Abstract
Background
The combination of CTLA-4 and PD-L1 inhibitors has a manageable adverse effect profile, although rare immune-related adverse events (irAE) can occur.
Case presentation
We describe an autoimmune polymyositis following a partial response to combination tremelimumab and durvalumab for the treatment of recurrent lung adenocarcinoma. Radiography revealed significant reduction in all metastases; however, the patient developed progressive neuromuscular hypoventilation due to lymphocytic destruction of the diaphragmatic musculature. Serologic testing revealed a low level of de novo circulating antibodies against striated muscle fiber. Immunohistochemistry revealed type II muscle fiber atrophy with a mixed CD8+ and CD4+ lymphocyte infiltrate, indicative of inflammatory myopathy.
Conclusions
This case supports the hypothesis that muscle tissue is a target for lymphocytic infiltration in immune checkpoint inhibitor-associated polymyositis. Further insights into the autoimmune mechanism of PM will hopefully contribute to the prevention and treatment of this phenomenon.
Electronic supplementary material
The online version of this article (doi:10.1186/s40425-017-0258-x) contains supplementary material, which is available to authorized users.
Keywords: Immune-related adverse event, Non-small cell lung cancer, Programmed death protein 1, Programmed death-ligand 1, Cytotoxic T-lymphocyte-associated-protein 4, Immune checkpoint inhibitor, Myasthenia gravis, Striated muscle antibody, MEDI4736
Background
Combination therapy with anti-cytotoxic T-lymphocyte-associated-protein 4 (CTLA-4) and anti-programmed death ligand 1 (PD-L1) monoclonal antibodies holds incredible potential for the treatment of solid tumors [1]. Despite their robust activity, these immune checkpoint inhibitors can have rare but important immune-related adverse events (irAEs). Specifically, CTLA-4 inhibitors have been associated with irAEs in most organ systems, including enterocolitis, hepatitis, and endocrinopathy [2]. Among these irAEs, there are several cases of inflammatory myopathies [3].
Polymyositis, dermatomyositis, and inclusion body myositis (IBM) are among a group of inflammatory myopathies associated with muscle weakness and inflammatory infiltrates within skeletal muscle. Polymyositis (PM) is a subacute myopathy which differs from other subgroups by perifascicular atrophy and the absence of vacuoles [4]. In animal models, PM is induced by clonal expansion of cluster of differentiation (CD) 8+ cells of specific T cell receptor (TCR) families targeting muscle tissue. High levels of interferon gamma (IFN-γ) lead to up-regulation of MHC class I in myotubes, even in areas far from sites of inflammation [5]. Perforin-dependent cytotoxicity mediated by CD8+ T cells causes muscle fiber atrophy and necrosis [6]. Diaphragmatic weakness is one of the most prominent and life-threatening consequences of autoimmune myositis [7].
Herein we describe a patient treated with a single infusion of combination anti-CTLA-4 and anti-PD-L1 antibody, and subsequently incurred a fatal destructive lymphocytic myositis involving the inspiratory muscles. We performed a retrospective analysis of this subject with the primary objective to further characterize the immunopathology of this rare adverse event.
Case presentation
A 64-year-old female former smoker presented with lung adenocarcinoma originally treated with right pneumonectomy. Follow-up imaging four years later revealed a recurrence involving the omentum, confirmed by biopsy. Primer-extension mass spectrometry genotyping of the tumor revealed a KRAS G12C mutation, and immunohistochemistry showed no PD-L1 expression in tumor cells. She was seronegative for HIV and viral hepatitis. The patient expressed a preference for immunotherapy instead of palliative chemotherapy. She began a phase 1 clinical trial (NCT02000947) consisting of tremelimumab (1 mg/kg) and durvalumab (10 mg/kg) IV over 1 h on Day 1 [8]. At Day 28, she reported progressive dysphagia and a barium esophagram demonstrated hypomotility. Soon thereafter, she developed respiratory acidosis. Magnetic resonance imaging (MRI) was normal, and lumbar puncture revealed normal cerebrospinal fluid (CSF) cell count with negative cytology. Computed tomography (CT) scan demonstrated a significant reduction in the size of the omental metastases (Fig. 1). Further tremelimumab -durvalumab was stopped, and she was treated empirically with high-dose prednisone at 120 mg/day. Despite this, she experienced progressive neuromuscular hypoventilation requiring continuous bilevel positive airway pressure (BiPAP) ventilation, with declined forced vital capacity (0.73 L) and impaired negative inspiratory force (−41 cm H2O). Intravenous polyvalent immunoglobulin (1 g/kg, Day 42–46), plasma exchange (Days 54–61), and pyridostigmine (Day 49–65) were added, with no effect. Eventually she requested de-escalation to comfort care, and died peacefully after withdrawal of BiPAP support on Day 65.
Fig. 1.
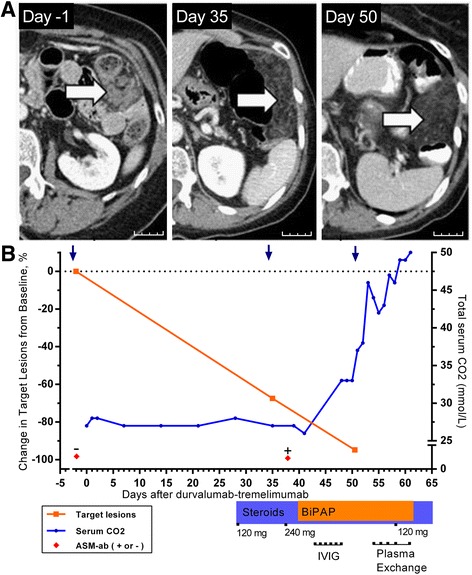
a Change in representative omental metastasis on CT scan after treatment with durvalumab- tremelimumab on Day 1. Arrows indicate omental metastasis replaced by fatty tissue. Scale bar indicates 25 mm. b Clinical course of patient until death from neuromuscular respiratory failure on Day 65. Serum CO2 retention is indicative of hypoventilation
Although the patient was initially suspected to have a drug-related myasthenia syndrome, consequent testing for acetylcholine receptor (AChR) immunoglobulin (IgG), voltage gated calcium channel (VGCC) IgG, and muscle-specific kinase protein (MuSK) IgG was negative. Anti-striated muscle (ASM) IgG was detected at a low titer of 1:40; it was not detected in archived pretreatment serum. Since the ASM IgG was only detectable at low titer, we could not conclude a diagnosis based solely upon the serology findings.
Upon autopsy, gross examination showed mild atrophy of the intercostal and diaphragm muscle. There was no evidence of interstitial lung disease. An additional file describes the methods used (Additional file 1) and controls used (Additional file 2). Microscopic examination of the inspiratory musculature showed an inflammatory mononuclear infiltrate most pronounced in the diaphragm, with only scattered preserved fibers (Fig. 2). Immunohistochemistry showed no increased lipid stores, and an attenuated mosaic pattern with type II fiber specific atrophy. Transactive response (TAR) DNA-binding protein 43 and trichrome evaluation were negative for inclusion bodies [9]. Mononuclear cells infiltrating the diaphragm and intercostal muscle consisted of a mixed phenotype of CD8+ and CD4+ lymphocytes. CD68+ macrophages were also observed in necrotic myofibers [10]. No residual metastases were identified on gross examination, indicating a complete pathological response to tremelimumab-durvalumab.
Fig. 2.
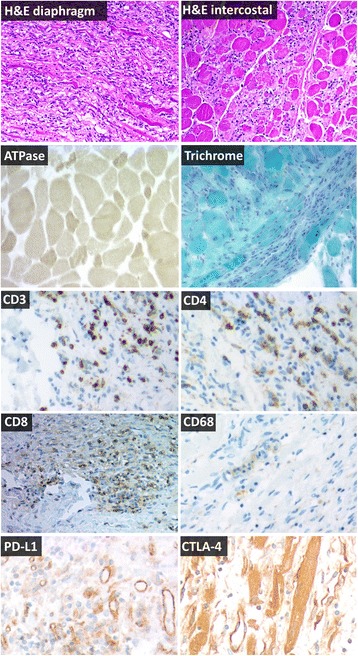
Representative immunohistochemistry of inspiratory muscles at autopsy, 66 days after tremelimumab-durvalumab treatment. Hematoxylin and eosin (H&E) sections show inflammatory myopathy in the diaphragm and intercostal muscles without rimmed vacuoles and without perifascicular atrophy, consistent with polymyositis. A mononuclear infiltrate is present which invades otherwise normal myofibers and completely effaces the background muscle fiber architecture in some areas. ATPase shows intense staining in small fibers compared to surrounding lighter, normal sized fibers in preserved areas of muscle, indicative of type II fiber atrophy. Trichrome shows mildly increased connective tissue, but shows no rimmed vacuoles, rods, or other inclusions. T cell co-receptor staining (CD3, CD4, CD8) revealed a mixed T-cell infiltrate which often completely effaced the myofascicular architecture. CD68 highlights necrotic myofibers scattered within the larger inflammatory infiltrate. PD-L1 expression was observed in blood vessels of dying muscle. Weak CTLA-4 expression was detected in necrotic myofibers. All images are 100× magnification
Discussion
As CTLA-4 and PD-1 axis inhibition becomes adopted for more cancer types, rare irAE presentations are becoming more common. Severe PM has been reported with monoclonal antibodies against both CTLA-4 and PD-1 (Table 1). This disease classically affects the proximal muscles and in severe cases, such as this one, is associated with dysarthria and dysphagia [11]. Although diaphragmatic PM has been previously reported [9], our case is prominent for its acute onset following immune-checkpoint inhibitor therapy.
Table 1.
Select cases of severe myositis associated with CTLA-4 and/or PD-1 axis inhibitors
| Drug(s) | Description | ↑CK? | ASM IgG? | Onset (wks) | Treatment | Trial | Reference |
|---|---|---|---|---|---|---|---|
| Ipilimumab + nivolumab | Polymyositis with respiratory involvement | Yes | Yes | 3 | Corticosteroid, infliximab, IVIG, Pex | NCT01928394 | [35] |
| Nivolumab | Myositis with respiratory failure | Yes | – | 7 | Corticosteroid | – | [36] |
| Nivolumab | Myasthenia crisis and polymyositis requiring ventilation | Yes | No | 2 | Corticosteroid, Pex, IVIG pyridostigmine | – | [19] |
| Pembrolizumab | Polyarticular tenosynovitis and proximal myositis | No | – | 56 | Sulfasalazine | NCT01295827 | [37] |
| Pembrolizumab | Exacerbation of preexisting myositis | Yes | – | 1 | IVIG | – | [38] |
| Ipilimumab | Dermatomyositis | Yes | – | 2 | Corticosteroid | – | [39] |
| Pembrolizumab / ipilimumab | Rhabdomyolysis associated with hypothyroidism | Yes | – | 6 | Levothyroxine | – | [40] |
| Ipilimumab | Combined myasthenia and myositis with AChR | Yes | Yes | 7 | Corticosteroid, IVIG | – | [26] |
| Ipilimumab | Retrobulbar weakness with proximal myositis. | Yes | Yes | 7 | Corticosteroid, IVIG | – | [3] |
| Ipilimumab | Orbital myositis associated with ipilimumab | – | – | 12 | Corticosteroid | – | [41] |
| Tremelimumab + durvalumab | Described in text. | No | Yes | 4 | Corticosteroid, Pex, IVIG | NCT02000947 |
Abbreviations: ASM, anti-striated muscle antibody, NCT national clinical trials identifier number, Pex plasma exchange, IVIG intravenous immunoglobulin G, AChR acetylcholine receptor antibody, CK creatine kinase, wks weeks
The pathogenesis of idiopathic PM has intrinsic mechanistic overlap with the action of CTLA-4 blockade. Anti-CTLA-4 monoclonal antibody treatment increases IFN-γ production in draining lymph nodes [12], which may induce higher MHC class I expression on adjacent cells. Like the drug-related PM reported here, idiopathic PM is identified by disorganized muscle fibers of variable sizes coupled with endomysial T cell infiltrates [13]. CD8+ cells preferentially invade fibers which express MHC class I and trigger necrosis via the perforin pathway [4]. Although terminally differentiated, these CD8+ T cell infiltrates are predominantly CD28null [14]. In patients, this CD28null phenotype correlates with resistance to corticosteroid therapy [15]. Thus, aberrant B7-family receptor function appears to have a contributory role in idiopathic PM, not unlike the CTLA-4 drug-related PM described.
Targeting of muscle fiber by autoreactive T cells has been proposed as the pathogenesis of idiopathic PM [16]. Muscle-related antigens have been recognized in TCRs sequenced from idiopathic PM lesions [17], and a focused T cell repertoire is observed in these lesions [18]. Consistent with idiopathic PM, TCR sequencing of inflamed muscle in a nivolumab-related PM case revealed a clonally expanded T cell population in the muscle, as compared to either blood or tumor [19]. Like most irAEs associated with CTLA-4 or PD-1 inhibition, PM is also a recognized complication of up to 7.8% of allogeneic stem cell transplants, due to graft-versus-host disease (GvHD) [20]. It occurs after onset of hematopoietic full chimerism, and is associated with an increase in circulating CD8+ T cells. Muscle biopsy often reveals a mononuclear infiltrate of donor T cells and macrophages at endomysial sites, as in our case [21]. However, GvHD PM usually responds promptly to high-dose corticosteroids or cyclosporine [22].
The PD-1 axis is also implicated in the development of idiopathic myositis. Skeletal muscle cells normally express PD-L1, which induces T cell anergy. PD-L1 had a immunoprotective role against myositis in coculture experiments of MHC class I/II labeled myoblasts with CD4+ or CD8+ T cells [23]. Thus, PD-1 or PD-L1 monoclonal antibody may theoretically contribute to the autoimmune myositis cascade by attenuating the protection of skeletal muscle cells against autoreactive T cells [24].
The pathological findings in our case seem most indicative of PM, although a seronegative myasthenia overlap condition may also have been present. A Murine B6 model suggests that anti-CTLA-4 treatment stimulates AChR auto-antibody production and enhances the T cell response to provoke severe autoimmune myasthenia gravis [25]. Several cases of myasthenia gravis have been reported with ipilimumab [26, 27], as well as ipilimumab-nivolumab [28].
Optimal treatment of KRAS-mutant lung adenocarcinoma remains an emerging field. At present, therapy with PD-L1 axis blockade appears to be at least as effective in KRAS-mutant lung adenocarcinomas, compared to wild-type [29, 30]. Although KRAS point mutations do not appear to result in highly antigenic proteins, there has been preclinical success in inducing T-cell responses against Ras-associated epitopes [31]. In addition, TCRs reactive to the mutant KRASG12D peptide have been isolated within CD8+ TIL cultured from colon adenocarcinoma [32, 33]. Likewise, TCRs reactive to KRASG12D/G12V peptides have been isolated from immunized, HLA-specific transgenic mice [34]. Thus, adoptive cell transfer may be a viable treatment option for the proportion of KRAS-mutant patients who do not respond to PD-L1 / CTLA-4 blockade.
This study was conducted post-mortem, and is characterized by important limitations. Unfortunately, electromyography was not conducted. In addition, sufficient volumes of blood to determine the specific epitope of striated muscle protein were not collected, or determine the putative autoantigens involved. Absence of properly preserved PBMCs precluded lymphocyte reactivity testing. In addition, no blood samples suitable for creatine kinase measurement were collected. The ASM IgG was only weakly positive in our case, which is inconclusive as a standalone finding. Nonetheless, PM was unmistakable on pathologic examination.
Conclusion
PM remains a rare but important irAE associated with anti-CTLA-4 and PD-L1 combination therapy. The case reviewed in this report supports the hypothesis that muscle tissue is a target for lymphocytic infiltration in tremelimumab - durvalumab associated PM. Fatality was an unfortunate outcome in this case, perhaps tied in part to delayed recognition. In similar cases, prompt initiation of corticosteroids and additional measures have limited the severity of PM. Further insights into the autoimmune mechanism of PM will hopefully contribute to the prevention and treatment of this phenomenon.
Additional files
Methods Section. This describes the methods used for deriving the clinical and pathological data presented. (DOCX 19 kb)
This figure shows the positive and negative controls for immune cell immunohistochemistry. Benign lymph node tissue serving as positive (+) control for presence of T cell lineage cells (CD3, CD4, CD8) and macrophage lineage cells (CD68), as well as benign smooth muscle tissue serving as a negative (−) control for these antibodies. All images are 20× magnification. (TIFF 4756 kb)
Acknowledgements
This work has been supported in part by the Tissue Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). We gratefully acknowledge the patient and her family who consented for autopsy.
Funding
Correlative studies were supported by 2013 Conquer Cancer Foundation Young Investigator Award (to BCC), NCI P50 CA119997 (to SJA). The funding organizations had no direct role in the preparation, review, or approval of the manuscript.
Authors’ contributions
SJ collected and assembled data into a rough draft, including figures. SJA treated the patient and conceived of the study. TAR interpreted and contributed the CT scan figure. RPS and BAC carried out autopsy study and immunohistochemistry, including microscopy photographs. ASW provided specialized immunohistochemistry, including microscopy photographs. BCC designed the report, provided financial support, and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors BCC and SJA have served as a paid consultant and/or received honoraria from AstraZeneca Pharmaceuticals. The remaining authors have no potential competing interests to disclose.
Consent for publication
Written informed consent was obtained from the patient for publication of their individual details and accompanying images. The consent form is held by the authors’ institution in the patient clinical notes.
Ethics approval and consent to participate
Yes, the subject described provided written informed consent. Approved by Liberty IRB; IRB00003411.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AChR
Acetylcholine receptor
- ASM
Anti-striated muscle
- BiPAP
Bilevel positive airway pressure
- CD
Cluster of differentiation
- CO2
Carbon dioxide level
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- CTCAE
Common toxicity criteria for adverse events
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- GvHD
Graft-versus-host disease
- H&E
Hematoxylin and eosin
- HIER
High pH heat induced epitope retrieval
- ICH –GCP
International conference on harmonization good clinical practice
- IFN-γ
Interferon gamma
- IHC
Immunohistochemistry
- irAE
Immune-related adverse events
- IV
Intravenous
- IVIG
Intravenous immunoglobulin G
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- MHC
Major histocompatibility
- MRI
Magnetic resonance imaging
- MuSK
Muscle specific kinase protein
- NCT
National clinical trials
- PBMCs
Peripheral blood mononuclear cells
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed death ligand 1
- Pex
Plasma exchange
- PM
Polymyositis
- RECIST
Response evaluation criteria in solid tumor
- TAR DP-43
Transactive response DNA binding protein 43 kDa
- TCR
T cell receptor
- VGCC
Voltage gated calcium channel
- wks
Weeks
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s40425-017-0258-x) contains supplementary material, which is available to authorized users.
Contributor Information
Sooraj John, Email: sjohn2@health.usf.edu.
Scott J. Antonia, Email: scott.antonia@moffitt.org
Trevor A. Rose, Email: trevor.rose@moffitt.org
Robert P. Seifert, Email: rseifert@health.usf.edu
Barbara A. Centeno, Email: Barbara.Centeno@moffitt.org
Aaron S. Wagner, Email: brainpathology@gmail.com
Ben C. Creelan, Phone: 813.745.3050, Email: ben.creelan@moffitt.org
References
- 1.Rizvi N, Chaft J, Balmanoukian A, Goldberg SB, Sanborn RE, Steele KE, et al. Tumor response from durvalumab (MEDI4736)+ tremelimumab treatment in patients with advanced non-small cell lung cancer (NSCLC) is observed regardless of PD-L1 status. J Immunother Cancer. 2015;3:1. doi: 10.1186/s40425-014-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 3.Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. Can J Neurol Sci. 2009;36:518–520. doi: 10.1017/S0317167100007939. [DOI] [PubMed] [Google Scholar]
- 4.Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989;20:224–231. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 5.Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998;113:407–414. doi: 10.1046/j.1365-2249.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugihara T, Okiyama N, Suzuki M, Kohyama K, Matsumoto Y, Miyasaka N, et al. Definitive engagement of cytotoxic CD8 T cells in C protein–induced myositis, a murine model of polymyositis. Arthritis Rheum. 2010;62:3088–3092. doi: 10.1002/art.27625. [DOI] [PubMed] [Google Scholar]
- 7.Blumbergs PC, Byrne E, Kakulas BA. Polymyositis presenting with respiratory failure. J Neurol Sci. 1984;65:221–229. doi: 10.1016/0022-510X(84)90086-8. [DOI] [PubMed] [Google Scholar]
- 8.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braidy JF, Poulson JM. Diaphragmatic weakness and myositis associated with systemic juvenile rheumatoid arthritis. Can Med Assoc J. 1984;130:47–49. [PMC free article] [PubMed] [Google Scholar]
- 10.Holness CL, da Silva RP, Fawcett J, Gordon S, Simmons DL. Macrosialin, a mouse macrophage-restricted glycoprotein, is a member of the lamp/lgp family. J Biol Chem. 1993;268:9661–9666. [PubMed] [Google Scholar]
- 11.Stenzel W, Goebel HH, Aronica E. Review: immune-mediated necrotizing myopathies--a heterogeneous group of diseases with specific myopathological features. Neuropathol Appl Neurobiol. 2012;38:632–646. doi: 10.1111/j.1365-2990.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- 12.Paradis TJ, Floyd E, Burkwit J, Cole SH, Brunson B, Elliott E, et al. The anti-tumor activity of anti-CTLA-4 is mediated through its induction of IFN gamma. Cancer Immunol Immunother. 2001;50:125–133. doi: 10.1007/s002620100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve. 2015;51:638–656. doi: 10.1002/mus.24566. [DOI] [PubMed] [Google Scholar]
- 14.Fasth AE, Dastmalchi M, Rahbar A, Salomonsson S, Pandya JM, Lindroos E, et al. T cell infiltrates in the muscles of patients with dermatomyositis and polymyositis are dominated by CD28null T cells. J Immunol. 2009;183:4792–4799. doi: 10.4049/jimmunol.0803688. [DOI] [PubMed] [Google Scholar]
- 15.Pandya JM, Loell I, Hossain MS, Zong M, Alexanderson H, Raghavan S, et al. Effects of conventional immunosuppressive treatment on CD244+ (CD28null) and FOXP3+ T cells in the inflamed muscle of patients with polymyositis and dermatomyositis. Arthritis Res Ther. 2016;18:1. doi: 10.1186/s13075-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franck E, Bonneau C, Jean L, Henry J-P, Lacoume Y, Salvetti A, et al. Immunological tolerance to muscle autoantigens involves peripheral deletion of autoreactive CD8+ T cells. PLoS One. 2012;7:e36444. doi: 10.1371/journal.pone.0036444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiendl H, Malotka J, Holzwarth B, Weltzien H-U, Wekerle H, Hohlfeld R, et al. An autoreactive γδ TCR derived from a polymyositis lesion. J Immunol. 2002;169:515–521. doi: 10.4049/jimmunol.169.1.515. [DOI] [PubMed] [Google Scholar]
- 18.Mantegazza R, Andreetta F, Bernasconi P, Baggi F, Oksenberg J, Simoncini O, et al. Analysis of T cell receptor repertoire of muscle-infiltrating T lymphocytes in polymyositis. Restricted V alpha/beta rearrangements may indicate antigen-driven selection. J Clin Investig. 1993;91:2880. doi: 10.1172/JCI116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura T, Fukushima S, Miyashita A, Aoi J, Jinnin M, Kosaka T, et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci. 2016;107:1055–1058. doi: 10.1111/cas.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeppen S, Thirugnanasambanthan A, Koldehoff M. Neuromuscular complications after hematopoietic stem cell transplantation. Support Care Cancer. 2014;22:2337–2341. doi: 10.1007/s00520-014-2225-0. [DOI] [PubMed] [Google Scholar]
- 21.Parker P, Chao NJ, Ben-Ezra J, Slatkin N, Openshaw H, Niland JC, et al. Polymyositis as a manifestation of chronic graft-versus-host disease. Medicine. 1996;75:279–285. doi: 10.1097/00005792-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Maillard-Lefebvre H, Morell-Dubois S, Lambert M, Charlanne H, Launay D, Hachulla E, et al. Graft-versus-host disease-related polymyositis. Clin Rheumatol. 2010;29:431–433. doi: 10.1007/s10067-009-1350-5. [DOI] [PubMed] [Google Scholar]
- 23.Wiendl H, Mitsdoerffer M, Schneider D, Chen L, Lochmüller H, Melms A, et al. Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: a novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. FASEB J. 2003;17:1892–1894. doi: 10.1096/fj.03-0039fje. [DOI] [PubMed] [Google Scholar]
- 24.Xiaoyu D, Yunxia W, Qi F, Dapeng W, Xiuying C, Jianhua J, et al. Expression of B7-homolog 1 in Polymyositis. Ann Clin Lab Sci. 2011;41:154–160. [PubMed] [Google Scholar]
- 25.Wang HB, Shi FD, Li H, Chambers BJ, Link H, Ljunggren HG. Anti-CTLA-4 antibody treatment triggers determinant spreading and enhances murine myasthenia gravis. J Immunol. 2001;166:6430–6436. doi: 10.4049/jimmunol.166.10.6430. [DOI] [PubMed] [Google Scholar]
- 26.Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro-Oncology. 2014;16:589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson DB, Saranga-Perry V, Lavin PJ, Burnette WB, Clark SW, Uskavitch DR, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2015;33:e122–e124. doi: 10.1200/JCO.2013.51.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone or in combination with ipilimumab in recurrent small-cell lung cancer. Lancet Oncol. 2016;17:883–95. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 29.Gadgeel S, Ciardiello F, Rittmeyer A, Barlesi F, Cortinovis D, Barrios C, et al. PL04a. 02: OAK, a randomized ph III study of atezolizumab vs docetaxel in patients with advanced NSCLC: results from subgroup analyses. J Thorac Oncol. 2017;12:S9–S10. doi: 10.1016/j.jtho.2016.11.011. [DOI] [Google Scholar]
- 30.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quandt J, Schlude C, Bartoschek M, Cid-Arregui A, Beckhove P, Momburg F. T cell responses against mutations in oncoproteins/tumor suppressor proteins and their induction by vaccination with long peptides. Cancer Res. 2013;73:1261. doi: 10.1158/1538-7445.AM2013-1261. [DOI] [Google Scholar]
- 32.Tran E, Ahmadzadeh M, Lu Y-C, Gros A, Turcotte S, Robbins PF, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E, Robbins PF, Lu Y-C, Prickett TD, Gartner JJ, Jia L, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang QJ, Yu Z, Griffith K, Hanada K-I, Restifo NP, Yang JC. Identification of T-cell receptors targeting KRAS-mutated human tumors. Cancer Immunol Res. 2016;4:204–214. doi: 10.1158/2326-6066.CIR-15-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilen MA, Subudhi SK, Gao J, Tannir NM, Tu S-M, Sharma P. Acute rhabdomyolysis with severe polymyositis following ipilimumab-nivolumab treatment in a cancer patient with elevated anti-striated muscle antibody. J Immunother Cancer. 2016;4:36. doi: 10.1186/s40425-016-0139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshioka M, Kambe N, Yamamoto Y, Suehiro K, Matsue H. Case of respiratory discomfort due to myositis after administration of nivolumab. J Dermatol. 2015;42:1008–1009. doi: 10.1111/1346-8138.12991. [DOI] [PubMed] [Google Scholar]
- 37.Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. 2015;38:37–39. doi: 10.1097/CJI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Ali SS, Goddard AL, Luke JJ, Donahue H, Todd DJ, Werchniak A, et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol. 2015;151:195–199. doi: 10.1001/jamadermatol.2014.2233. [DOI] [PubMed] [Google Scholar]
- 40.Min L, Hodi FS. Anti-PD1 following ipilimumab for mucosal melanoma: durable tumor response associated with severe hypothyroidism and rhabdomyolysis. Cancer Immunol Res. 2014;2:15–18. doi: 10.1158/2326-6066.CIR-13-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecouflet M, Verschoore M, Giard C, Gohier P, Le Corre Y, Milea D, et al. Annales de dermatologie et de venereologie. 2012. Orbital myositis associated with ipilimumab; pp. 448–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods Section. This describes the methods used for deriving the clinical and pathological data presented. (DOCX 19 kb)
This figure shows the positive and negative controls for immune cell immunohistochemistry. Benign lymph node tissue serving as positive (+) control for presence of T cell lineage cells (CD3, CD4, CD8) and macrophage lineage cells (CD68), as well as benign smooth muscle tissue serving as a negative (−) control for these antibodies. All images are 20× magnification. (TIFF 4756 kb)


