Abstract
Mesalamine serves as the gold standard in treating ulcerative colitis. However, its precise mechanism(s) of action remains unclear. Here, we show that mesalamine treatment rapidly decreases polyphosphate levels in diverse bacteria, including members of the human gut microbiome. This decrease sensitizes bacteria towards oxidative stress, reduces colonization and attenuates persister cell and biofilm formation, suggesting that mesalamine aids in diminishing the capacity of bacteria to persist within chronically inflamed environments.
Polyphosphates (polyPs) are universally conserved polymers composed of up to 1,000 inorganic phosphate monomers1. Microbial polyP synthesis is catalysed by polyP kinase (PPK), an enzyme almost exclusively found in bacteria1. Bacteria lacking PPK are defective in virulence, biofilm formation, persistence and oxidative stress response1,2, making PPK a potentially powerful antimicrobial drug target. We therefore developed an in vitro PPK assay and screened small molecule libraries for PPK inhibitors (see Supplementary Data). One of our identified PPK inhibitors turned out to be 5-aminosalicylic acid (also known as mesalamine) (Fig. 1a), a drug that has been used to treat patients with mild to moderate ulcerative colitis (UC) for over 70 years3,4.
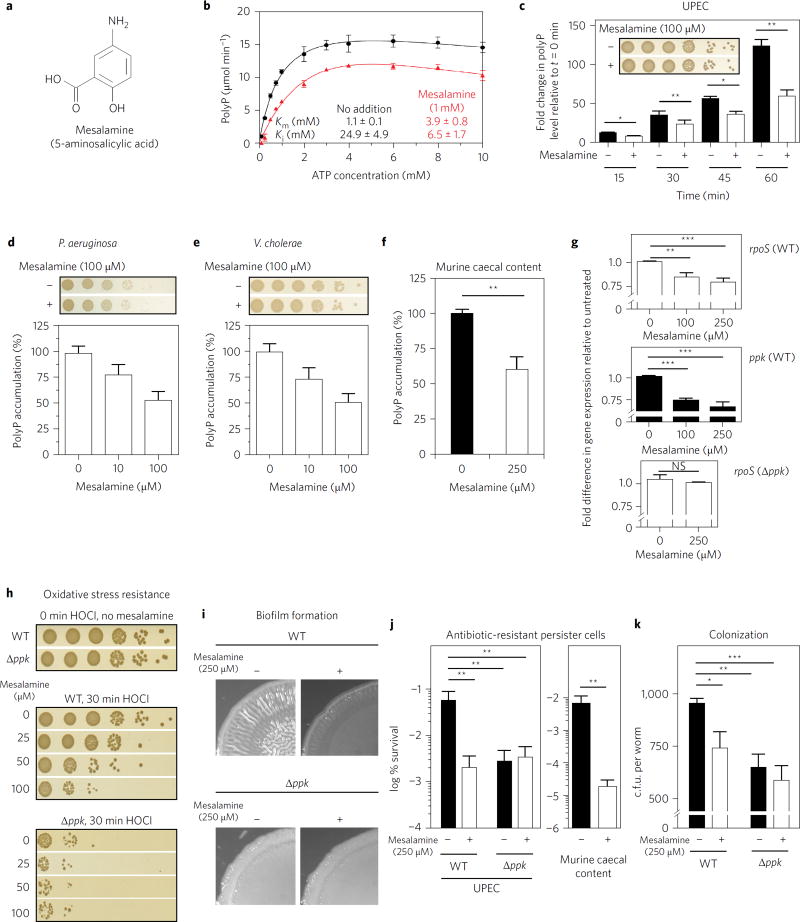
Figure 1. Mesalamine mimics ppk mutant phenotypes.
a, Structure of mesalamine. b, Michaelis–Menten kinetics of E. coli PPK with (red triangles) or without (black circles) 1 mM mesalamine. Data represent the mean ± s.d. c–f, Effect of mesalamine on nutrient-shift-induced polyP accumulation in UPEC (c), P. aeruginosa (d), V. cholera (e), or bacteria cultivated from caecal contents of healthy BL/6 mice (f). In c, *0.01 < P < 0.05, **0.001 < P < 0.01 (unpaired t-test). In d,e, data represent the mean ± s.d. In f, **P = 0.0058 (ratio paired t-test). Insets: bacterial survival was tested 60 min after nutrient shift. g–k, Effect of mesalamine on: expression of rpoS (top) and ppk (middle) in UPEC wild-type cells or rpoS in UPEC ppk− (bottom) 20 min after shift to low phosphate medium (g); oxidative stress resistance of UPEC wild-type and ppk− strains (h); macrocolony biofilm formation of UPEC wild-type and ppk− strains (i); formation of ampicillin-resistant persister cells of UPEC wild-type and ppk− strains or of bacteria cultivated from caecal contents of healthy BL/6 mice (j); and colonization of P. aeruginosa wild-type and ppk− strains in C. elegans (k). In g, NS, P > 0.01, **0.001 < P <0.01, ***0.0001 < P < 0.001 (one-way ANOVA). In j, UPEC: **0.001 < P < 0.01 (two-way ANOVA); murine caecal content: **P = 0.008 (ratio paired t-test). In k, *P < 0.05, **P < 0.01, ***P < 0.001 (two-way ANOVA). All data in bar diagrams represent mean ± s.d. Detailed information about the statistical analyses is provided in the Methods.
Kinetic studies using purified E. coli PPK confirmed that mesalamine inhibits PPK in vitro by increasing the Michaelis constant (Km) of PPK for adenosine triphosphate (ATP) and lowering its inhibitory constant (Ki) for substrate inhibition by ATP (Fig. 1b). To determine whether mesalamine treatment had any effect on the in vivo activity of PPK, we measured cellular polyP levels in a variety of PPK-containing pathogens following a shift to low-phosphate medium, a condition known to induce polyP accumulation5. Each of the tested strains showed a 50–60% reduction in polyP levels when the nutrient shift was conducted in the presence of non-lethal doses of mesalamine (Fig. 1c–e and Supplementary Fig. 2a). Similar results were obtained when we treated bacteria cultivated from the caecal content of healthy mice (Fig. 1f). These results suggested that mesalamine directly affects bacterial polyP levels and also works at concentrations lower than those needed to significantly inhibit purified PPKin vitro. Because polyP regulates its own production by inducing the transcription of rpoS, the gene encoding the general stress response sigma factor RpoS that also controls ppk expression6, we tested transcript levels of rpoS and ppk in wild-type uropathogenic Escherichia coli (UPEC) upon nutrient shift in the absence and presence of mesalamine. Indeed, we found that mesalamine significantly reduced both rpoS and ppk expression (Fig. 1g, top and middle panels) while not altering rpoS expression levels in nutrient-shifted ppk-deficient strains (Fig. 1g, bottom panel). These results suggest that mesalamine attenuates the in vivo feedback regulation of polyP via rpoS, probably contributing to the more pronounced effect of mesalamine treatment on in vivo polyP levels. Future mechanistic studies are needed to reveal the mechanism of mesalamine action on PPK and explore the possibility that bacteria themselves might convert mesalamine into a more PPK-reactive compound7.
Mesalamine, which works on the luminal side of the inflamed colonic mucosa, effectively decreases inflammation. Although its mechanisms of action(s) are still largely unknown3, recent work has suggested that in addition to affecting host responses, mesalamine might also alter the adhesion and persistence of intestinal gut bacteria, leading to a reduced load of mucosal bacteria in mesalamine-treated UC patients8. Based on our results, we now reasoned that by reducing the polyP content of bacteria, mesalamine might sensitize bacteria to inflammatory oxidants like hypochlorous acid (HOCl) (ref. 2) and/or alter their colonizing and biofilm-forming properties, thereby potentially directly contributing to gut microbiotic changes. Indeed, we found that mesalamine treatment (1) caused a dose-responsive increase in HOCl sensitivity of UPEC that was comparable to the increased HOCl sensitivity of a ppk mutant strain (Fig. 1h); (2) quite faithfully reproduced in wild-type UPEC and Pseudomonas aeruginosa the defect in macrocolony and pellicle biofilm formation previously observed in the corresponding ppk deletion mutants (Fig. 1i and Supplementary Fig. 2b,c); (3) reduced formation of ampicillin-resistant persister cells by two to three orders of magnitude in both UPEC and bacteria cultivated from healthy mouse caecal content (Fig. 1j); and (4) decreased the ability of P. aeruginosa to colonize Caenorhabditis elegans to an extent that was comparable to the colonization defect observed in a ppk deletion strain (Fig. 1k). In neither case did mesalamine treatment affect the phenotype of the respective ppk deletion strain, indicating that the observed effects are indeed PPK-dependent. Taken together, these results strongly argue for polyP production to be a relevant in vivo target of mesalamine and suggest a potential application of mesalamine for treating diseases that are caused by pathogens whose virulence and colonization depend on PPK activity1.
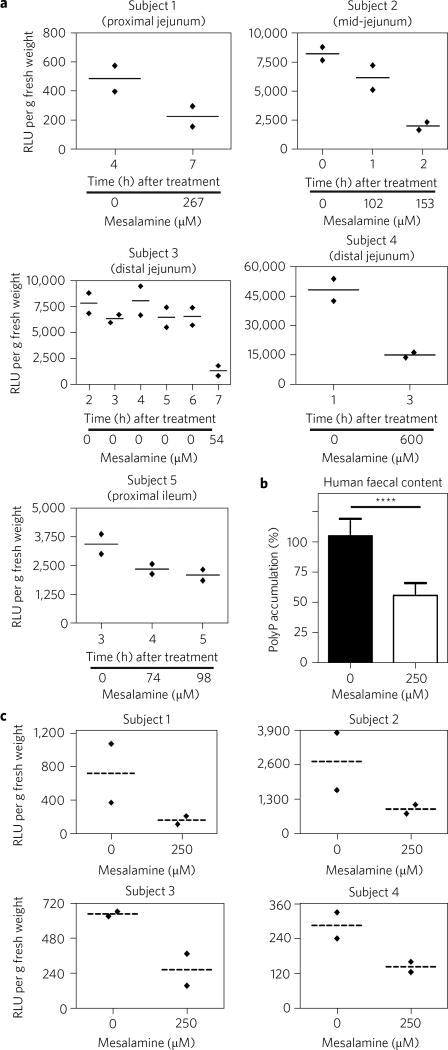
To determine the levels of mesalamine that gut bacteria encounter during treatment phases and how they affect microbial polyP levels in situ, we treated healthy human subjects with clinically relevant doses of two commonly used mesalamine-based drugs (see Supplementary Data)9. At hourly intervals, we took gastrointestinal (GI) luminal samples and tested for mesalamine and polyP contents. Not unexpectedly, the GI luminal samples of the tested subjects showed markedly different steady-state polyP levels (Fig. 2a and Supplementary Fig. 2d). These polyP levels remained largely constant as long as no mesalamine was detected. However, the moment measurable amounts of mesalamine were detected in the samples, the polyP levels were dramatically decreased (Fig. 2a, Supplementary Data and Supplementary Fig. 2d). Bacteria cultivated from human faecal content, a commonly used substitute for colon microbiome samples10, were equally responsive to mesalamine treatment. Ex vivo treatment with mesalamine caused a 50% reduction in polyP content upon nutrient shift (Fig. 2b), and an up to 70% reduction in steady-state polyP levels (Fig. 2c). These results strongly suggest that microbiota-encoded PPK is indeed a physiologically relevant target of mesalamine in humans.
Figure 2. Mesalamine reduces polyP levels in gut microbiota.
a, Steady-state polyP levels and mesalamine concentrations in GI luminal samples of healthy human subjects following oral mesalamine administration. Data for each subject represent two technical replicates and the mean. b, PolyP levels in nutrient-shifted bacteria cultivated from faecal content of healthy human subjects following ex vivo mesalamine treatment. Data represent the mean ± s.d. ****P < 0.0001 (ratio paired t-test). c, Steady-state polyP levels in stool samples of healthy human subjects after 60 min of ex vivo treatment with 250 µM mesalamine. Data for each subject represent two technical replicates and the mean. Detailed information about the statistical analyses is provided in the Methods.
Our study demonstrated that mesalamine affects polyP levels in a wide variety of different bacteria ranging from clinically isolated UPEC and P. aeruginosa strains to GI luminal samples. These results suggest that mesalamine’s effects in treating UC are not only due to its anti-inflammatory action, but are also due to it directly altering the ability of bacteria to colonize, survive and persist within an environment of chronic inflammation. Our results by no means imply that this is the sole mechanism by which mesalamine acts on UC, especially given that mesalamine is much less effective in patients suffering from Crohn’s disease (CD)11. The latter might be related to the different pathology of the two diseases: whereas inflammation is confined to the mucosa in UC, the whole thickness of the gut wall is inflamed in CD3. However, given the many roles of polyP in stress resistance, biofilm formation, persister cell formation and virulence, our findings suggest that mesalamine might have a much wider range of therapeutic applications than previously anticipated.
Methods
Ethics statements
The University of Michigan laboratory animal care policies follow the Public Health Service policy on humane care and use of laboratory animals. Animals were assessed twice daily for physical condition and behaviour and those assessed as moribund were humanely euthanized by CO2 asphyxiation. Trained animal technicians performed animal husbandry in an AAALAC-accredited facility. Five- to eight-week-old C57BL/6 wild-type (WT) mice (male or female) were obtained from a breeding colony at the University of Michigan that was originally established using animals purchased from Jackson Laboratories. Mice were housed with autoclaved food, bedding and water. Cage changes were performed in a laminar flow hood. Mice had a 12-hour cycle of light and darkness.
The human small intestinal samples used for bacterial polyphosphate quantification were obtained as part of clinical trial NCT01999400 (https://clinicaltrials.gov/show/NCT01999400). The study protocol for this clinical trial was approved by the Institutional Review Board (IRB) at the University of Michigan (IRBMED, HUM00053912) and the Research Involving Human Subjects Committee at the Food and Drug Administration (RIHSC, #11–072D). The study protocol involving the human-derived stool samples was approved by the IRB at the University of Michigan (IRBMED, HUM00120526). Informed consent was given by each subject and the study was carried out accordance with the protocol, International Conference on Harmonization Good Clinical Practice guidelines and any applicable local regulatory requirements.
Chemicals and reagents
Unless otherwise indicated, chemicals were from Sigma-Aldrich or Fisher. PolyP standards were prepared using sodium polyP from Acros Organics. Stocks (100 mM) of mesalamine and other potential inhibitors were prepared in dimethylsulfoxide (DMSO), diluted in DMSO and used immediately.
Protein purification
C-terminally His6-tagged PPK from E. coli MG1655 and N-terminally His6-tagged PPX from Saccharomyces cerevisiae were purified as previously described2.
Bacterial strains and growth conditions
All strains and plasmids used in this study are listed in Supplementary Table 1. DNA manipulations were carried out by standard methods12. UPEC strain UTI89 and its ppk::kan+ derivative, which was constructed using the lambda red recombinase method13, were provided by M. R. Chapman (University of Michigan). P. aeruginosa PA14 and its ppk1-TnMrT7::aacC1+ (GnR) derivative were from the defined transposon insertion collection of Liberati et al.14 and were provided by B. Boles (University of Iowa). V. cholerae El Tor C6706lacZ and its ppk1-TnFGL3::kan+ derivative were from the defined transposon insertion collection of Cameron et al.15 and were provided by Victor DiRita (University of Michigan).
Unless specified otherwise, UPEC, P. aeruginosa and V. cholera were grown aerobically at 37 °C in lysogenic broth (LB) or potassium morpholinopropanesulfonate (MOPS) minimal medium (Teknova) containing 0.2% glucose, 1.32 mM potassium phosphate and 10 µM thiamine. Bacteria cultivated from the caecal contents of mice or from human-derived stool samples were grown under anaerobic conditions in LB. Respective antibiotics were added when appropriate (Supplementary Table 1). To analyse the effect of mesalamine on polyP content in vivo, polyP accumulation was determined upon nutritional deprivation5 of UPEC, P. aeruginosa and V. cholerae, respectively. Briefly, cells were grown aerobically to mid-log phase in LB at 37 °C in the absence of mesalamine and collected by centrifugation. The pellet was resuspended in MOPS low-phosphate media containing 0.2% glucose, 100 µM potassium phosphate and 10 µM thiamine in the presence of various concentrations of mesalamine and incubated for 60 min. To exclude the toxic effects of mesalamine, serial dilutions in 0.9% NaCl and spot-titering on LB agar were performed using Precision XS Microplate Sample Processor (Bio-Tek) before cell harvest for polyP measurements. The same procedure was applied when the polyP content was analysed from bacteria cultivated from murine caecal contents and human-derived stool samples, respectively, with the only difference that all cultivation steps were performed under anaerobic conditions at 37 °C.
Quantification of bacterial polyP levels following nutrient deprivation
To properly assess the effects of mesalamine on in vivo polyP levels, the influence of mesalamine on nutrient deprivation-induced polyP accumulation was monitored. Under these conditions, endogenous polyP levels are sufficiently high to allow us to use previously published methods with slight variations2,16. Cells sufficient to yield 200 µg total cellular protein were collected by centrifugation and lysed by incubation for 10 min at 95 °C in 0.25 ml GITC lysis buffer (4 M guanidine isothiocyanate, 50 mM Tris-HCl, pH 7). The protein content of each sample was determined using the Bradford assay (Bio-Rad) on 5 µl aliquots. PolyP was extracted by sequential addition of 15 µl 10% sodium dodecyl sulfate, 0.5 ml 95% ethanol and 5 µl glassmilk (0.1 g ml−1 acid-washed silicon dioxide in GITC lysis buffer). This mixture was applied (1 min at 3,000g) to silica membrane spin columns (Econospin, Epoch Life Science) and rinsed twice with 0.75 ml NW Buffer (5 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM EDTA, 50% ethanol). After centrifuging once more to dry the membrane, polyP was eluted by adding 50 µl 50 mM Tris-HCl (pH 8), incubated for 15 min at room temperature, then centrifuged to collect eluate. PolyP extracts and standards of known polyP content (0–10 µM) that also went through the GITC extraction procedure were incubated 1 h at 37 °C with 250 µM adenosine diphosphate (ADP), 50 mM HEPES (pH 7.5), 50 mM ammonium sulfate, 5 mM MgCl2 in the presence or absence of 50 nM E. coli PPK. The resulting ATP was quantified with QuantiLum Recombinant Luciferase (Promega; reactions contained 50 mM Tricine buffer, pH 7.8, 10 mM MgSO4, 0.2 mM EDTA, 0.2 mM sodium azide, 1 mM dithiothreitol (DTT), 100 µM luciferin, 25 nM luciferase) in a FLUOstar Omega microplate reader (BMG Labtech). Values obtained from samples digested in the absence of PPK were subtracted from the values of samples digested in the presence of PPK to calculate the amount of polyP in each sample. Total nanomoles of polyP (in terms of individual Pi monomers) for each sample were normalized to total cellular protein.
Ex vivo mesalamine treatment of murine caecal contents and human-derived stool samples
Directly after extraction of the caecum from five- to eight-week-old C57BL/6 WT mice (male or female), the caecal contents were resuspended in 50 mM Tris/HCl (pH 8.5) and incubated in the presence or absence of 250 µM mesalamine at 37 °C for 60 min. The same procedure was applied for freshly secreted human stool samples. Subsequently, polyP was extracted and quantified as described in the subsequent section. Quantification of polyP from the caecal contents of mice and human-derived stool samples was performed for at least five individuals per condition.
Quantification of steady-state polyP levels in murine caecal content, human GI luminal samples and human-derived stool samples upon mesalamine treatment
For detection of the physiological low levels of polyP that are present in caecal contents of healthy mice, stool samples of healthy human subjects and GI luminal samples of healthy human subjects, we developed an assay consisting of four steps modified from published protocols for extraction and quantification of polyanions.
Cell lysis and extraction of polyP. Cells were lysed by alkaline lysis17. In brief, samples were resuspended in 100 mM Tris, pH 8.5, 2 mM EDTA and two volumes of lysis buffer (200 mM NaOH, 1% SDS) were added, vigorously vortexed and incubated for 10 min. Samples were neutralized by addition of one volume 8 M ammonium acetate and precipitates were spun down for 10 min at 20,000g. PolyP was then extracted by the addition of an equal volume of phenol: chloroform (pH 4) to the supernatant18. The pH of the phenol affects the distribution of DNA and RNA between the organic and aqueous phases and at lower pH DNA is removed from the aqueous phase efficiently19. The phases were separated by centrifugation at 14,000g for 10 min. The aqueous phase was transferred to another tube and the organic layer was back extracted with 1 volume 100 mM Tris-HCl, pH 8.5, 2 mM EDTA. The pooled aqueous phase was then extracted with one volume of chloroform to remove organic solvents. Subsequently, polyP was precipitated with 2.5 volumes of ice-cold ethanol overnight at −80 °C. After centrifugation, supernatants were removed and the remaining pellet was dried in a speedvac without heating. Precipitants were resuspended in the desired volume of 100 mM Tris (pH 8.5).
Apyrase treatment. To reduce the background ATP signal, samples were incubated 1/20 volume 40 mM Hepes, pH 7.5, 76 mM CaCl2 and 0.5 U ml−1 apyrase for 20 min at 30 °C. The reaction was inactivated for 20 min at 60 °C.
PPK reaction. PolyP extracts were incubated for 1 h at 37 °C with 250 µM ultrapure ADP, 50 mM HEPES (pH 7.5), 50 mM ammonium sulfate, 5 mM MgCl2 in the presence or absence of 50 nM E. coli PPK.
Detection of ATP. The resulting ATP was quantified with QuantiLum Recombinant Luciferase (Promega) (reactions contained 50 mM Tricine buffer, pH 7.8, 10 mM MgSO4, 0.2 mM EDTA, 0.2 mM sodium azide, 1 mM DTT, 100 µM luciferin, 25 nM luciferase) in a FLUOstar Omega microplate reader (BMG Labtech). Values obtained from samples digested in the absence of PPK were subtracted from the values of samples digested in the presence of PPK to calculate the amount of polyP in each sample. As additional control to see whether the detected signal represents in fact polyP-derived ATP, we digested the polyP of each sample with PPX before the PPK reaction as described elsewhere2. Only samples, whose relative luminescence units (RLUs) were reduced by incubation with PPX were considered to be polyP. The RLUs of polyP for each sample were normalized to gram fresh weight.
Phenotypic analyses
Transcriptional studies using quantitative real-time polymerase chain reaction
Because polyP in Gram-negative bacteria has been shown to induce the transcription of rpoS, a sigma factor that regulates not only expression of general stress response genes but also of ppk6,20, we measured ppk and rpoS mRNA levels in phosphate-starved WT UPEC cells. Gene expression analysis by real-time polymerase chain reaction (RT–PCR) was performed upon nutritional deprivation5 of UPEC strains UTI89 and UTI89 ppk, respectively. Briefly, cells were grown aerobically to mid-log phase in LB at 37 °C in the absence of mesalamine and collected by centrifugation. The pellet was resuspended in MOPS low-phosphate media in the presence of the indicated concentrations of mesalamine and incubated for 20 min. RNA was prepared using the NucleoSpin RNA kit (Macherey&Nagel) and DNA-free kit (Ambion). A PrimeScript 1st strand cDNA Synthesis Kit (Takara) was used to generate cDNA, and RT–PCRs were set up with SYBR GreenER qRT–PCR mix (Invitrogen) and a Mastercycler ep realplex2 real-time PCR system (Eppendorf). Expression ratios were calculated compared with the expression of each gene in untreated UTI89 and UTI89 ppk cultures, respectively, by the ΔΔCT method21, and normalized to the expression of rrsD (encoding 16S rRNA), the expression of which did not change under the conditions tested. Primers used for RT–PCR analysis were: rrsD, 5′ AGA GTT TGA TCC TGG CTC AG 3′ and 5′ TTA CTC ACC CGT CCG CCA CTC 3′; ppk, 5′ GGT GCG TTT TGT TTA TCA GCG CG 3′ and 5′ CCA GAT TGG CTT TGC CGACAT 3′; rpoS, 5′ GGTAGA GAA GTT TGACCC GGA ACG 3′ and 5′ CGG TTC GCA GGT AAA CGT T 3′.
HOCl sensitivity assay
The survival of UTI89 and UTI89 ppk under HOCl stress in the presence and absence of various mesalamine concentrations was investigated as described previously22. UPEC UTI89 WT and UTI89 ppk were grown in the presence and absence of various concentrations of mesalamine at 37 °C with shaking in MOPS glucose medium to mid-log phase. Once absorbance at 600 nm had reached 0.45, 2.5 mM HOCl was added to 125 ml baffled flasks and incubated at 37 °C with shaking (200 r.p.m.) for 30 min. Cells (0.5 ml) were collected by centrifugation immediately before and 30 min after HOCl addition, then rinsed with MOPS medium containing 10 mM Na2S2O3, but no glucose, K2HPO4 or thiamine. Dilutions in 0.9% NaCl and spot-titering on LB agar were performed using a Precision XS Microplate Sample Processor (Bio-Tek). Each strain was tested at least four times.
Biofilm formation
To monitor the macrocolony biofilm, overnight cultures of E. coli UTI89 and UTI89 ppk were diluted to an optical density at 600 nm (OD600) of approximately 1 in YESCA broth as described previously23. A 6 µl volume of each culture was spotted onto YESCA agar plates (1.2 g l−1 yeast extract, 10 g l−1 casamino acids, 20 g l−1 agar) containing either no or 250 µM mesalamine. The plates were incubated at 26 °C and the wrinkled colony phenotype was followed over time. Pictures were taken after 30 h with an Olympus DP72 camera mounted on an Olympus SZX16 research stereomicroscope using bright-field microscopy. Each strain was tested at least three times.
For pellicle biofilm formation of UPEC, 1 µl of an overnight culture of E. coli strains UTI89 or UTI89 ppk was used to inoculate 1 ml YESCA broth (1.2 g l−1 yeast extract, 10 g l−1 casamino acids) supplemented with or without the indicated concentrations of mesalamine in sterile 24-well plates. The plates were incubated at 26 °C. After 5 days of growth, pellicle biofilms were rinsed with double-distilled H2O and stained with crystal violet using a published protocol24. P. aeruginosa biofilms were grown in M63 media (2 g l−1 (NH4)2SO4, 13.6 g l−1 KH2PO4, 0.5 mg l−1 FeSO4× 7H2O, 1 mM MgSO4, 0.2% glucose, 0.1% casamino acids, 10 µM thiamine, pH 7.0) with a starting OD600 = 0.001 in sterile 96-well plates for 24 h at 37 °C without shaking. Biofilms were solubilized in 30% acetic acid for quantification by absorbance at 550 nm. Each strain was tested at least three times.
Antibiotic persistence
Persistence was measured as described previously25. Overnight cultures of UTI89 and UTI89 ppk as well as the murine caecal content were diluted 100-fold into 10 ml fresh LB medium supplemented with or without the indicated concentrations of mesalamine and incubated for 2.5 h at 37 °C in a closed falcon tube under constant shaking. Ampicillin (Amp) was then added to a final concentration of 100 µg ml−1 and cells were incubated for 5 h at 37 °C in a closed falcon tube under constant shaking. Aliquots (1 ml) were removed before and after antibiotic treatment, spun down, and washed once with LB fresh medium either containing or not containing the indicated concentration of mesalamine, serially diluted and plated on LB solid medium. Persistence was defined as follows: % persistence (log % survival) = c.f.u./ml (surviving cells after Amp treatment)/c.f.u./ml (surviving cells before Amp treatment). Each strain was tested at least three times.
Bacterial colonization assay
Colonization of C. elegans with P. aeruginosa was performed as described previously, with slight modifications26. In brief, nematodes were age-synchronized by bleaching, and embryos were incubated at 25 °C on nematode growth media (NGM) agar plates containing P. aeruginosa PA14 and PA14 ppk. After 3 days, worms were washed twice with M9 + 25 mM levamisole for paralysis and inhibition of pharyngeal pumping and expulsion. Subsequently, worms were washed twice with M9 + 25 mM levamisole + 300 µg ml−1 carbenicillin to remove surface bacteria. Finally, worms were washed twice in M9 buffer alone. The washed nematodes were then counted in the BioSorter (Union Biometrica). A total of 50 worms were collected and resuspended in 125 ul PBS buffer with 1% Triton X-100 and mechanically disrupted using a motor pestle. Worm lysates were serial-diluted in PBS buffer, plated onto LB agar plates and incubated at 37 °C. c.f.u. were counted the next day. Each strain was tested at least three times.
Statistics
The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment. No statistical methods were used to predetermine samples size. All experiments were repeated at least three independent times. The statistical significance of variance is reported per experiment in figure legends. All centre values are means. Error bars represent standard deviation (s.d.). Figure 1b: mean ± s.d., n = 3 individual experiments. Fig. 1c: mean ± s.d., n = 3 independent experimental replicates per condition, unpaired t-test, *0.01 < P < 0.05, **0.001 < P < 0.01. Fig. 1d: mean ± s.d., n = 3 independent experimental replicates per condition. Fig. 1e: mean ± s.d., n = 3 independent experimental replicates per condition. Fig. 1f: mean ± s.d., n = 3 independent experimental replicates per condition involving five individuals, ratio paired t-test, **P = 0.0058. Fig. 1g: mean ± s.d., n = 3 biological replicate bacterial cultures per condition, one-way analysis of variance (ANOVA), NS, P > 0.01, **0.001 < P < 0.01, ***0.0001 < P < 0.001. Fig. 1h: each strain/condition was tested three times in independent experiments. Fig. 1i: Each strain/condition was tested four times in independent experiments. Fig. 1j: UPEC: mean ± s.d., n = 3 biological replicate bacterial cultures per condition, two-way ANOVA, **0.001 < P < 0.01; caecal content: mean ± s.d., n = 3 biological replicate bacterial cultures per condition, ratio paired t-test, **P = 0.0088. Fig. 1k: mean ± s.d., n = 3 independent experimental replicates per condition, two-way ANOVA, *P < 0.05, **P < 0.01, ***P < 0.001. Fig. 2a: each subject represents a biological replicate; two technical replicates were measured for subjects 1 to 5 and the mean is shown. Fig. 2b: mean ± s.d., n = 3 independent experimental replicates per condition, ratio paired t-test, ****P < 0.0001. Fig. 2c: each subject represents a biological replicate; two technical replicates per subject were measured and the mean is shown.
Supplementary Material
Acknowledgments
The authors acknowledge the University of Michigan Center for Chemical Genomics for assistance with high-throughput screening. The authors thank K. Vendrov for killing the mice and preparing caecal samples, D. Knoefler for help with the statistical analyses and C.M. Cremers for help with establishing the assay for quantification of steady-state polyP levels. The authors thank L. Xie and K. Wan for their help with the purification of PPX and PPK, respectively. This work was funded by the National Institute of Health grants GM065318 (to U.J.), AI090871 and AI24255 (to V.B.Y.) and by FDA grants HHSF223201000082C and HHSF223201300460A (to D.S.). Clinical samples were collected with help from the Michigan Institute for Clinical & Health Research (MICHR) NIH grant UL1TR000433. J.-U.D. is supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft grant DA1697/1-1.
Footnotes
Data availability. The data that support the findings of this study are available from the corresponding author upon request.
Author contributions
J.-U.D., M.J.G., D.B. and F.B. designed and carried out experiments. M.J.K., Y.W., J.R.B., W.L.H. and D.S. designed and carried out the biopsies of the human GI samples and the subsequent analyses of mesalamine concentrations. J.-U.D., M.J.G. and U.J. conceived the study, interpreted the results and wrote the manuscript. V.B.Y. was involved in conceiving the experiments for detection of polyP in the caecal contents of mice. J.L. was involved in establishing the assay for quantification of steady-state polyP levels.
Supplementary information is available for this paper.
Competing interests
The authors declare no competing financial interests.
References
- 1.Rao NN, Gómez-García MR, Kornberg A. Annu. Rev. Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 2.Gray MJ, et al. Mol. Cell. 2014;53:689–699. doi: 10.1016/j.molcel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauso Ø, Martinsen TC, Waldum H. Scand. J. Gastroenterol. 2015;50:933–941. doi: 10.3109/00365521.2015.1018937. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, MacDonald JK. Inflamm. Bowel Dis. 2012;18:1785–1794. doi: 10.1002/ibd.23024. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda A, et al. Proc. Natl Acad. Sci. USA. 1999;96:14264–14269. doi: 10.1073/pnas.96.25.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiba T, Tsutsumi K, Yano H. Proc. Natl Acad. Sci. USA. 1997;94:11210–11215. doi: 10.1073/pnas.94.21.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hogezand RA, et al. Eur. J. Clin. Pharmacol. 1992;43:189–192. doi: 10.1007/BF01740669. [DOI] [PubMed] [Google Scholar]
- 8.Andrews CN, et al. Aliment. Pharmacol. Ther. 2011;34:374–383. doi: 10.1111/j.1365-2036.2011.04732.x. [DOI] [PubMed] [Google Scholar]
- 9.Yu A, et al. Mol. Pharmaceutics. (in the press) [Google Scholar]
- 10.Jandhyala SM, et al. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim WC, Wang Y, MacDonald JK, Hanauer S. Cochrane Database Syst. Rev. 2016 doi: 10.1002/14651858.CD008870.pub2. http://doi.org/bwwv. [DOI] [PMC free article] [PubMed]
- 12.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 13.Datsenko KA, Wanner BL. Proc. Natl Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati NT, et al. Proc. Natl Acad. Sci. USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron DE, Urbach JM, Mekalanos JJ. Proc. Natl Acad. Sci. USA. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ault-Riché D, et al. J. Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnboim HC. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumble KD, Kornberg A. J. Biol. Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 19.Brawerman G, Mendecki J, Lee SY. Biochemistry. 1972;11:637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- 20.Maciag A, et al. Nucleic Acids Res. 2011;39:5338–5355. doi: 10.1093/nar/gkr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaffl MW. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray MJ, Wholey W-Y, Parker BW, Kim M, Jakob U. J. Biol. Chem. 2013;288:13789–13798. doi: 10.1074/jbc.M113.454421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremers CM, et al. Mol Cell. 2016;63:768–780. doi: 10.1016/j.molcel.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y, Smith DR, Hufnagel DA, Chapman MR. Methods Mol. Biol. 2013;966:53–75. doi: 10.1007/978-1-62703-245-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maisonneuve E, Gerdes K. Cell. 2014;157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 26.Portal-Celhay C, Blaser MJ. Infect. Immun. 2012;80:1288–1299. doi: 10.1128/IAI.05522-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.