Abstract
Objective
To objectively and subjectively compare nonionic iohexol and ionic diatrizoate iodinated oral contrast as part of a cathartic bowel regimen within the same CT colonography (CTC) cohort, with otherwise identical preparations.
Materials and Methods
In our IRB-approved retrospective study, 46 asymptomatic adults (mean age, 59.4 years; 26M/20F) returning for follow-up CTC over a 9-month interval underwent the same bowel preparation with the exception of 75 ml iohexol 350 (Omnipaque) in place of 60 ml diatrizoate (Gastrografin). All other preparation components (bisacodyl, magnesium citrate, and 2% barium) remained constant. Objective volumetric analysis of residual colonic fluid volume and fluid attenuation was performed. Additionally, two radiologists experienced with CTC, blinded to the specific bowel preparation, scored each of 6 colonic segments for adherent residual solid stool using a previously validated 4-point scale (0 for no stool; 1–3 for increasing residual stool). Paired t-test was used for comparison of the cohorts.
Results
No clear clinically-meaningful difference was found between the two preparations on overall objective or subjective evaluation. Mean (±SD) residual fluid volume was 173±126 ml with the iohexol preparation and 130±79 ml with the diatrizoate prep (p=0.02). Mean total colonic stool score was 2.5 (0.42/segment) with iohexol and 2.3 (0.38/segment) with diatrizoate (p=0.69). Mean (±SD) fluid attenuation was higher with iohexol (849±270HU) compared with diatrizoate (732±168HU) (p=0.03).
Conclusions
Based on this direct intra-patient comparison, we found that oral iohexol is a suitable alternative to diatrizoate for fluid tagging as part of a cathartic bowel preparation at CTC. Because this nonionic tagging agent is more palatable, less expensive, and likely safer than ionic diatrizoate, our CTC program now utilizes iohexol as the standard recommended regimen.
Introduction
Colorectal cancer screening with CT colonography (CTC) has proven effective in terms of accurate detection of advanced neoplasia, safety, patient acceptance, and cost effectiveness [1–4]. One important feature of high quality CTC is adequate bowel preparation prior to the examination to ensure both removal of bulk stool and contrast tagging of any residual material remaining in the colon [5–13]. Tagging of solid residual stool can be accomplished with dilute (2% w/v) barium sulfate, whereas uniform tagging of residual luminal fluid requires iodinated water-soluble agents [14]. Sodium diatrizoate/diatrizoate meglumine (Gastrografin, Bracco Diagnostics) has served in this role for many years. This ionic agent also has a cathartic effect that may further improve cleansing of any solid debris [9]. Nonionic iodinated contrast agents including iohexol (Omnipaque, GE Healthcare) are generally considered safer than ionic agents and are more palatable for oral consumption [15–17]. Furthermore, while nonionic agents were historically more expensive than ionic agents, iohexol now costs less than diatrizoate in our practice. For these reasons, our department recently switched from diatrizoate to iohexol for all routine CT examinations utilizing positive oral contrast. For CTC, a similar switch from ionic to nonionic iodinated contrast was slowly introduced. One potential issue for CTC is whether these benefits of the nonionic agent might be offset by undesirable effects on bowel preparation quality, including increased solid adherent stool and luminal fluid possibly associated with nonionic agents due to their presumed decreased cathartic-like action.
Kim et al. [15] recently reported their experience with diatrizoate versus iohexol for CTC by comparing two separate patient cohorts. Beyond a higher frequency of intraluminal bubbles in the iohexol cohort, examination quality was found to be similar. In our screening CTC program, we have asymptomatic adults who are returning for repeat screening after an initial negative screening CTC examination. This provided the opportunity to perform a head-to-head intra-patient comparison of the efficacy of iohexol versus diatrizoate, with all other elements of the bowel preparation regimen remaining the same. This objective comparison included both automated assessment of the density and volume of residual luminal fluid, as well as scoring of residual adherent stool by interpreting radiologists. The specific purpose of our study, therefore, was to determine if nonionic iohexol contrast results in comparable bowel preparation as compared with the current standard utilizing ionic diatrizoate contrast, leaving all other preparation components constant.
Materials and Methods
Study design
Our retrospective study complied with the Health Insurance and Portability and Accountability Act and was approved by our institutional review board; the need for informed consent was waived. As noted in the introduction, our department recently switched from diatrizoate to iohexol for oral contrast at routine CT based on emerging data regarding cost, safety, and patient preference. This clinical decision was also gradually introduced for CTC. A total of 46 consecutive asymptomatic adults were identified who underwent initial CTC for colorectal cancer screening using diatrizoate as a tagging agent for residual fluid, with follow-up CTC using iohexol (mean follow-up interval = 4.1 years; range 1.5–7.5 years). The other elements of the cathartic bowel preparation regimen remained constant, as described below. The bowel preparation utilizing diatrizoate was administered to all individuals undergoing the initial CTC between 2006 and 2012 and the iohexol regimen was used for their follow-up examination performed between February 2014 and November 2014. The supine series was used for all analyses unless fluid subtraction was inadequate, in which the case prone images were used. The mean patient age was 59.4 years (range 49–88 years) at the time of initial CTC and 63.5 years (range 52–93 years) at the time of the follow-up CTC. There were 20 women and 26 men.
CTC Bowel Preparation Regimens
With the exception of the specific oral iodinated contrast agent being used (ionic versus nonionic), the bowel preparation regimen was otherwise identical between the two visits. Starting the day before the scheduled CTC examination, patients were restricted to a clear liquid diet and received two 5 mg bisacodyl tablets, which were taken before 11 am. The evening before CTC, one 296 mL bottle of magnesium citrate was ingested, followed in 2–3 hours by a second bottle of magnesium citrate and 250 ml of 2% w/v barium sulfate to tag residual solid stool. Lastly, either 60 ml diatrizoate (diatrizoate meglumine and diatrizoate sodium solution; Gastrografin, Bracco Diagnostics) (initial CTC) or 75 ml iohexol (Omnipaque 350, GE Healthcare) (follow-up CTC) was given 2–3 hours later to tag residual fluid. The difference in volume of diatrizoate and iohexol provided for roughly equal iodine loads (22.0 vs. 26.3 g iodine, respectively). The slight volume increase and iodine load for the nonionic prep was chosen to potentially counteract the presumed decrease in cathartic-like action of the ionic prep. In terms of hydration, patients were instructed to take one glass (8 oz.) of a clear liquid with the bisacodyl (step 1), four to six glasses during steps 2 and 3 (cathartic and barium), and one glass with step 4 (diatrizoate or iohexol).
Colonic distention and CT acquisition protocol
Automated CO2 delivery (PROTOCO2L, Bracco) was used to achieve colonic distention. [18] Spasmolytics were not administered. Scanning commenced after equilibrium intraluminal pressure was achieved. Technologists immediately reviewed images to ensure adequate insufflation. CT image acquisition was performed on 16- or 64-section multi-detector CT machines (GE Healthcare). Acquisition parameters consisted of 1.25 mm collimation, 1 mm reconstruction interval, 120 kVp, and tube current modulation (noise index set at 50, 30–300 mA range).
Analysis of Bowel Preparation Quality
Assessment of the fidelity of colonic preparation included volume and density measurement of residual tagged fluid, as well as scoring of residual adherent solid tagged stool. Based on our previous experience with volumetric analysis of residual colonic fluid at CTC, we prefer to have less than 200 ml of residual luminal fluid to allow for efficient and effective 3D fly-through evaluation [5, 6]. In terms of fluid density for 2D evaluation, we prefer an attenuation value greater than 500 HU [5, 6]. Finally, adherent stool must be minimized to allow for a primary 3D polyp evaluation [14].
The total volume and mean attenuation of the residual luminal colonic fluid for each patient were derived using an automated quality assessment (QA) tool available on a beta version of our CTC software system (V3D Colon, Viatronix) that provides the luminal fluid and gas volumes [5, 6, 19]. This portion of the study was conducted by one of the co-authors (B.J.), who is a board-certified radiologist with CTC training and 5 years of experience. Proper processing requires correct centerline generation and colonic segmentation. The colon segmentation algorithm detects the gas-filled luminal cavity by initial thresholding. After visual confirmation and approval of correct segmentation, the QA software uses a region growing technique to delineate all tagged fluid regions connected to the gas-filled lumen. This software algorithm has been validated by the manufacturer on two established phantoms used for their product validation. According to the manufacturer, the accuracy for volume and attenuation measurement is within the range of 98%. The luminal fluid volume and its average attenuation (CT attenuation number in HU) were recorded. The efficiency of the automated fluid detection was verified online on axial 2D CTC image review with and without digital subtraction of the tagged fluid (electronic cleansing). This step confirmed that all residual tagged colonic luminal fluid was captured by the automated volumetric tool. As stated above, in cases where the software did not properly subtract all tagged luminal fluid on supine views, analysis was attempted using prone views. Cases where subtraction was inadequate on both views were excluded.
For adherent stool assessment, a previously established 4-point scoring system was employed by segment, consisting of a score of zero for no adherent tagged stool, score of one for solid tagged stool ≤5 mm, score of two for 1–3 particles 6–9 mm, and score of three for >3 particles 6–9 or any ≥10 mm [7, 8]. Two experienced fellowship-trained abdominal radiologists with extensive CTC experience (J.L.H. and J.B.R., 9 and 6 years, respectively; case experience >1,000 CTC examinations) each separately scored all examinations in random order, blinded to the contrast type. The endoluminal 3D fly-through in concert with 2D correlation was utilized for optimal detection of residual adherent stool. The supine series was used as the default view; the prone view was employed only for cases where the supine view was inadequate. The views were matched such that each iohexol/diatrizoate CTC pair utilized the same position.
Statistical analyses
Continuous data were expressed as means with standard deviations or 95% confidence intervals (CI). The paired t-test was used for intra-individual comparison of fluid volumes and fluid attenuations between the diatrizoate and iohexol regimens. A p-value of <0.05 indicated a statistically significant difference.
Results
The intra-individual head-to-head comparison revealed that the diatrizoate regimen resulted in less residual fluid in 27 of the 46 patient examinations (58.7%, 95% CI 44.3–73.1%) examinations, whereas iohexol resulted in less residual fluid in the remaining 19 patients (41.3%, 95% CI: 26.9–55.7%). The overall mean residual fluid volume was higher for the iohexol regimen (173±126 ml) than for the diatrizoate regimen (130±79 ml; p=0.02). Residual fluid volumes ranged from 1–593 ml with iohexol and 4–398 ml with diatrizoate. However, the intra-patient differences tended to be small, with a residual fluid difference between the diatrizoate and iohexol studies of less than 70 ml in 30 of 46 patients (65.2%). That is, those with small fluid volumes with diatrizoate tended to have small volumes with iohexol, and the same for larger residuals. In general, both regimens resulted in excellent bowel preparation (Figure 1), with volumes<200 ml in 39 (84.7%) of diatrizoate cases and 33 (72.7%) of iohexol examinations. Figure 2 shows a bow-and-whisker plot for residual luminal fluid volumes for the two cohorts.
Figure 1.
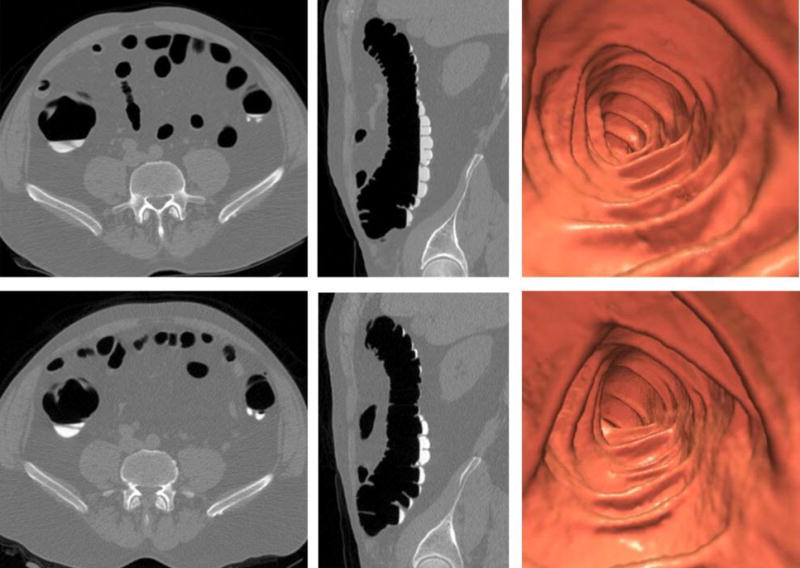
Initial and follow-up CTC in 61-year-old (at time of index study) man.
Top row (initial CTC with diatrizoate preparation): 2D transverse, 2D sagittal, and 3D endoluminal CTC images show excellent preparation quality with minimal tagged fluid (90 ml; mean attenuation, 589 HU) and almost no adherent tagged stool (pooled total colonic stool score = 0.5).
Bottom row (follow-up CTC with iohexol preparation): Matching CTC images show very similar preparation results (63 ml; 852 HU; pooled total colonic stool score = 0.5).
In general, intra-patient preparation results at follow-up CTC tended to mirror initial results with diatrizoate (see text).
Figure 2.
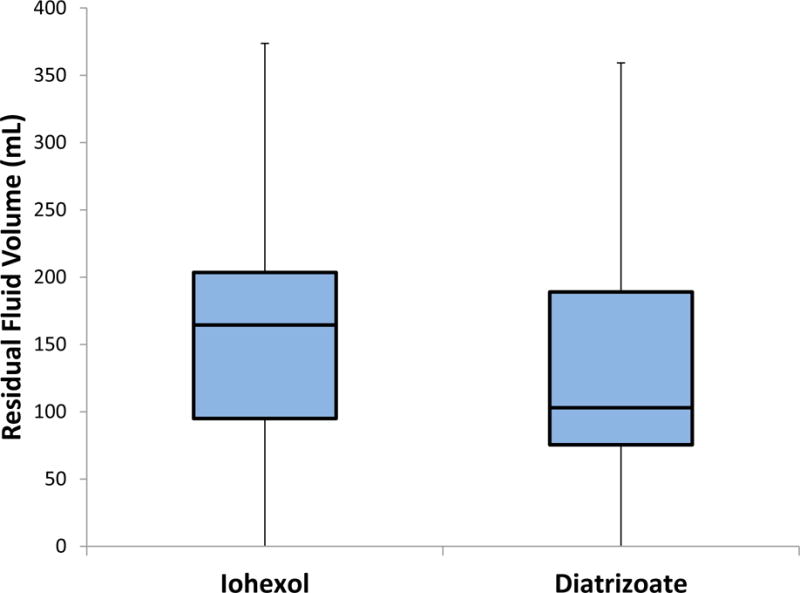
Standard box-and-whisker plots of residual colonic fluid volumes. Results are clinically comparable. Boxes include all measurements in the 2nd and 3rd quartiles with the line inside the box denoting the median value. Whiskers indicate 1.5 × the inter-quartile range (portions of the lower whiskers are cropped because they extend below 0).
The intra-individual head-to-head comparison revealed that the diatrizoate regimen resulted in higher fluid attenuation in 15 of the 46 patients (32.6%, 95% CI: 18.2–46.4%), whereas iohexol resulted in higher fluid attenuation in the remaining 31 patients (67.4%, 95% CI: 53.0–81.8%). The overall mean fluid attenuation was significantly higher for the iohexol regimen (849±270HU) than the diatrizoate regimen (732±168HU; p=0.03). Mean fluid attenuation ranged from 353–1546 HU with iohexol and 314–1088 with diatrizoate. The distribution of mean fluid density is shown on Figure 3.The intra-patient difference in mean attenuation was >100 HU between the diatrizoate and iohexol studies in all but 11 cases (23.9%). The iohexol regimen resulted in five examinations (10.9%) with residual colonic fluid attenuation of less than 500 HU, whereas the diatrizoate regimen resulted in four examinations (8.7%) with attenuation less than 500 HU. The remaining examinations (n = 41 for iohexol and n = 42 for diatrizoate) resulted in attenuation values >500 HU.
Figure 3.
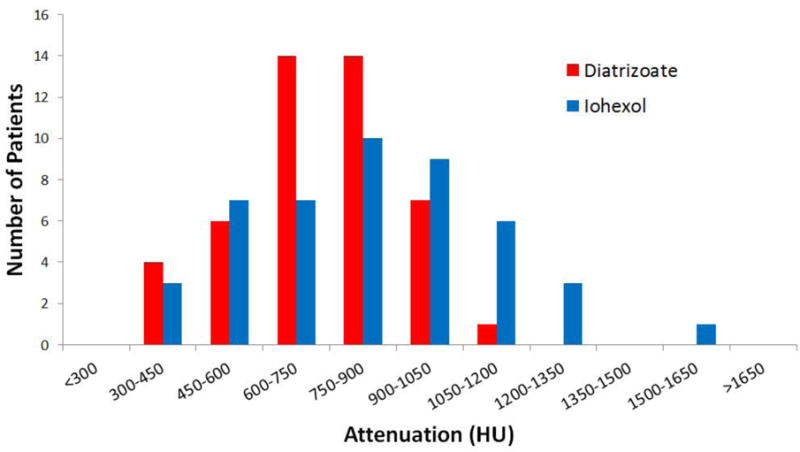
Distribution of mean attenuation of luminal fluid.
The intra-individual head-to-head comparison revealed an equal distribution of diatrizoate and iohexol regimens resulting in less residual adherent stool, with 23 of the 46 patients for each (50.0%, 95% CI 35.6–64.4%).The average total colonic residual stool score was not statistically significantly different between the iohexol group and the diatrizoate group (2.5±2.8 vs. 2.3±2.5; p=0.69). Mean total colonic stool scores ranged from 0–13.0 with iohexol and 0–10.5 with diatrizoate. The intra-patient differences in total stool scores between the diatrizoate and iohexol studies was 3.5 or less in 37 of 46 cases (80.4%) and 0.5 or less in 16 of 46 cases (34.8%). More detailed data on segmental adherent stool scores according to each radiologist are provided in Table 1.
Table 1.
Scoring* of tagged adherent solid stool at CTC
| Radiologist 1 | Radiologist 2 | ||||
|---|---|---|---|---|---|
| Segment | Iohexol | Diatrizoate | Iohexol | Diatrizoate | p value |
| Rectum | 0.26 ± 0.53 | 0.52 ± 1.03 | 0.37 ± 0.64 | 0.28 ± 0.66 | 0.46 |
| Sigmoid | 0.57 ± 0.98 | 0.48 ± 0.86 | 0.52 ± 0.86 | 0.63 ± 0.93 | 0.94 |
| Descending | 0.24 ± 0.64 | 0.3 ± 0.66 | 0.43 ± 0.81 | 0.43 ± 0.72 | 0.78 |
| Transverse | 0.41 ± 0.83 | 0.3 ± 0.66 | 0.74 ± 0.91 | 0.76 ± 0.92 | 0.75 |
| Ascending | 0.39 ± 0.74 | 0.2 ± 0.45 | 0.41 ± 0.75 | 0.15 ± 0.47 | 0.04 |
| Cecum | 0.35 ± 0.67 | 0.28 ± 0.69 | 0.17 ± 0.61 | 0.17 ± 0.57 | 0.72 |
|
| |||||
| Total | 2.22 ± 3.16 | 2.09 ± 2.88 | 2.65 ± 2.82 | 2.41 ± 2.46 | 0.69 |
See text for detail regarding the 4-point scoring scale for adherent stool
Discussion
In our retrospective study, both the iohexol- and diatrizoate-based CTC regimens resulted in excellent bowel preparation quality in terms of residual fluid volume, residual fluid attenuation, and residual solid adherent stool. The small differences observed in both the direct intra-patient and overall average comparisons are not felt to be of clinical relevance in terms of lesion detection. The strength of our study design was that all cases of the diatrizoate and iohexol versions of the CTC prep had a direct intra-patient comparison. Interestingly, residual fluid volumes and amount of adherent stool tended to closely match according to individual patients, such that patients with an excellent initial prep quality with diatrizoate tended to have an equally good result with iohexol (and the same for poor prep quality). The results of our study suggest that iohexol produces nearly equivalent and consistently excellent bowel preparation as compared with diatrizoate when utilized as part of a proven CTC bowel preparation regimen.
There was no statistically significant difference in residual adherent solid stool score between diatrizoate and iohexol groups, with each demonstrating very little residue. The acceptable equivalence of diatrizoate and iohexol by this metric is of utmost importance as abundant residual adherent stool precludes a primary 3D evaluation due to the pseudopolyp appearance of adherent stool, whether tagged or not. There was a statistically significant difference in residual colonic fluid volume between diatrizoate and iohexol groups, with iohexol resulting in slightly more fluid on average. Despite this difference, both regimens resulted in residual fluid volumes predominantly in the optimal range of less than 200 mL and therefore our group did not consider this difference to be clinically meaningful [5, 6].
There was a statistically significant difference in residual colonic fluid attenuation between the diatrizoate and iohexol examinations, with iohexol resulting in higher attenuation. In our experience, residual colonic fluid with attenuation less than 500 HU leads to suboptimal examinations and the risk of missing submerged polyps [5, 6, 14]. When this criterion is used, the majority of examinations in both groups resulted in optimal attenuation of residual colonic fluid and the difference was not felt to be clinically meaningful. One might argue that there should be an upper limit to the optimal attenuation range, but in our experience, higher attenuation values are more easily adjusted for by setting appropriate window width and level during interpretation. There is a “blooming effect” with higher attenuation fluid which could lead to error in size measurements of submerged polyps, but polyp measurement should generally utilize series where the lesion is outlined by air – not fluid. In our experience, polyps are rarely submerged on all views, given the relatively low volume of residual fluid associated with our preparations. The higher attenuation of residual colonic fluid on iohexol exams may be due in part from the slightly higher iodine load and contrast volume in the iohexol prep. In our study, the iohexol preparation consisted of 75 mL of iohexol 350 which provides a total of 26.3 g iodine (75 mL × 350 mg iodine/mL). The diatrizoate preparation consisted of 60 mL diatrizoate which provides a total 22.0 g iodine (60 mL × 367 mg iodine/mL). Going forward, we have since adjusted the total volume of iohexol in the preparation to 50 mL (to further reduce residual fluid volumes) with good results. This amount of iohexol matches that used by Kim et al [15]. Future investigation after we have a much larger clinical experience with iohexol at CTC will help determine whether this change results in a slight reduction in residual colonic fluid attenuation.
A major attribute in favor of oral iohexol is that it is generally found to be more palatable than diatrizoate by patients [16, 17]. This is especially true for our CTC preparation as both iodinated agents are given in an undiluted form, with the option to dilute only if desired. One study showed that 39% of patients would refuse to take diatrizoate for CTC, compared with only 9% for iohexol [17]. Historically, diatrizoate has been less expensive than iohexol, but the price of iohexol has recently dropped below that of diatrizoate in our pharmacy system, which removes the previous cost barrier to its use. Finally, although diatrizoate has been very safe in our experience with over 10,000 CTC examinations, there are at least potential risks related to aspiration, hypovolemia, and anaphylactic reaction. In all respects, iohexol is presumably even safer.
The main limitation of our study is the relatively small patient cohort (n=46), but we believe this is more than compensated for by the fact that intra-patient comparison was available for all 46 patients. This direct one-to-one comparison of diatrizoate and iohexol by patient allowed for more critical assessment, and removes many potential biases. Going forward, we plan to reassess our larger experience with iohexol after more patients have been evaluated. Another group of investigators have compared oral iohexol and diatrizoate as part of a CTC bowel preparation utilizing polyethylene glycol (PEG) [15]. In this study, Kim et al. reported that the iohexol preparation resulted in a higher rate of frothy colonic bubble formation compared with the diatrizoate preparation. A key difference between this study and our current study is the use of polyethylene glycol PEG as a lavage agent and lack of intra-patient comparison in their study. In our study, all patients received magnesium citrate as a cathartic agent and we did not note significant bubble formation in any of our examinations, regardless of the contrast agent used. Although unproven, this leads us to believe that this bubble formation is most likely associated with the PEG component of the preparation. Another limitation of our study was the unavoidable delay between the two CTC studies for each individual patient, as well as the necessarily fixed order of the ionic and nonionic agents, which could conceivably lead to a systematic bias.
In conclusion, our initial experience with iohexol at CTC shows that it represents a suitable alternative to diatrizoate for fluid tagging as part of a cathartic bowel preparation. Based on the results of our study, in conjunction with iohexol being more palatable, less expensive, and presumably safer than diatrizoate, we believe that iohexol can replace diatrizoate in routine clinical practice, and have implemented this change in our program.
Acknowledgments
Dr. Pickhardt is co-founder of VirtuoCTC, shareholder in Cellectar Biosciences, and has previously received support from Check-Cap, Bracco, and Philips.
References
- 1.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 2.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 3.Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. American Journal of Roentgenology. 2012;198:1361–1366. doi: 10.2214/AJR.11.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickhardt PJ. CT Colonography for Population Screening: Ready for Prime Time? Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannas P, Bakke J, del Rio AM, Pickhardt PJ. Intra-individual comparison of magnesium citrate and sodium phosphate for bowel preparation at CT colonography: Automated volumetric analysis of residual fluid for quality assessment. Clin Radiol. 2014;69:1171–1177. doi: 10.1016/j.crad.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bannas P, Bakke J, Patrick JL, Pickhardt PJ. Automated volumetric analysis for comparison of oral sulfate solution (SUPREP) with established cathartic agents at CT colonography. Abdominal Imaging. 2015;40:11–18. doi: 10.1007/s00261-014-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel Preparation for CT Colonography: Blinded Comparison of Magnesium Citrate and Sodium Phosphate for Catharsis. Radiology. 2010;254:138–144. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective blinded trial comparing 45-mL and 90-mL doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. Journal of Computer Assisted Tomography. 2007;31:53–58. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 10.Chang KJ, Rekhi SS, Jr, Anderson SW, Soto JA. Fluid tagging for CT colonography: effectiveness of a 2-hour iodinated oral preparation after incomplete optical colonoscopy. Journal of Computer Assisted Tomography. 2011;35:91–95. doi: 10.1097/RCT.0b013e3181f5a610. [DOI] [PubMed] [Google Scholar]
- 11.Eddy M, Stevenson G, Mathieson J, Behrens C, Eddy R. Bowel Preparation Suitable for Same Day Computed Tomography Colonography and Colonoscopy. Can Assoc Radiol J. 2011;62:256–259. doi: 10.1016/j.carj.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Juchems MS, Hoffmann MHK, Schmidt SA, Apostel A, Brambs HJ, Aschoff AJ. Bowel preparation for CT colonography: Comparison of two different cleansing protocols. European Journal of Radiology. 2006;60:460–464. doi: 10.1016/j.ejrad.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218:274–277. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 14.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: Advantages and pitfalls with primary three-dimensional evaluation. American Journal of Roentgenology. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 15.Kim B, Park SH, Hong GS, et al. Iohexol versus diatrizoate for fecal/fluid tagging during CT colonography performed with cathartic preparation: comparison of examination quality. Eur Radiol. 2015 doi: 10.1007/s00330-014-3568-0. [DOI] [PubMed] [Google Scholar]
- 16.McNamara MM, Lockhart ME, Fineberg NS, Berland LL. Oral Contrast Media for Body CT: Comparison of Diatrizoate Sodium and Iohexol for Patient Acceptance and Bowel Opacification. American Journal of Roentgenology. 2010;195:1137–1141. doi: 10.2214/AJR.09.3968. [DOI] [PubMed] [Google Scholar]
- 17.Pollentine A, Ngan-Soo E, McCoubrie P. Acceptability of oral iodinated contrast media: a head-to-head comparison of four media. British Journal of Radiology. 2013;86 doi: 10.1259/bjr.20120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. American Journal of Roentgenology. 2006;186:1491–1496. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ, Bakke J, Kuo J, et al. Volumetric Analysis of Colonic Distention According to Patient Position at CT Colonography: Diagnostic Value of the Right Lateral Decubitus Series. American Journal of Roentgenology. 2014;203:W623–W628. doi: 10.2214/AJR.13.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]


