Abstract
A K99-negative mutant of the K99 reference strain Escherichia coli B41 (O101:K99) was isolated (strain B41M). It did not react with OK antiserum to a K12 K99+ recombinant or with OK antiserum to K99-positive organisms from the O8, O20, or O64 serogroups, but it did react with OK antiserum to K99-positive organisms from the O101 and O9 serogroups. The mutant hemagglutinated sheep erythrocytes and attached in vitro to calf enterocytes when cultured at 37 degrees C but not when grown at 18 degrees C. Attachment was mannose resistant but susceptible to heating and formaldehyde. These properties were associated with the presence of fimbriae. The isolated hemagglutinin migrated to the anode in immunoelectrophoresis experiments, competitively inhibited attachment of strain B41M to calf enterocytes, and could be demonstrated adhering to these cells in vitro by indirect immunofluorescent staining. The anionic hemagglutinin is referred to provisionally as F41. Germfree piglets infected with strain B41M developed diarrhea within 16 h. Scanning electron microscopy showed groups of bacteria adherent to the microvilli of villous enterocytes. Indirect immunofluorescent staining demonstrated the presence of F41 antigen in vivo.
Full text
PDF

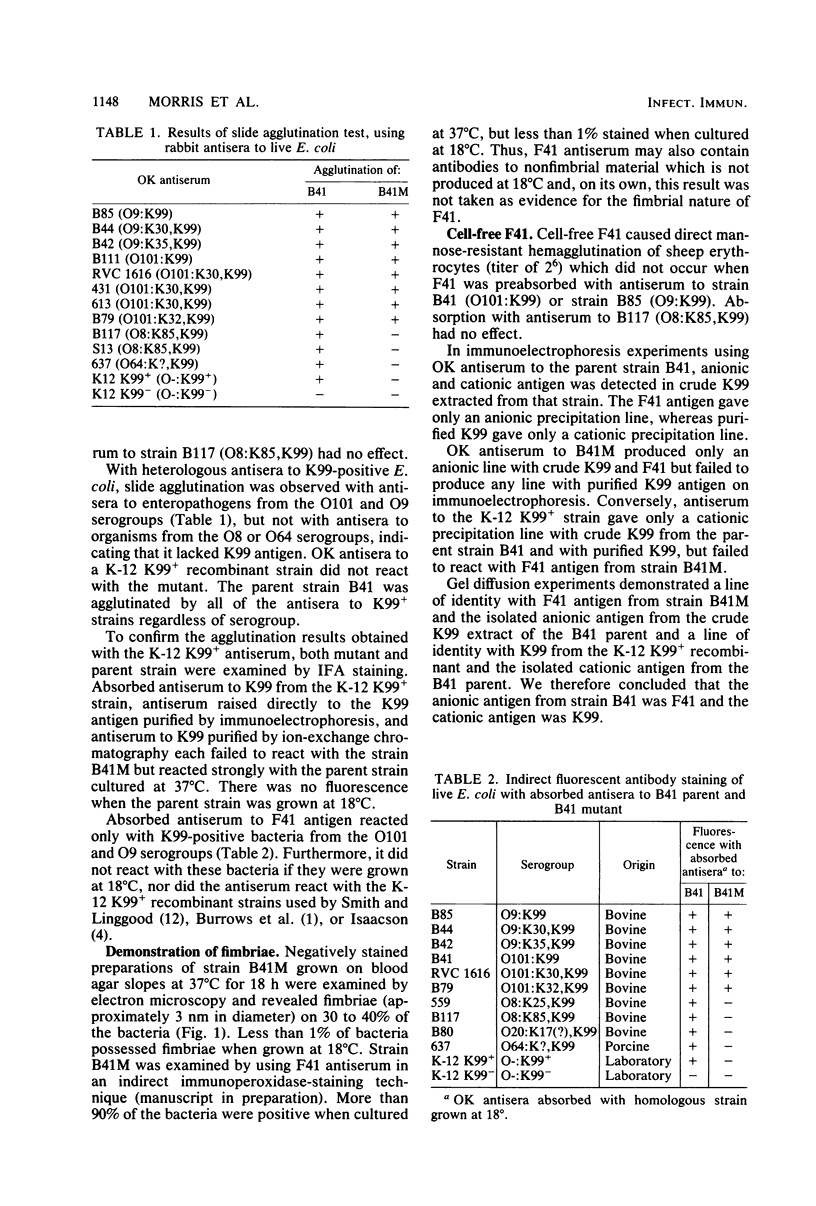
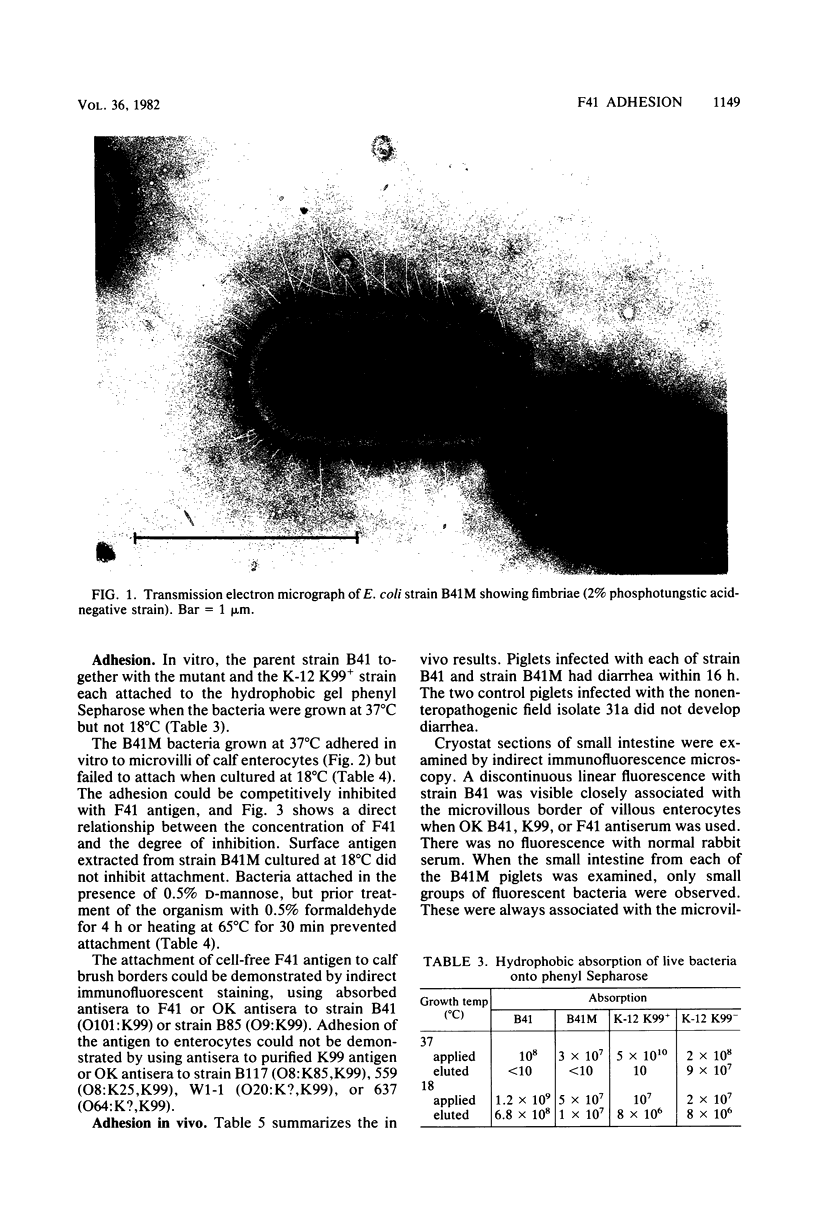
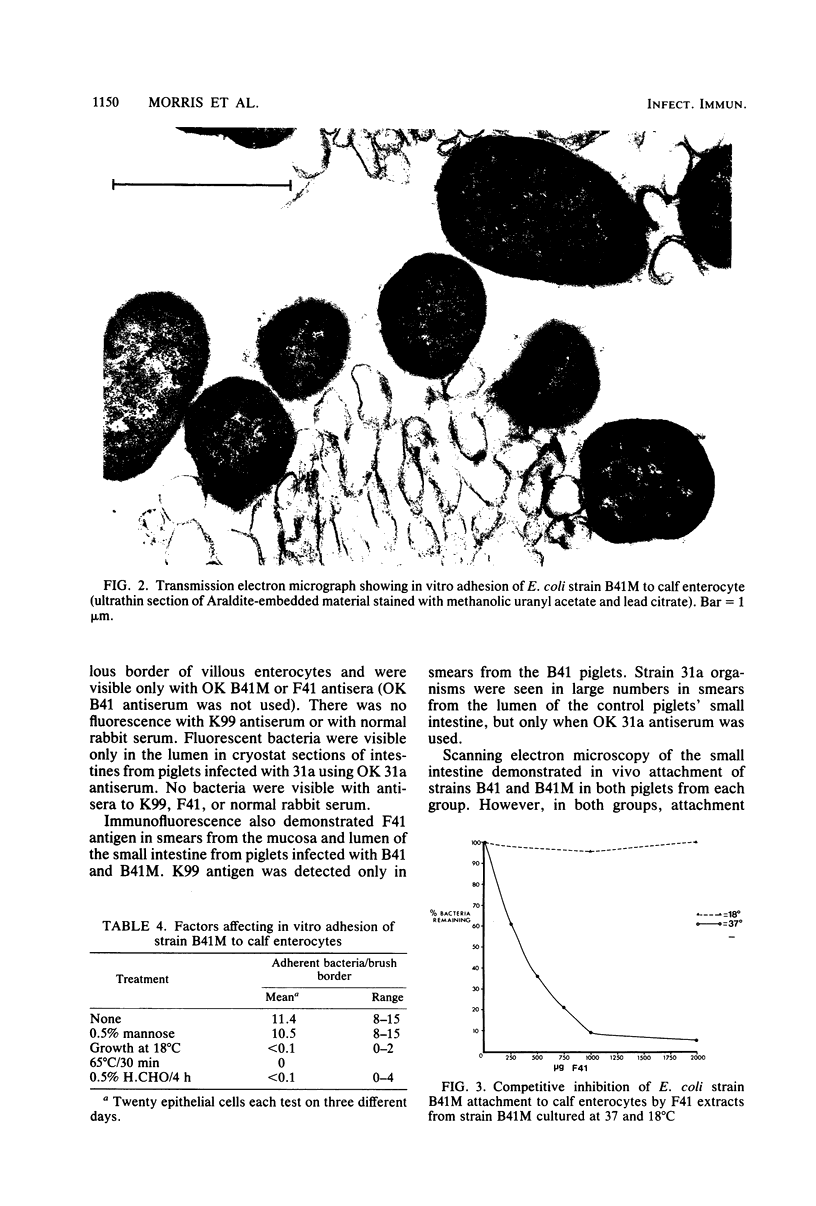
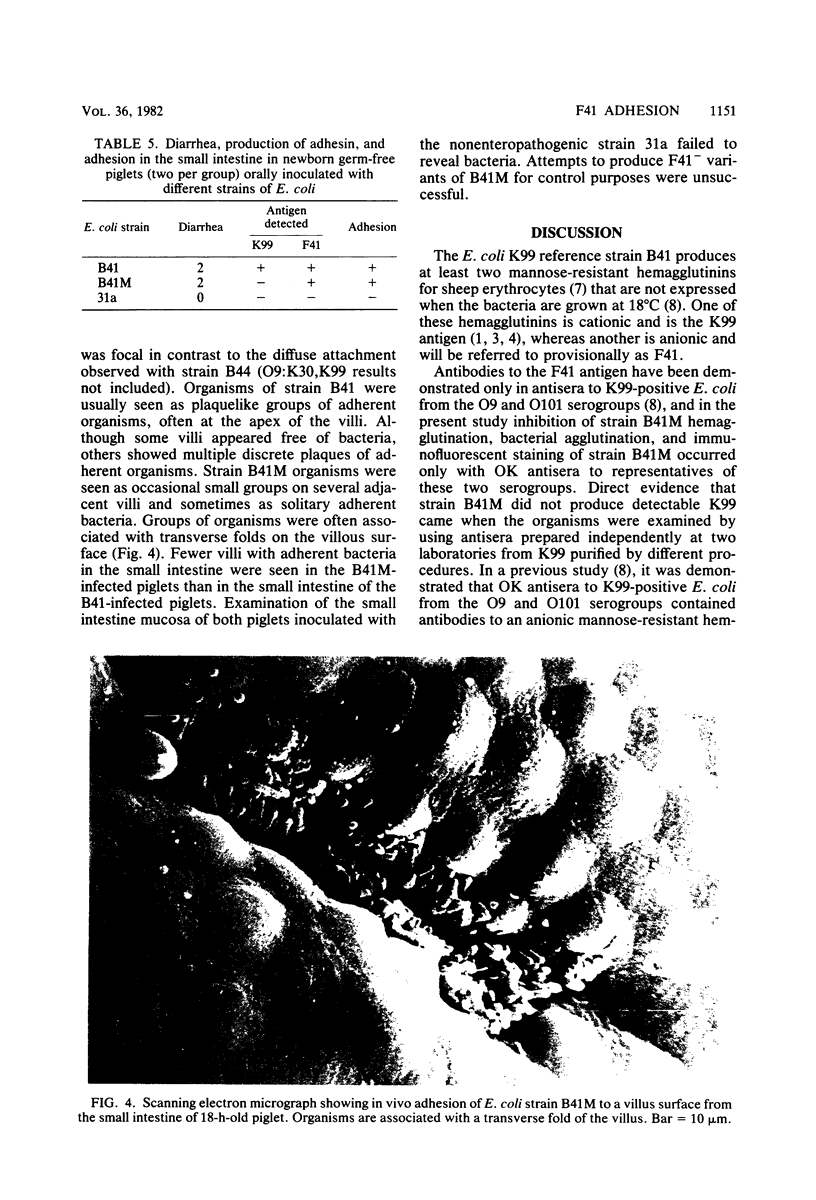


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows M. R., Sellwood R., Gibbons R. A. Haemagglutinating and adhesive properties associated with the K99 antigen of bovine strains of Escherichia coli. J Gen Microbiol. 1976 Oct;96(2):269–275. doi: 10.1099/00221287-96-2-269. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Nagy B., Isaacson R. E., Orskov I. Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect Immun. 1977 Feb;15(2):614–620. doi: 10.1128/iai.15.2.614-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Sojka W. J. Anionic and cationic components of the K99 surface antigen from Escherichia coli B41. J Gen Microbiol. 1978 Jul;107(1):173–175. doi: 10.1099/00221287-107-1-173. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Sojka W. J. Preliminary characterization of cell-free K99 antigen isolated from Escherichia coli B41. J Gen Microbiol. 1977 Apr;99(2):353–357. doi: 10.1099/00221287-99-2-353. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Thorns C. J., Sojka W. J. Evidence for two adhesive antigens on the K99 reference strain Escherichia coli B41. J Gen Microbiol. 1980 May;118(1):107–113. doi: 10.1099/00221287-118-1-107. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]