Abstract
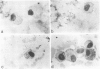
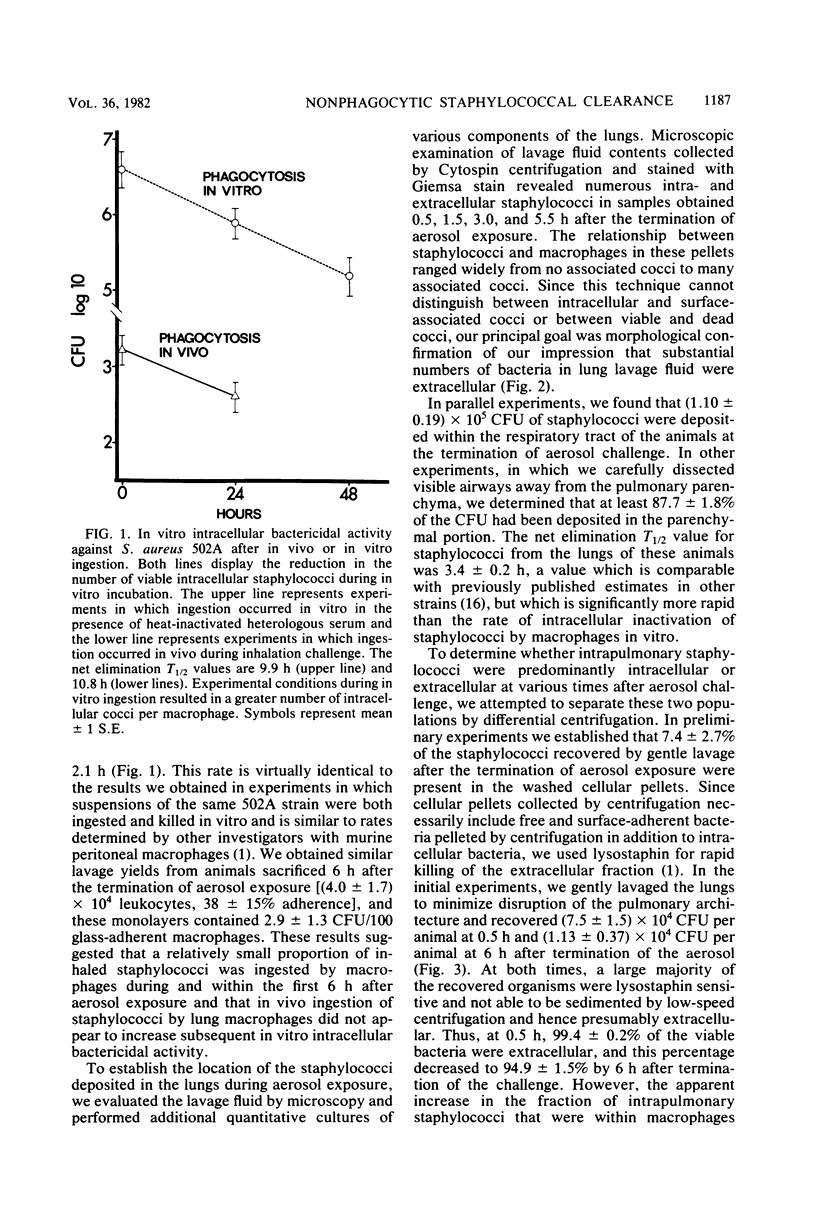
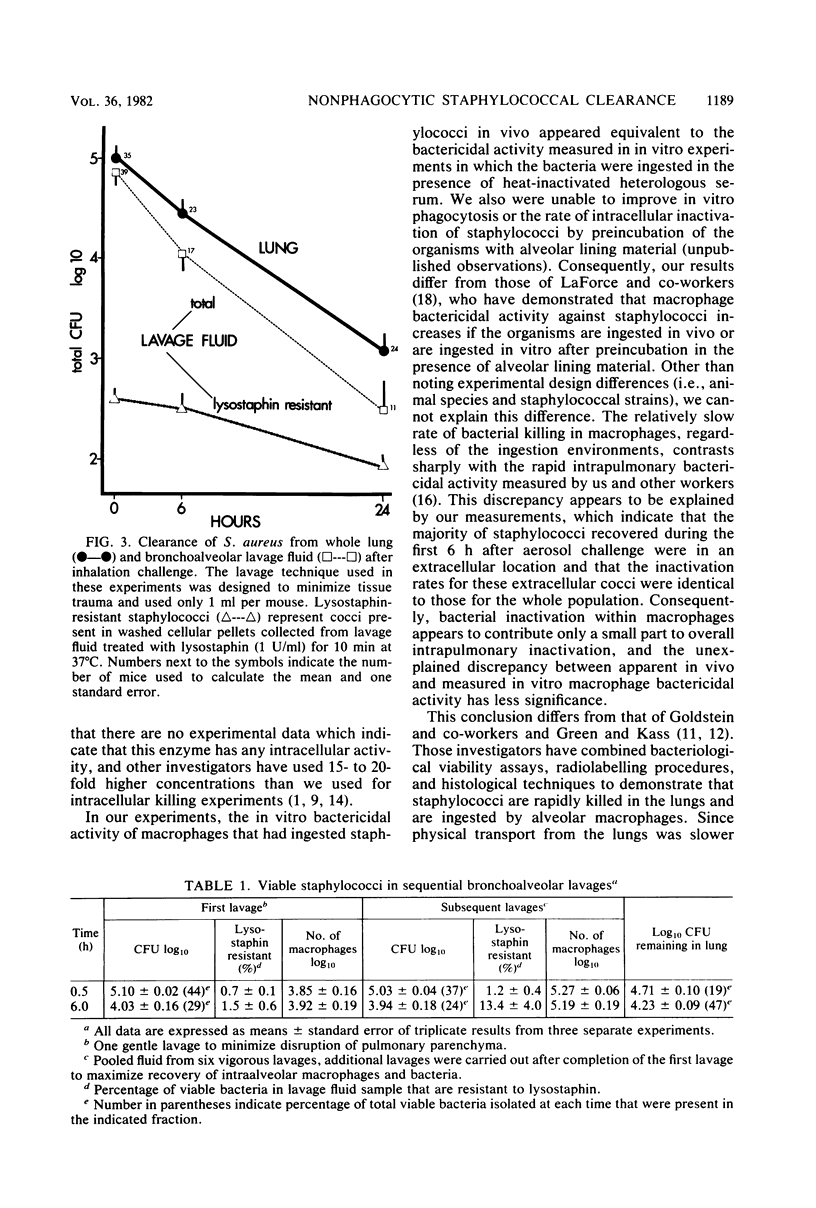
Several investigations of host and bacterial factors critical to staphylococcal clearance from lungs suggest that alveolar macrophages may not provide the principal defense against inhaled staphylococci. We evaluated possible contributions of extracellular bactericidal activities in lungs with a standard aerosol challenge model by using methods which allowed recovery of macrophages for in vitro bactericidal assays and recovery of intrapulmonary staphylococci for clearance studies. Macrophages recovered by a gentle lavage technique immediately after aerosol exposure contained 6.0 +/- 2.3 colony-forming units of viable staphylococci per 100 glass-adherent macrophages. These intracellular staphylococci were killed in vitro with a half-life of 10.8 +/- 2.1 h, which is identical to our results with a completely in vitro system for ingestion and killing. However, 99.4 +/- 0.2% and 94.9 +/- 1.5% of the viable cocci recovered in bronchoalveolar lavage at 0.5 and 6.0 h after aerosol exposure were sensitive to lysostaphin, a rapidly bactericidal enzyme with no demonstrable activity against intracellular organisms and therefore, presumably extracellular. Photomicrographs from lavage pellets obtained 0.5, 1.5, 3.0, and 5.5 h after aerosol exposure confirmed the presence of numerous extracellular cocci. These extracellular cocci were eliminated at the same rate as whole lung cocci (half-life = 3.07 and 3.14 h, respectively) and at a much faster rate than intracellular cocci. In summary, we found large numbers of extracellular staphylococci in bronchoalveolar spaces during the first 6 h after aerosol exposure that are inactivated at the same rate as the whole lung bacterial population. Since only a small number of staphylococci are ingested by macrophages and intracellular bactericidal activity appears too slow to explain intrapulmonary killing, we conclude that an as yet unidentified extracellular killing process contributed to staphylococcal clearance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R., Bonventre P. F. Phagocytosis and intracellular killing of Staphylococcus aureus by normal mouse peritoneal macrophages. Infect Immun. 1975 Aug;12(2):346–352. doi: 10.1128/iai.12.2.346-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Role of lysozyme in the microbicidal activity of rat alveolar macrophages. Infect Immun. 1977 Jun;16(3):974–982. doi: 10.1128/iai.16.3.974-982.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D. Pulmonary opsonins in Klebsiella pneumoniae pneumonia in rats. Infect Immun. 1981 Aug;33(2):533–539. doi: 10.1128/iai.33.2.533-539.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber J. H., Daniele R. P. Chemotactic activity of guinea pig alveolar macrophages. Am Rev Respir Dis. 1978 Apr;117(4):673–684. doi: 10.1164/arrd.1978.117.4.673. [DOI] [PubMed] [Google Scholar]
- DeMaria T. F., Kapral F. A. Pulmonary infection of mice with Staphylococcus aureus. Infect Immun. 1978 Jul;21(1):114–123. doi: 10.1128/iai.21.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S., Lanyon H., Cole P. J. Use of lysostaphin to remove cell-adherent staphylococci during in vitro assays of phagocyte function. Br J Exp Pathol. 1978 Aug;59(4):381–385. [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S. N., Hollinger M. A. Effects of lung toxins on PGF2 alpha and PGE2 levels in plasma and combined pleural effusion and lung lavage of rats. Adv Prostaglandin Thromboxane Res. 1980;7:953–956. [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G. N., Rehm S. R., Toews G. B., Hart D. A., Pierce A. K. Lung clearance of Staphylococcus aureus strains with differing protein A content: protein A effect on in vivo clearance. Infect Immun. 1978 Jul;21(1):7–9. doi: 10.1128/iai.21.1.7-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington-Fowler L., Henson P. M., Wilder M. S. Fate of Listeria monocytogenes in resident and activated macrophages. Infect Immun. 1981 Jul;33(1):11–16. doi: 10.1128/iai.33.1.11-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J. Factors influencing the immune enhancement of intrapulmonary bactericidal mechanisms. Infect Immun. 1976 Aug;14(2):389–398. doi: 10.1128/iai.14.2.389-398.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S. J., Johanson W. G., Jr, Pierce A. K., Reisch J. S. Determinants of lung bacterial clearance in normal mice. J Clin Invest. 1976 Apr;57(4):811–817. doi: 10.1172/JCI108356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Egan R. W. Prostaglandins, arachidonic acid, and inflammation. Science. 1980 Nov 28;210(4473):978–984. doi: 10.1126/science.6254151. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Marom Z., Shelhamer J. H., Kaliner M. Effects of arachidonic acid, monohydroxyeicosatetraenoic acid and prostaglandins on the release of mucous glycoproteins from human airways in vitro. J Clin Invest. 1981 Jun;67(6):1695–1702. doi: 10.1172/JCI110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Effect of influenza infection on the phagocytic and bactericidal activities of pulmonary macrophages. Infect Immun. 1979 Nov;26(2):651–657. doi: 10.1128/iai.26.2.651-657.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeling D. J., Peterson P. K., Hammerschmidt D. E., Kim Y., Verhoef J., Wilkinson B. J., Quie P. G. Chemotaxigenesis by cell surface components of Staphylococcus aureus. Infect Immun. 1979 Oct;26(1):57–63. doi: 10.1128/iai.26.1.57-63.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. P., Brain J. D. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. Anat Rec. 1975 Mar;181(3):581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- Territo M. C., Golde D. W. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):111–120. [PubMed] [Google Scholar]
- Watson J. A., Auld J. A., Meyer G. C. Clearance and inactivation of the vegetative and spore forms of Bacillus subtilis Var niger in rat lungs. Am Rev Respir Dis. 1973 Jun;107(6):975–984. doi: 10.1164/arrd.1973.107.6.975. [DOI] [PubMed] [Google Scholar]
- Way C. F., Dougherty W. J., Cook J. A. Immunohistochemical demonstration of lysozyme in the major reticuloendothelial organs of glucan-stimulated rats. J Reticuloendothel Soc. 1980 Jun;27(6):585–594. [PubMed] [Google Scholar]