glycoproteins from the blood of an antarctic fish. The structure of the proline-containing glycopeptides. J Biol Chem. 1978 Jul 25;253(14):5155–5162. [PubMed] [Google Scholar]
- Ng N. F., Trinh K. Y., Hew C. L. Structure of an antifreeze polypeptide precursor from the sea raven, Hemitripterus americanus. J Biol Chem. 1986 Nov 25;261(33):15690–15695. [PubMed] [Google Scholar]
- O'Grady S. M., Clarke A., DeVries A. L. Characterization of glycoprotein antifreeze biosynthesis in isolated hepatocytes from Pagothenia borchgrevinki. J Exp Zool. 1982 Apr 10;220(2):179–189. doi: 10.1002/jez.1402200207. [DOI] [PubMed] [Google Scholar]
- Osuga D. T., Feeney R. E. Antifreeze glycoproteins from Arctic fish. J Biol Chem. 1978 Aug 10;253(15):5338–5343. [PubMed] [Google Scholar]
- Raymond J. A., Lin Y., DeVries A. L. Glycoprotein and protein antifreezes in two Alaskan fishes. J Exp Zool. 1975 Jul;193(1):125–130. doi: 10.1002/jez.1401930112. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag J. D., Cheng C. H., Panico M., Morris H. R., DeVries A. L. Primary and secondary structure of antifreeze peptides from arctic and antarctic zoarcid fishes. Biochim Biophys Acta. 1987 Oct 15;915(3):357–370. doi: 10.1016/0167-4838(87)90021-5. [DOI] [PubMed] [Google Scholar]
- Scott G. K., Davies P. L., Kao M. H., Fletcher G. L. Differential amplification of antifreeze protein genes in the pleuronectinae. J Mol Evol. 1988;27(1):29–35. doi: 10.1007/BF02099727. [DOI] [PubMed] [Google Scholar]
- Scott G. K., Davies P. L., Shears M. A., Fletcher G. L. Structural variations in the alanine-rich antifreeze proteins of the pleuronectinae. Eur J Biochem. 1987 Nov 2;168(3):629–633. doi: 10.1111/j.1432-1033.1987.tb13462.x. [DOI] [PubMed] [Google Scholar]
- Scott G. K., Hayes P. H., Fletcher G. L., Davies P. L. Wolffish antifreeze protein genes are primarily organized as tandem repeats that each contain two genes in inverted orientation. Mol Cell Biol. 1988 Sep;8(9):3670–3675. doi: 10.1128/mcb.8.9.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. K., Hew C. L., Davies P. L. Antifreeze protein genes are tandemly linked and clustered in the genome of the winter flounder. Proc Natl Acad Sci U S A. 1985 May;82(9):2613–2617. doi: 10.1073/pnas.82.9.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Lin Y., De Vries A. L. Structure and mode of action of glycoproteins from an antarctic fish. Biochim Biophys Acta. 1972 Apr 15;263(2):406–413. doi: 10.1016/0005-2795(72)90092-x. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Lin Y., DeVries A. L. Structure of the carbohydrate of antifreeze glycoproteins from an antartic fish. FEBS Lett. 1975 Jun 15;54(2):135–138. doi: 10.1016/0014-5793(75)80060-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]