Abstract
Purpose
For patients undergoing CT colonography, an opportunity exists for concurrent osteoporosis screening without additional radiation exposure or patient time using proximal femur quantitative CT (QCT) “CTXA”.
Materials and Methods
This cohort included 129 female and 112 male adults (mean age, 60.1±8.2 years; range, 50–95 years) who underwent CT colonography between March 2013 and September 2014. Areal BMD in g/cm2 and resultant left femoral neck T-score was prospectively measured on the supine CT series using QCT Pro Version 5.1 (Mindways Software, Austin, TX). QCT results were reported with the CT colonography. Chart review evaluated if the patients were eligible for BMD screening according to the United States Preventative Service Task Force (USPSTF) and National Osteoporosis Foundation (NOF) guidelines, had undergone prior BMD testing, and to assess if QCT results changed patient management.
Results
Overall, 68.0% (164/241) of patients from this cohort had not previously undergone BMD screening. According to the NOF guidelines, 44.0% (106/241) of patients were eligible for screening. T-scores within the osteopenic and osteoporotic range were detected in 32.3% (78/241) and 5.0% (12/241) of patients respectively. Of these patients with low bone mineral density, 66.7% (60/90) had not previously undergone screening or were eligible for BMD testing. Reporting of CTXA T-scores altered management in 9 patients (3.7%) with low bone mineral density.
Conclusion
Maximizing the pre-existing value from imaging studies is crucial in the current era of healthcare reform. We demonstrate the ability to combine colorectal and osteoporosis screening at CT, adding clinical and likely economic value.
Keywords: Screening, CT colonography, osteoporosis, bone mineral density
Introduction
Osteoporosis is a common condition, affecting more than 10 million people in the United States, and is associated with a lifetime fracture risk of ~50% in females and ~20% in males [1, 2]. Osteoporosis-related fractures affect quality and quantity of life, with hip fractures in particular associated with high morbidity and mortality [3, 4]. Despite these risks and the availability of proven treatments to reduce fractures, osteoporosis is underdiagnosed and therefore under-treated in the United States [5–7]. The reasons for underdiagnosis are multifactorial and include non-adherence to screening guidelines, with approximately half of female Medicare beneficiaries never having been screened, as well as conflicting guidelines, with United States Preventative Services Task Force (USPSTF) guidelines stating that screening males is of indeterminate benefit, due predominantly to insufficient data and resource costs [7–9].
Abdominal CT scans obtained for other indications can be used in an opportunistic fashion to screen for osteoporosis without significant additional cost [10–12]. The most pertinent measurement for opportunistic screening would utilize bone mineral density (BMD) at the femoral neck, as this can be used in conjunction with the WHO FRAX tool to estimate fracture risk and guide treatment. Previous work documents that dual-energy QCT Computed Tomography X-Ray Absorptiometry (CTXA) at CT colonography (CTC) is equivalent to standard Dual X-Ray Absorptiometry (DXA) in identifying low bone mineral density [13]. As there is significant overlap in patient population between those undergoing CT colonography and those at risk for low BMD, adding CTXA evaluation to CTC could increase identification of patients at increased fracture risk with minimal additional cost. To this end, we have added femoral neck CTXA BMD evaluation to CTC screening in our clinical practice. The purpose of this study was to evaluate the clinical impact of this practice, including the detection of patients with previously unrecognized low BMD. A secondary endpoint was determining if this identification of low BMD had an effect on patient management.
Materials and Methods
Patient Cohort
The University of Wisconsin Health Sciences Institutional Review Board approved this Health Insurance Portability & Accountability Act (HIPAA) compliant retrospective study. The need for obtaining signed informed consent was waived for this retrospective analysis. Beginning in March 2013 all patients undergoing CTC at 2 (of 7) clinical sites with a phantom available underwent CTXA BMD assessment as part of their extracolonic evaluation. All patients over the age of 50 who had clinical CTXA BMD assessment at the time of CTC between March 2013 and September 2014 were identified. Patients who were referred from outside the institution were excluded from analysis due to limited information available in the electronic medical record. The final study cohort consisted of 241 patients (129 female, 112 male) ranging in age from 50–95, with mean (standard deviation [SD]) age of 60.1 (8.2) years at the time of CTC.
Computed Tomography Acquisition
Multi-detector Computed Tomography (MDCT) scanning for standard CTC screening was performed using low-dose technique. Immediately before MDCT imaging, the colon is distended with carbon dioxide using a continuous automated low-pressure delivery system. Noncontrast supine and prone MDCT acquisitions of the abdomen and pelvis then were obtained utilizing 16- or 64-detector scanners (GE Healthcare) with a 1.25-mm collimation, 120 kVp, and low-dose modulated tube current technique (noise index 50; range, 30–300 mA). Images are reconstructed using a standard soft tissue algorithm with 1.25-mm slice thickness at 1-mm intervals. For extracolonic evaluation (including BMD), the supine series is also reconstructed with a 5-mm slice thickness at 3-mm intervals. Each patient had a QCT calibration phantom on the CT table, centered at the hips (Figure 1) to allow HU calibration for BMD measurement.
Figure 1.
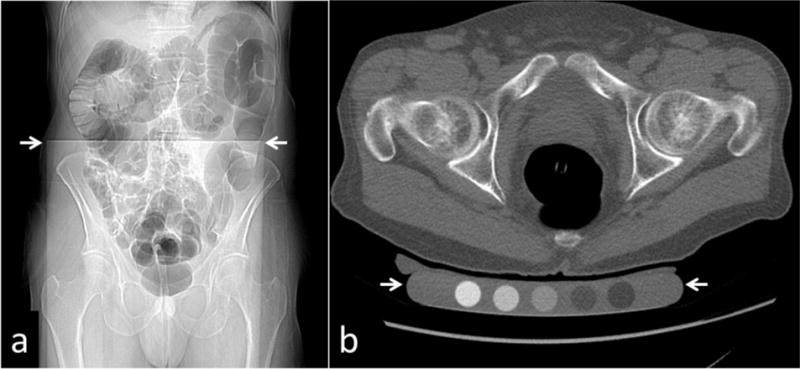
(a) CT scout image depicting phantom (top of phantom at white arrows) underneath patient. (b) Axial CT image depicting phantom (between white arrows) underneath patient on CT table.
Computed Tomography X-Ray Absorptiometry Image Analysis
The procedure for QCT hip BMD acquisition has been previously described [13]. Briefly, CTC volume images were sampled using the software “Slicepick” (Mindways Software Inc.) to produce a simulated projection anteroposterior image to locate the femoral head and lesser trochanter in the same way as using a localizer. A contiguous set of slices was then chosen covering this region for BMD analysis (Figure 2).
Figure 2.
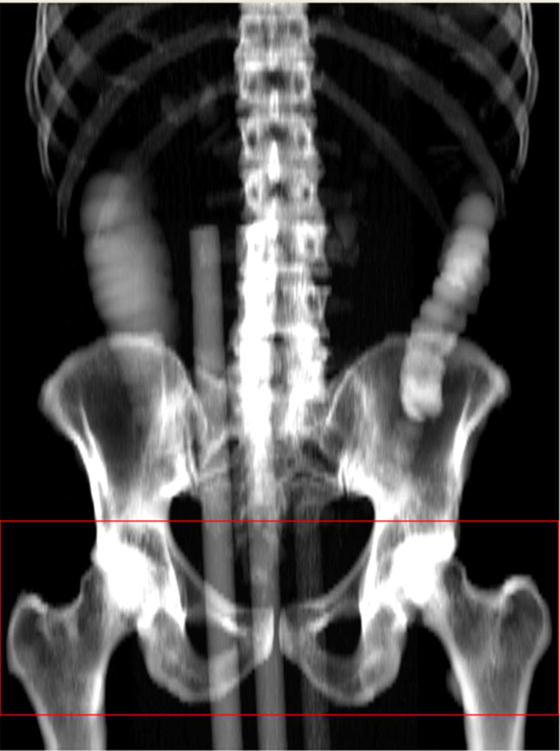
Simulated projection image used for localization. Localization region is defined between the red lines.
The QCT-derived aBMD was determined using Mindways CTXA Hip software, version 5.0, according to manufacturer’s directions (Mindways Software Inc.) This has been described in detail elsewhere [14]. Regions of interest (ROIs) similar to those used in Hologic DXA devices (Hologic Inc., Bedford, MA) for proximal femur analysis (total hip, FN, intertrochanter, and trochanter) were identified automatically on the projected image by the software (Figure 3). The left hip was chosen for analysis (n=240) unless the femoral shaft was found to be inadequately imaged for the entire lesser trochanter to be analyzed, in which case the right hip was chosen (n=1).
Figure 3.
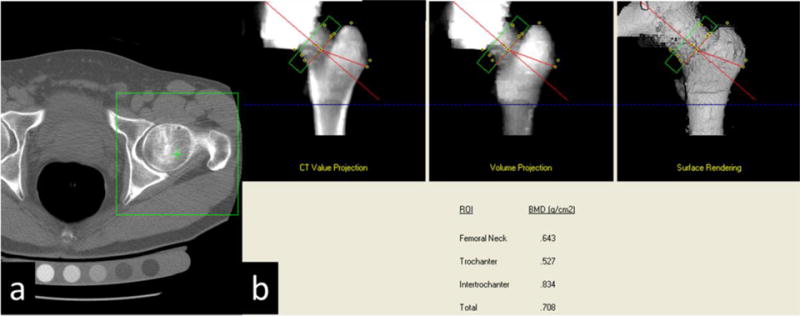
(a) Green box is utilized to select the hip for automated analysis. (b) Green box defines the femoral neck for bone mineral density determination.
The automatically identified ROIs were visually checked by dedicated CT technologists to verify that the lower extent of the intertrochanter ROI was set at the lower junction of the lesser trochanter and the femoral shaft and that the femoral neck axis and femoral neck ROI position was appropriate. The ROIs were adjusted by the technologists as required, with verification of the final selection by the physician interpreting the CT colonography to determine need for repeat analysis. Final resultant aBMD (g/cm2) were stored in the QCT Pro database for export as text files. Femoral neck T-scores were calculated using the manufacturer’s CTXA reference database. The results were prospectively reported along with colorectal and other extracolonic findings, such as AAA screening.
Chart Review
Patient electronic medical records were reviewed by a single author (Blinded) at a median of 9 months (range 2–19 months) post reporting of T-score from CTXA. The date of prior BMD, eligibility for screening according to USPSTF or National Osteoporosis Foundation (NOF) guidelines [8, 9], and management decisions made based on BMD results were recorded.
Results
Prevalence of Low Bone Mineral Density
The patient population is outlined in Figure 4. Of the study cohort, 37.3% (90/241) of patients had low BMD with 32.3% (78/241) classified as osteopenic and 5.0% (12/241) as osteoporotic. Female patients had low BMD 48.8% (63/129) of the time with 43.4% (56/129) classified as osteopenic and 5.4% (7/129) as osteoporotic. For male patients 24.1% (27/112) had low BMD with 19.6% (22/112) classified as osteopenic and 4.5% (5/112) as osteoporotic. For patients >65 years of age, 50.9% (28/55) had low BMD with 41.8% (23/55) osteopenic and 9.1% (5/55) osteoporotic. Patients 64 or younger had low BMD 33.3% (62/186) of the time with 29.7% (55/185) osteopenic and 3.8% (7/185) osteoporotic.
Figure 4.
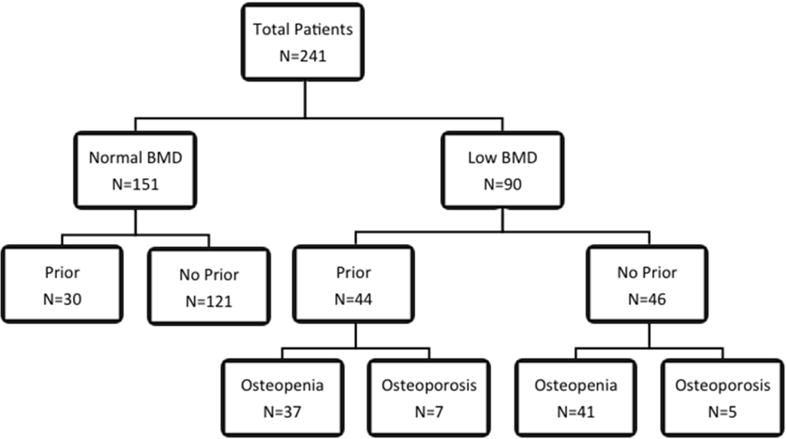
Patient Cohort breakdown. Low BMD are patients that are osteopenic (defined as T-score ≤ −1.0) or osteoporotic (defined as T-score ≤ −2.5).
Eligibility for Screening
According to USPSTF guidelines 39.8% (96/241) of patients were eligible for screening, which was 74.4% (96/129) of the female cohort. Criteria for eligibility included being female and >65 years for 29 patients and having a FRAX estimated fracture risk greater than that of a 65-year-old female (9.3% estimated 10-year risk) in 67 patients. When National Osteoporosis Foundation guidelines, which advocate screening males aged 70 and older, were used, an additional 10 males would be eligible for screening, yielding 44.0% (106/241) of the population and 8.9% (10/112) of the male cohort. Of the patients eligible for screening, 32.1% (34/106) had not previously undergone screening.
Low Bone Mineral Density Detection
Of the patients with low BMD detected at CTC, 48.9% (44/90) had not previously undergone screening. An additional 14 patients were eligible for repeat screening. The reported low BMD resulted in 1 patient starting bisphosphonate treatment, 1 patient beginning Ca++ and vitamin D supplementation, and 4 patients having follow-up DXA. An additional 3 patients with normal BMD were eligible for follow-up DXA which was cancelled due to the CTXA. In total, patient management was altered in 9 patients (3.7%) due to the opportunistic BMD evaluation at CTC.
Case example 1
A 55-year-old male who had undergone a negative CTC in 2008 has a 5 year follow-up CTC in 2013. The patient is a smoker, but is otherwise healthy and therefore would not meet current guidelines for BMD screening. CTXA was performed as an add-on to CTC with a resultant T-score of −1.9, in the osteopenic range. The patient began calcium and vitamin D supplementation and received a nutrition consultation.
Case example 2
A 59-year-old healthy female who underwent CTC in 2006 due to incomplete colonoscopy was overdue for repeat CTC in 2014. She had also previously undergone DXA in 2011 which demonstrated osteopenia, with left femoral neck T-score of −2.4. No specific plan for BMD re-evaluation was notated in the chart. CTXA was performed as an add-on to CTC with a resultant T-score of −2.7. Due to the diagnosis of osteoporosis, she was initiated on alendronate therapy with follow-up DXA scheduled in 1 year.
Discussion
CTXA is a useful addition to CTC as it allows for simultaneous BMD screening in appropriate patients without an additional study and in addition can identify those with low BMD who do not meet current screening guidelines. The addition of CTXA to a CTC examination requires minimal change to the workflow as the processing required can be efficiently performed by CT technologists after a short training period with the interpreting physician performing a visual quality control. The CTXA results can be reported by providing the BMD, T-score, and WHO classification into a template. This study also suggests that CTXA can alter patient management.
Increasing rates of BMD screening fills a clinical need as previous studies have demonstrated adherence rates to screening guidelines of 12 to 56% across groups and 19 to 97% among individual physicians [15, 16]. While one might presume providers and patients adhering to screening recommendations for colon cancer and polyps would also adhere to osteoporosis screening guidelines, 32.1% of the patients for whom BMD screening was indicated in this study had not undergone screening. In addition, this low-cost addition to CTC could potentially expand BMD screening guidelines, although further research into cost-benefit analysis would be necessitated prior to taking that step. The addition of BMD evaluation in this cohort did help identify patients with low BMD who would have otherwise gone undetected, as approximately half of patients with low BMD in this study had never been screened.
This study has the most potential to improve osteoporosis detection in men. The most recent USPSTF guidelines give an “I” rating to osteoporosis screening in males, meaning that there is insufficient evidence to support screening. Within the rationale for this rating the task force concedes that BMD testing in males could potentially prevent a substantial fracture burden, while opportunity cost is cited as the only harm. This opportunity cost is determined by the substantial number of DXA scanners that would be required to screen the male population who would benefit [8]. The addition of CTXA to CTC stands to decrease this opportunity cost of screening males, many of whom would otherwise go unscreened. The cost-effectiveness of this approach is beyond the scope of this manuscript, but is a potential area for future investigation.
BMD screening adds to a growing list of extracolonic screening opportunities at CTC. The detection of abdominal aortic aneurysms (AAA) and extracolonic cancers is a known benefit of CTC [17, 18] and is a cost-effective use of resources [19]. Other potential opportunities for added-value at CTC include screening for non-alcoholic fatty liver disease (NAFLD) via liver attenuation, measurement of visceral fat, and quantification of abdominal aortic calcification [20–22]. Beyond femoral neck CTXA, using lumbar spine attenuation measurements has also been proposed as a way to screen for osteoporosis at both CTC and routine CT [10, 11], however this method requires additional time without additional economic incentive, which could limit adoption. Comparatively, CTXA is a post-processing technique requiring only dedicated software and minimal technologist and radiologist time plus is eligible for an add-on charge, potentially increasing adoption.
An area of future investigation is to evaluate the effect of offering CTXA with CT examinations performed for any indication on adherence to BMD screening guidelines. Awareness of the problem of osteoporosis would need to increase within the abdominal radiology community as the scans which would allow co-existent screening are predominantly those of the abdomen and pelvis, and previous work has demonstrated that vertebral body compression fractures are missed in up to 84% of cases on these examinations when sagittal reconstruction are not specifically reviewed [23]. In addition, while non-contrast examinations have shown correlation with DXA and therefore would be reasonable to use for screening [11], data is still needed to prove whether the same is true for contrast-enhanced studies. Early work has demonstrated that CT enterography examinations with biomechanical image-analysis can predict low BMD in patients with inflammatory bowel disease [24]. Further work is needed to validate CTXA as a screening modality with contrast-enhanced CT. Of note, a newer version of the QCT software that does not require prospective placement of the phantom under the patient was recently approved by the FDA, which may allow for wider adoption of the opportunistic CT screening approach.
This study has several limitations, most notably in that the population was limited to those undergoing CTC. However, this patient population has a large overlap with patients recommended to undergo screening and the radiologists performing CTC are already of the screening mindset, making this an appropriate group of physicians for early adoption. An additional limitation is that follow-up action on BMD reporting was limited to chart review via the retrospective nature of the study. It is possible that action occurred on the BMD reporting that was not documented in our EMR, but occurred outside this healthcare system. We also did not actively advertise availability of this screening to referring providers, which may explain the relatively low rate of action on the results. These limitations may account for the low rate of altered patient management despite the high prevalence of abnormal results. A prospective trial that involved direct follow-up with patients would allow a more accurate evaluation of the true effects of the addition of CTXA to CTC. Finally, given the short follow-up time of this study, no evaluation can be made on fracture rates for those screened in this fashion. Again, a prospective trial comparing those who undergo CTXA at CTC with those who undergo optical colonoscopy or those who undergo CTC without CTXA would allow a more robust evaluation.
In summary, adding CTXA to CTC adds value in that it increases the number of patients screened in addition to detecting those with osteoporosis who are not within current screening guidelines. Intervention within this patient population could reduce fractures and their associated socioeconomic impact. In addition, normal BMD results at CTC could reduce the need for screening DXA. This opportunistic screen is performed with minimal cost and could replace DXA for initial screening evaluation in select populations.
Summary sentence.
Adding CTXA BMD evaluation to CT colonography adds value by increasing the number of eligible patients screened in addition to detecting patients with low bone mineral density who are not within current screening guidelines, thereby allowing improved identification of individuals at increased fracture risk.
Conclusion.
Osteoporosis is currently underscreened and underdiagnosed
CTXA bone mineral density evaluation is an easy addition to a CT colonography program
Opportunistic bone mineral density screening at CT can increase screening rates and identify low bone mineral density in those not otherwise eligible for screening
Footnotes
Disclosures: Dr. Pickhardt is co-founder of VirtuoCTC, shareholder in Cellectar Biosciences, and consultant for Check-Cap.
References
- 1.Burge R, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of Bone and Mineral Research. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.King AB, Fiorentino DM. Medicare Payment Cuts For Osteoporosis Testing Reduced Use Despite Tests’ Benefit In Reducing Fractures. Health Affairs. 2011;30(12):2362–2370. doi: 10.1377/hlthaff.2011.0233. [DOI] [PubMed] [Google Scholar]
- 3.Center JR, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA, et al. Risk of mortality following clinical fractures. Osteoporosis International. 2000;11(7):556–561. doi: 10.1007/s001980070075. [DOI] [PubMed] [Google Scholar]
- 5.Abraham A. Undertreatment of osteoporosis in men who have had a hip fracture. Archives of Internal Medicine. 2003;163(10):1236–1236. doi: 10.1001/archinte.163.10.1236-a. [DOI] [PubMed] [Google Scholar]
- 6.Kiebzak GM, et al. Undertreatment of osteoporosis in men with hip fracture. Archives of Internal Medicine. 2002;162(19):2217–2222. doi: 10.1001/archinte.162.19.2217. [DOI] [PubMed] [Google Scholar]
- 7.Wilkins CH, Goldfeder JS. Osteoporosis screening is unjustifiably low in older African-American women. Journal of the National Medical Association. 2004;96(4):461–467. [PMC free article] [PubMed] [Google Scholar]
- 8.Final Recommendation Statement: Osteoporosis: Screening. US Preventive Services Task Force. 2014 Oct; http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/osteoporosis-screening. February 27, 2015]
- 9.National Osteoporosis Foundation. [cited 2012 January 31]; Available from: http://www.nof.org/
- 10.Pickhardt PJ, et al. Opportunistic Screening for Osteoporosis Using Abdominal Computed Tomography Scans Obtained for Other Indications. Annals of Internal Medicine. 2013;158(8):588–+. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickhardt PJ, et al. Simultaneous Screening for Osteoporosis at CT Colonography: Bone Mineral Density Assessment Using MDCT Attenuation Techniques Compared With the DXA Reference Standard. Journal of Bone and Mineral Research. 2011;26(9):2194–2203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summers RM, et al. Feasibility of Simultaneous Computed Tomographic Colonography and Fully Automated Bone Mineral Densitometry in a Single Examination. Journal of Computer Assisted Tomography. 2011;35(2):212–216. doi: 10.1097/RCT.0b013e3182032537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickhardt P, et al. Comparison of Femoral Neck BMD Evaluation Obtained Using Lunar DXA and QCT With Asynchronous Calibration From CT Colonography. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2014 doi: 10.1016/j.jocd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Khoo BCC, et al. Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporosis International. 2009;20(9):1539–1545. doi: 10.1007/s00198-008-0820-y. [DOI] [PubMed] [Google Scholar]
- 15.DeJesus RS, et al. Predictors of Osteoporosis Screening Completion Rates in a Primary Care Practice. Population Health Management. 2011;14(5):243–247. doi: 10.1089/pop.2010.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen K, Maier D. Osteoporosis: Evaluation of Screening Patterns in a Primary-Care Group Practice. Journal of Clinical Densitometry. 2008;11(4):498–502. doi: 10.1016/j.jocd.2008.08.104. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, et al. Unsuspected extracolonic findings at screening CT colonography: Clinical and economic impact. Radiology. 2008;249(1):151–159. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, et al. Colorectal and Extracolonic Cancers Detected at Screening CT Colonography in 10 286 Asymptomatic Adults. Radiology. 2010;255(1):83–88. doi: 10.1148/radiol.09090939. [DOI] [PubMed] [Google Scholar]
- 19.Hassan PJ, Pickhardt, Kim DH. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: Model simulation with cost-effectiveness analysis (vol 168, pg 696, 2008) Archives of Internal Medicine. 2008;168(12):1269–1269. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 20.Boyce CJ, et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR American Journal of Roentgenology. 2010;194(3):623–8. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 21.Pickhardt PJ. CT Colonography for Population Screening: Ready for Prime Time? Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickhardt PJ, et al. Visceral Adiposity and Hepatic Steatosis at Abdominal CT: Association With the Metabolic Syndrome. American Journal of Roentgenology. 2012;198(5):1100–1107. doi: 10.2214/AJR.11.7361. [DOI] [PubMed] [Google Scholar]
- 23.Carberry GA, et al. Unreported Vertebral Body Compression Fractures at Abdominal Multidetector CT. Radiology. 2013;268(1):120–126. doi: 10.1148/radiol.13121632. [DOI] [PubMed] [Google Scholar]
- 24.Weber NK, et al. Validation of a CT-Derived Method for Osteoporosis Screening in IBD Patients Undergoing Contrast-Enhanced CT Enterography. American Journal of Gastroenterology. 2014;109(3):401–408. doi: 10.1038/ajg.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


