Abstract
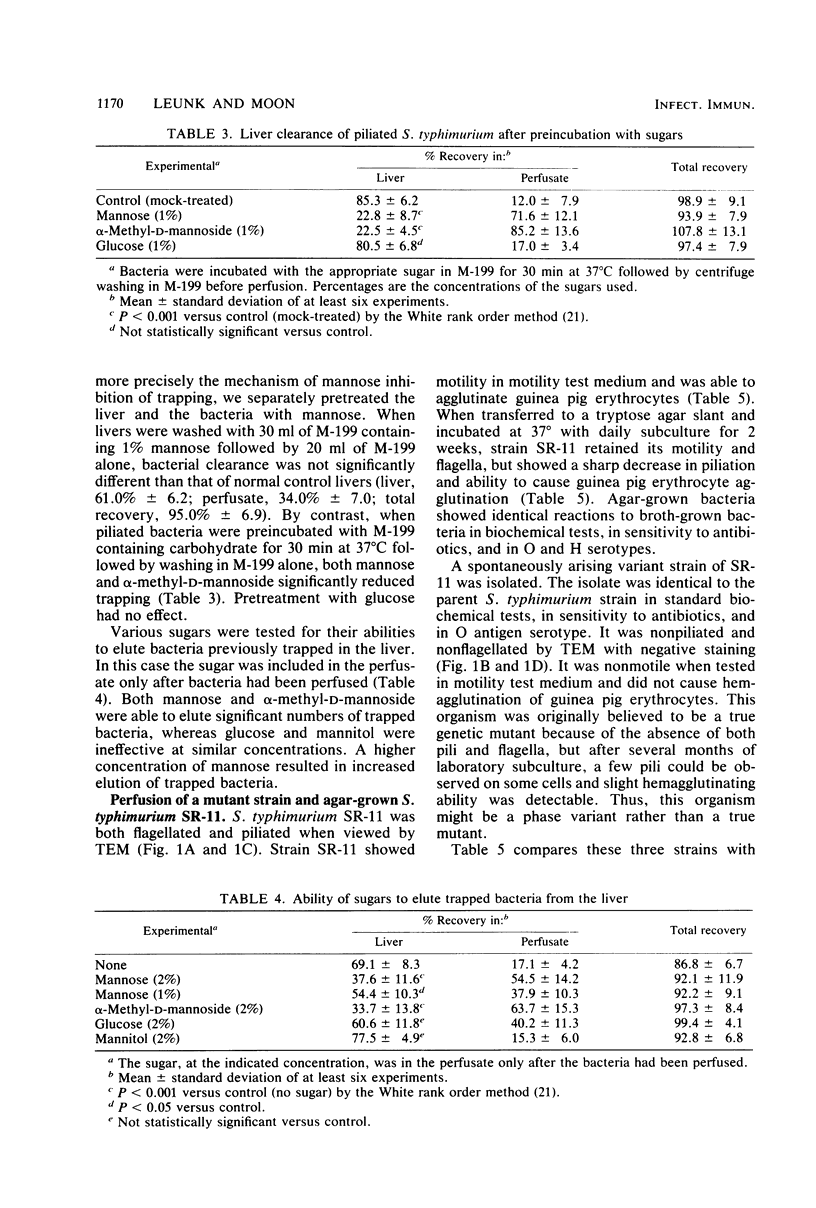
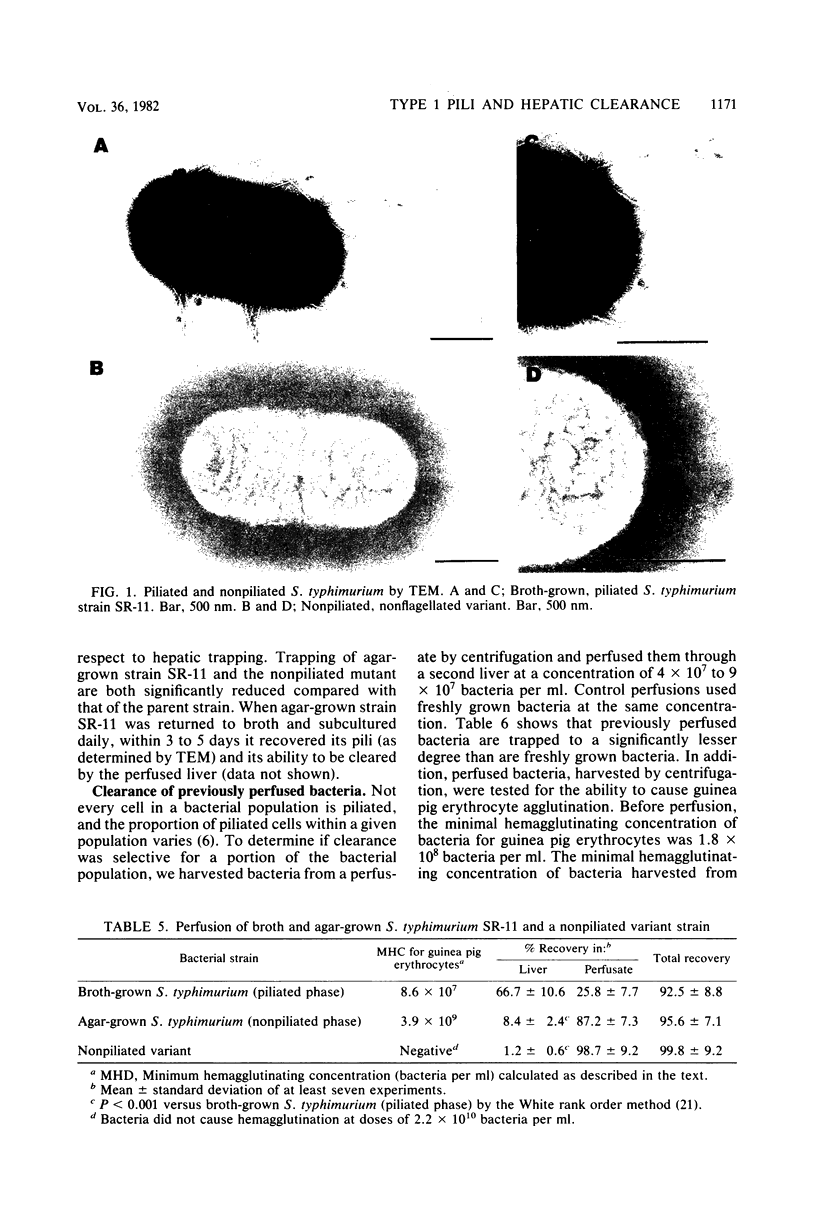
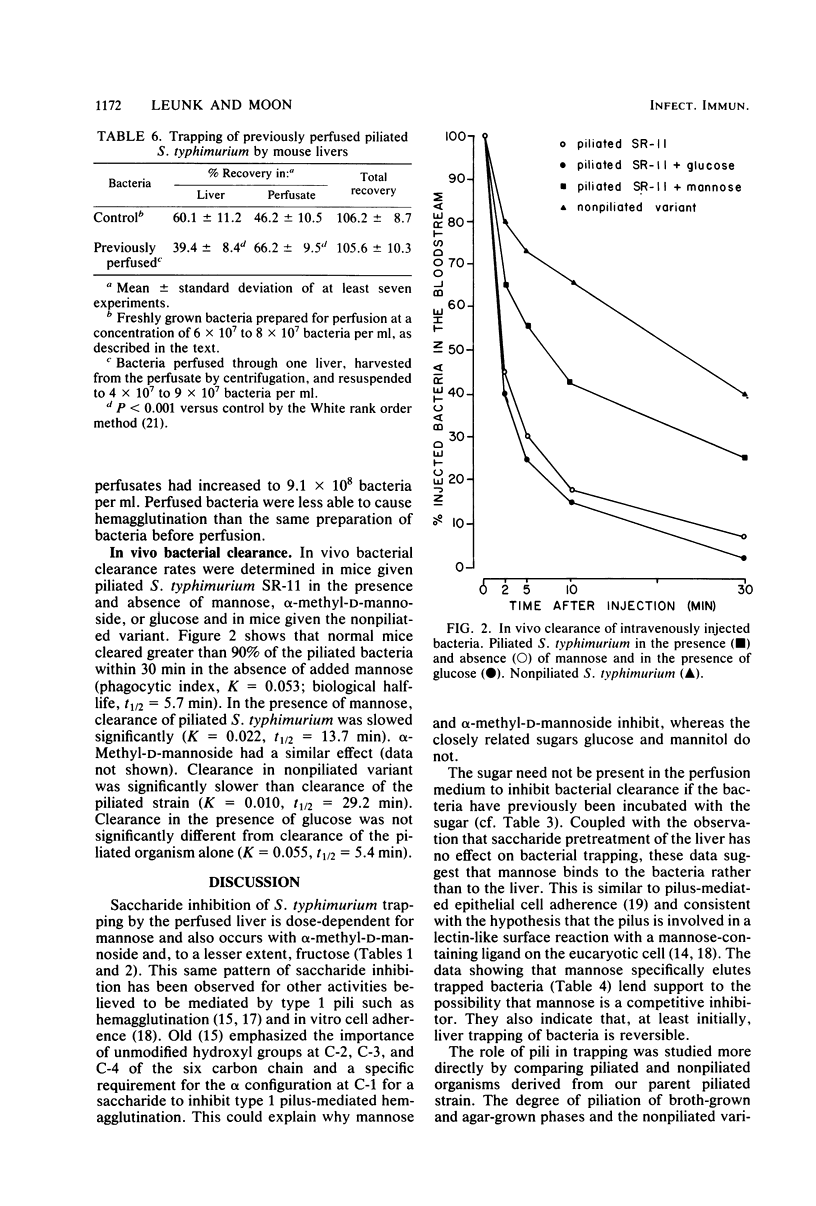
The role of type 1 pili in the adherence of Salmonella typhimurium strain SR-11 to hepatic sinusoidal cells was investigated. An average of 66.7% of piliated organisms was cleared by perfused livers on a single pass. Mannose and alpha-methyl-D-mannoside inhibited such trapping in a dose-dependent manner. Preincubation of the bacteria, but not the liver, with either sugar also inhibited trapping, suggesting that the sugar binds to bacterial, not hepatic, receptors. Significant numbers of previously trapped bacteria could be eluted by adding mannose to the wash medium. Bacteria with reduced piliation, obtained either by growing bacteria on agar or by using a nonpiliated variant of the parent strain, were trapped to a significantly lesser extent than the parent strain. The liver appears to selectively trap heavily piliated organisms since reperfusion of bacteria through a second liver results in significantly less trapping than occurs with the first perfusion. In vivo, the nonpiliated variant strain was cleared much more slowly than the piliated parent strain. Mannose and alpha-methyl-D-mannoside, but not glucose, decreased clearance rates of piliated organisms. Cumulatively, the data suggest that type 1 pili are a major factor in hepatic clearance of S. typhimurium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Atkinson H. M., Trust T. J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect Immun. 1980 Mar;27(3):938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959 Aug;21:271–286. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. J., Vrable R. A., Broka J. A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975 Aug;12(2):411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A. J., Amundsen S. K., Jones J. M. Effect of carbohydrates on adherence of Escherichica coli to human urinary tract epithelial cells. Infect Immun. 1980 Nov;30(2):531–537. doi: 10.1128/iai.30.2.531-537.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlydaszyk J. C., Moon R. J. Fate of 51Cr-labeled lipopolysaccharide in tissue culture cells and livers of normal mice. Infect Immun. 1976 Jul;14(1):100–105. doi: 10.1128/iai.14.1.100-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



