Abstract
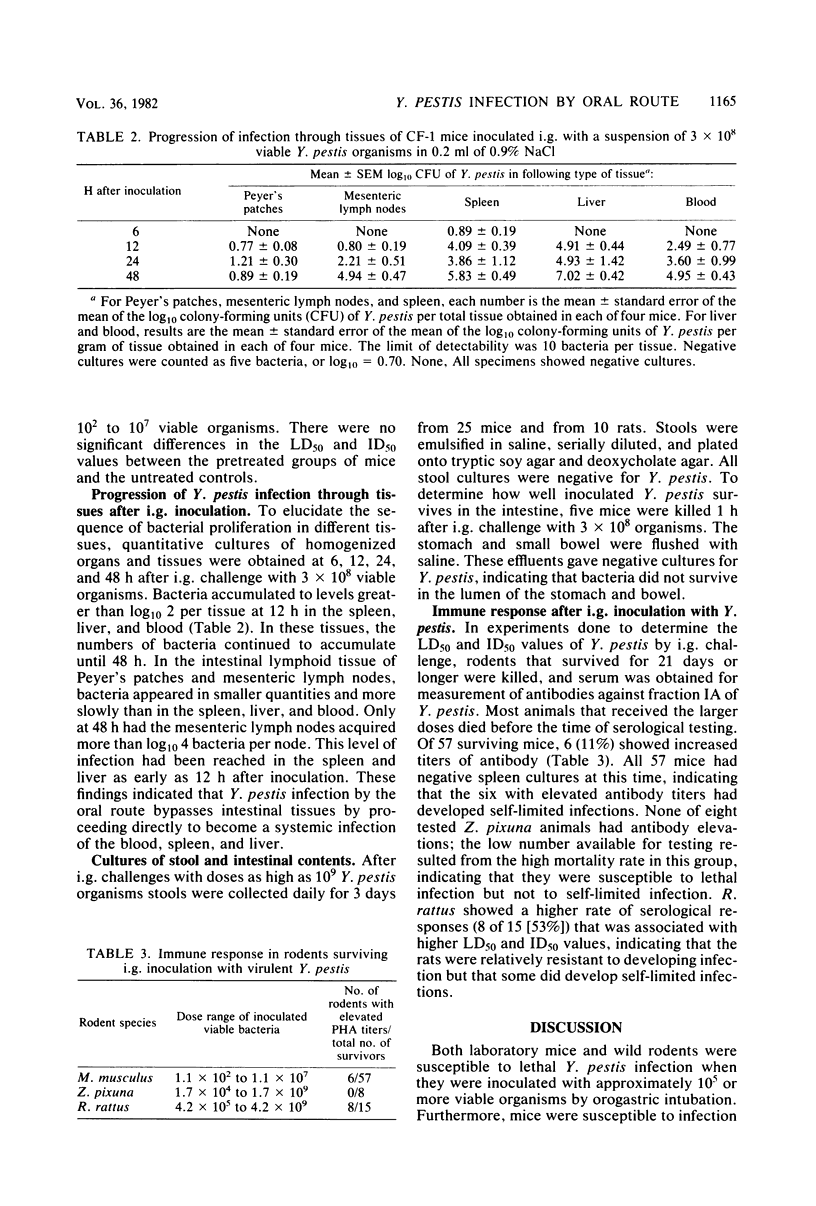
To clarify the pathogenesis of oral plague infection, we studied the susceptibility of three species of rodents to intragastric inoculation of Yersinia pestis, described the pathology and progression of infection, and measured antibody responses to fraction IA antigen of Y. pestis. The 50% lethal doses of bacteria by intragastric inoculation for Mus musculus, Zygodontomys pixuna, and Rattus rattus were log10 = 6.32, 5.46, and 9.62, respectively, which were at least 1,000-fold higher than the values obtained by subcutaneous inoculation. M. musculus was shown to be susceptible to lethal infection also when bacteria were ingested in drinking water. Microscopic pathology was consistent with heavy systemic infection. Quantitative cultures of tissues at different times after intragastric inoculation revealed that infections of blood, liver, and spleen preceded infections of Peyer's patches and mesenteric lymph nodes. Stool cultures were negative. The strain of Y. pestis used for inoculation was killed when exposed to a buffered solution at pH less than or equal to 3. Antibody responses were observed in some of the surviving rodents after intragastric challenge. These results showed that Y. pestis was an effective oral pathogen that produced fatal systemic infections and self-limited infections with immunity but did not produce enteric pathology or lead to fecal excretion of bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. II. FACTORS RESPONSIBLE FOR ITS LOSS FOLLOWING STREPTOMYCIN TREATMENT. J Exp Med. 1964 Nov 1;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R., Beesley E. D., Surgalla M. J. Pasteurella pestis: Role of Pesticin I and Iron in Experimental Plague. Science. 1965 Jul 23;149(3682):422–424. doi: 10.1126/science.149.3682.422. [DOI] [PubMed] [Google Scholar]
- Butler T., Hudson B. W. The serological response to Yersinia pestis infection. Bull World Health Organ. 1977;55(1):39–42. [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. Experimental Yersinia enterocolitica infection in mice: kinetics of growth. Infect Immun. 1974 May;9(5):851–857. doi: 10.1128/iai.9.5.851-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Collins F. M. The route of enteric infection in normal mice. J Exp Med. 1974 May 1;139(5):1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh D. C., Deoras P. J., Hunter D. H., Marshall J. D., Jr, Do-van-Quy, Rust J. H., Jr, Purnaveja S., Winter P. E. Some observations on the necessity for serological testing of rodent sera for Pasteurella pestis antibody in a plague control programme. Bull World Health Organ. 1970;42(3):451–459. [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Elbert S. S., Eisler D. M. Immunity in plague: protection induced in Cercopithecus aethiops by oral administration of live, attenuated Yersinia pestis. J Infect Dis. 1976 Mar;133(3):302–309. doi: 10.1093/infdis/133.3.302. [DOI] [PubMed] [Google Scholar]
- Christie A. B., Chen T. H., Elberg S. S. Plague in camels and goats: their role in human epidemics. J Infect Dis. 1980 Jun;141(6):724–726. doi: 10.1093/infdis/141.6.724. [DOI] [PubMed] [Google Scholar]
- Field L. H., Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun. 1981 Feb;31(2):783–791. doi: 10.1128/iai.31.2.783-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden H. D., Chang R. S., Lou J. J., Cooper T. Y. A filterable agent in throat washings of patients with infectious mononucleosis. J Infect Dis. 1971 Oct;124(4):422–424. doi: 10.1093/infdis/124.4.422. [DOI] [PubMed] [Google Scholar]
- Knop J., Rowley D. Antibacterial mechanisms in the intestine. Elimination of V. cholerae from the gastrointestinal tract of adult mice. Aust J Exp Biol Med Sci. 1975 Apr;53(2):137–146. [PubMed] [Google Scholar]
- MILLER C. P., BOHNHOFF M. CHANGES IN THE MOUSE'S ENTERIC MICROFLORA ASSOCIATED WITH ENHANCED SUSCEPTIBILITY TO SALMONELLA INFECTION FOLLOWING STREPTOMYCIN TREATMENT. J Infect Dis. 1963 Jul-Aug;113:59–66. doi: 10.1093/infdis/113.1.59. [DOI] [PubMed] [Google Scholar]
- Poland J. D., Barnes A. M., Herman J. J. Human bubonic plague from exposure to a naturally infected wild carnivore. Am J Epidemiol. 1973 May;97(5):332–337. doi: 10.1093/oxfordjournals.aje.a121513. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Moussa M. A., Cavanaugh D. C. Experimental plague in the California ground squirrel. J Infect Dis. 1979 Oct;140(4):618–621. doi: 10.1093/infdis/140.4.618. [DOI] [PubMed] [Google Scholar]
- von Reyn C. F., Weber N. S., Tempest B., Barnes A. M., Poland J. D., Boyce J. M., Zalma V. Epidemiologic and clinical features of an outbreak of bubonic plague in New Mexico. J Infect Dis. 1977 Oct;136(4):489–494. doi: 10.1093/infdis/136.4.489. [DOI] [PubMed] [Google Scholar]