Abstract
Purpose
To objectively compare the volume, density, and distribution of luminal fluid for same-day oral-contrast-enhanced CTC following incomplete optical colonoscopy (OC) versus deferred CTC on a separate day utilizing a dedicated CTC bowel preparation.
Methods
HIPAA-compliant, IRB-approved retrospective study compared 103 same-day CTC studies after incomplete OC (utilizing 30 ml oral diatrizoate) against 151 CTC examinations performed on a separate day after failed OC using a dedicated CTC bowel preparation (oral magnesium citrate/dilute barium/diatrizoate the evening before). A subgroup of 15 patients who had both same-day CTC and separate-day routine CTC was also identified and underwent separate analysis. CTC exams were analyzed for opacified fluid distribution within the GI tract, as well as density and volume. Data was analyzed utilizing Kruskal-Wallis and Wilcoxon Signed Rank tests.
Results
Opacified luminal fluid extended to the rectum in 56% (58/103) of same-day CTC versus 100% (151/151) of deferred separate-day CTC (p<0.0001). For same-day CTC, contrast failed to reach the colon in 11% (11/103) and failed to reach the left colon in 26% (27/103). Volumetric colonic fluid segmentation for fluid analysis (successful in 80 same-day and 147 separate-day cases) showed significantly more fluid in the same-day cohort (mean, 227 ml vs. 166 ml; p<0.0001); the actual difference is underestimated due to excluded cases. Mean colonic fluid attenuation was significantly lower in the same-day cohort (545 HU vs. 735 HU; p<0.0001). Similar findings were identified in the smaller cohort with direct intra-patient CTC comparison.
Conclusions
Dedicated CTC bowel preparation on a separate day following incomplete OC results in a much higher quality examination compared with same-day CTC.
Introduction
Computed tomography colonography (CTC) is an effective test for colorectal cancer screening (1–5). However, in the current absence of broad coverage by insurance providers, a much more common indication for CTC is in the setting of a “failed” or incomplete optical colonoscopy (OC) (6–10). In fact, CTC has largely replaced the double contrast barium enema for this indication in most practices, as studies have shown the superiority of CTC for both detection of polyps and significant extracolonic findings (11, 12). With the exception of a few radiology practices with an active screening program, incomplete OC examinations likely account for the vast majority of CTC requests (13).
Colorectal cancer remains the second-leading cause of cancer-related death in the United States even though it is largely preventable through screening (14). The main reason for this discrepancy is lack of adherence with screening. A major contributor to noncompliance is patient apprehension regarding bowel cleansing (15). Cathartic bowel cleansing is required for both optical colonoscopy and CTC screening with the option for same-day OC polypectomy (16).
There are two primary strategies regarding the timing of CTC following incomplete OC. The first and most common is same-day CTC utilizing the prior OC prep, often supplemented with oral contrast after recovery from OC (17). This is often the more convenient option for the patient as they do not have to undergo further cathartic bowel preparation (assuming bowel prep for OC was adequate) and return on a separate day. At our institution, 30 mL of ionic water-soluble iodinated contrast (diatrizoate) is given orally once the patient adequately recovers from sedation, and CTC is then performed a minimum 2–3 hours later. Another option we have utilized is to have the patient return for CTC at a later date utilizing a standard CTC bowel regimen with an osmotic cathartic and dual agent tagging protocol.
No consensus currently exists regarding the appropriate timing of CTC following incomplete OC. One prior study assessed colonic transit of oral contrast (diatrizoate) after incomplete OC at same-day CTC, but there was no cohort for comparison (17). To our knowledge, no objective comparison exists for same-day versus deferred separate-day strategies for CTC following incomplete OC.
We have access to an automated volumetric CTC software tool that allows for quantification of both luminal gas and residual opacified colonic fluid, including volumetric and density assessment (18–21). This tool provides both an objective and efficient means for comparison between patient cohorts. The purpose of this study is to compare, in the setting of incomplete OC, the opacified residual colonic fluid in terms of volume, density, and distribution at same-day salvage CTC versus deferred CTC on a separate day with dedicated CTC-specific bowel preparation.
Materials and Methods
Study Design
This retrospective study complied with the Health Insurance and Portability and Accountability Act and was approved by our institutional review board; the need for informed consent was waived. Over a period of 40 months from January of 2010 through August of 2014, 4546 CTC examinations were interpreted at the University of Wisconsin Hospital and Clinics (UWHC). A subset of 399 (8.8%) of these examinations were performed for the indication of recent incomplete optical colonoscopy. The most common reasons cited for failed colonoscopy were varying combinations of colonic tortuosity, redundancy, looping, diverticular narrowing, and patient discomfort). Patients were excluded from undergoing same-day CTC if bowel preparation was reported as poor at OC, a deep or extensive biopsy was performed at OC, or if the patient was symptomatic post-OC.
For same-day CTC following incomplete optical colonoscopy, patients were given 30 mL of water-soluble iodinated contrast (sodium diatrizoate/diatrizoate meglumine; Gastrografin) by mouth and CT imaging was performed a minimum 2–3 hours after contrast ingestion was completed. A total of 121 same-day CTC examinations were identified, of which 18 cases (15%) were excluded due to anatomic factors that would restrict transit of luminal contrast, including occlusive masses, strictures, and colon-containing hernias. Mean age (± SD) for the final same-day cohort of 103 patients was 63.5 ± 10.6 years; 73% were female (n=75). The colonoscope failed to reach beyond the splenic flexure in 48.5% of these cases.
We have utilized a number of bowel preparation regimens for CTC performed on a separate day following incomplete OC, but the standard CTC preparation at our institution for many years (including the study period) has been magnesium citrate for catharsis (22, 23). To maintain uniformity, we included only cases utilizing this magnesium citrate-based regimen for the comparison group in this analysis. Our standard magnesium citrate bowel preparation is as follows: Starting the day before the scheduled CTC examination, patients are restricted to a clear liquid diet and receive two 5-mg bisacodyl tablets, which are taken before 11 am. Three to six hours after the bisacodyl, patients ingest two 296 ml bottles of magnesium citrate solution separated by 2–3 hours. Patients are also given 250 mL of (2% w/v) barium sulfate to tag residual solid stool and 60 mL of Gastrografin to tag residual fluid.
A total of 176 deferred (ie, non-same-day) CTC cases for incomplete OC utilizing the magnesium citrate bowel prep were identified. There were 25 cases (14%) that were excluded due to inadequate bowel distention, again largely related to occlusive masses or hernias, yielding a final cohort of 151 patients. Mean patient age for this cohort was similar to the same-day group (60.9 ± 10.6years); 68% were female (n=102). The colonoscope failed to reach beyond the splenic flexure in 47.0% of these cases, a similar proportion to the same-day completion CTC cohort.
An additional cohort of 17 patients was identified in our CTC database that had undergone both a same-day CTC for incomplete colonoscopy and a separate CTC utilizing our standard bowel preparation with magnesium citrate. This cohort allowed for a direct intra-patient comparison of the two approaches. Patients for this cohort were identified from an extended time period of 2004 through 2014. Two patients in this group were excluded, one for an obstructing sigmoid mass and another for a colon-containing hernia that restricted colonic distention, yielding a final cohort of 15 patients. The mean time interval between the two CTC exams in this cohort was 4.1 years (range, 7–95 months); no major change in medical status was noted in these patients between the CTC exams that might significantly impact bowel motility. The average age at the time of initial CTC exam was 60.2 ± 10.4 years; the majority of the patients were male (9/15, 60%).
Automated CO2 delivery (PROTOCO2L, Bracco Diagnostics) was used to achieve colonic distention(24). No spasmolytics were utilized. After equilibrium intraluminal pressure was achieved, CT imaging was performed in the supine and prone positions. Distention was assessed on scout views and 2D axial imaging, with subsequent decubitus imaging performed if necessary (24). MDCT image acquisition was performed on 8, 16- or 64-section multi-detector CT scanners (GE Healthcare, Waukesha, WI). Acquisition parameters consisted of 1.25 mm collimation, 1 mm reconstruction interval, 120 kVp, and tube current modulation (noise index set at 50, 30–300 mA range).
The total volume and mean attenuation of the residual colonic fluid for each patient were derived using automated quality assessment (QA) software available on a beta version of our CTC software (V3D Colon, Viatronix, Stony Brook, NY). The algorithm for QA fluid detection has been described in prior publications (18, 19). In brief, proper processing required correct centerline generation and colonic segmentation. The system detects the gas-filled luminal cavity by initial thresholding. The user than approves or modifies correct segmentation. The system uses a region-growing technique to mark tagged fluid regions connected to the gas-filled lumen. A median filtering technique is applied to remove irregularity caused by image noise and artifacts.
The supine and prone series were loaded into the analysis software and the series with best colonic distention and contrast detection was utilized for volumetric analysis. Decubitus series were reviewed and analyzed if both supine and prone series were inadequately distended. One of the co-authors (J.T.) recorded the luminal fluid volume and its average attenuation (CT attenuation number in HU). Colonic length and total colonic volume was also recorded. The efficiency of the automated fluid detection was verified online on axial two-dimensional CTC image review with and without digital subtraction of the tagged fluid (electronic cleansing). This step confirmed that all residual tagged colonic luminal fluid was captured by the automated volumetric tool.
The presence of oral contrast in the colon and the distal extent of the contrast column were recorded for each examination, utilizing the series showing the most distal extent. In addition, the percentage of the total oral contrast volume in the stomach, small bowel, and colon was estimated visually. Of note, lesion detection was not employed as an endpoint for comparison in this study, primarily due to sample size considerations and the general lack of confirmation for potential proximal lesions in this incomplete OC population.
Statistical Analyses
For the larger cohorts of same-day failed OC patients and interval CTC following failed OC with CTC prep, Kruskal Wallis test was used to assess difference between groups for numerical variables. In the smaller cohort of 15 patients who had undergone both a CTC same day following failed OC as well as CTC with standard prep, a Wilcoxon signed rank test was used to test for difference between groups. P < 0.05 (two sided) was the criterion for statistical significance. There was no adjustment of p-values for multiple testing. All analyses were obtained in R 3.1.0 (R Core Team 2014).
Results
Opacified luminal fluid extended to the rectum in 56% (58/103) of same-day CTC following incomplete OC versus 100% (151/151) of deferred separate-day CTC (p<0.0001). For same-day CTC, colonic fluid opacification was completely absent in 11% (11/103) and failed to reach the left colon in 26% (27/103) (Figure 1). The automated QA software successfully calculated the total residual colonic fluid volumes in 78% (80/103) of same-day examinations and 97% (147/151) of the separate day exams. Residual colonic fluid analysis in these cases showed significantly more residual colonic fluid in the same-day cohort (mean, 227 ml vs. 166 ml; p<0.0001). The actual difference in residual fluid volume between the two cohorts is underestimated since the the primary reason for failure of the QA software in the same-day cohort was excessive unopacified or under-opacified fluid. Mean colonic fluid attenuation was significantly lower in the same-day cohort (545 ± 148 HU vs. 735 ± 209 HU; p<0.0001) but mean colonic length was similar between the two groups (211±42 cm vs. 220 ± 42 cm; (p=0.06). Mean colonic volume (an indicator of both anatomy and distention) was also similar between both groups (2228± 923 mL vs. 2243±1024 mL; p=0.9) (Table 1). Estimated percentage of oral contrast in the small bowel was markedly higher in the same-day group (mean, 38.9% versus 0.3%; p<0.00001). A typical case from the same-day CTC cohort is shown in Figure 2 that shows excessive residual fluid, much of which remains proximal to the colon.
Figure 1. Distal colonic segment opacified with oral contrast at same-day CTC.

Pie chart depicting most distal segment of positive oral contrast opacification in the 103 patient same-day CTC group. Contrast failed to reach the rectum in nearly half of the cases, whereas 100% of the 151 cases utilizing standard CTC prep with CTC performed on a separate day had contrast tagging to the rectum.
Table 1.
Results for Same-day CTC with OC prep versus Interval CTC with Standard prep.
| Same-Day CTC | Interval CTC | P- value | |
|---|---|---|---|
| Colonic fluid volume (mL) | 227 | 166 | <0.0001 |
| Colonic fluid attenuation (HU) | 545 | 735 | <0.0001 |
| Cases with fluid tagging to rectum | 56% | 100% | <0.0001 |
| Cases with oral contrast in small bowel | 38.9% | 0.3% | <0.0001 |
| Total colonic volume (mL) | 2228 | 2243 | 0.9 |
Figure 2. Images from same-day CTC following incomplete OC.

Scout image from CTC (A) obtained 2–3 hours after oral administration of 30 ml diatrizoate shows very dense residual fluid in the stomach. Less dense oral contrast is faintly seen in the small bowel and colon, but are better depicted on transverse CTC imaging (B). Although oral contrast extends to the descending colon, the attenuation is sub-optimally low for lesion detection. The overall colonic fluid volume is increased compared with the typical results obtained with our standard CTC prep.
In the 15-patient subgroup with both same-day and standard CTC, 20% (3/15) of the same-day exams following incomplete OC did not have contrast extend to the colon, 40% (6/15) had contrast extend to the transverse colon, and 33% (5/15) had contrast extend to the rectum. In this same 15 patient cohort, contrast extended to the rectum in all cases at standard CTC with dedicated CTC bowel preparation. Examples of cases with this intra-patient comparison are shown in Figures 3 and 4. Volume analysis was performed for cases where the oral contrast extended to the left colon on the same-day CTC. The mean residual fluid volume (±SD) was 223 ± 108 mL for same-day CTC versus 177 ± 121 mL for the dedicated CTC bowel prep (p=1.0). Mean fluid attenuation (±SD) was 579 ± 152 HU for same-day CTC versus 631 ± 224 HU for standalone CTC (p<0.05). The estimated percentage of the total oral contrast volume that remained in the small bowel was 44% for the same-day CTC and <1% for the dedicated CTC prep (p<0.05).
Figure 3. Selected images from CTC exams performed on same patient – the initial study performed same day after incomplete OC and the second study representing our standard CTC regimen.
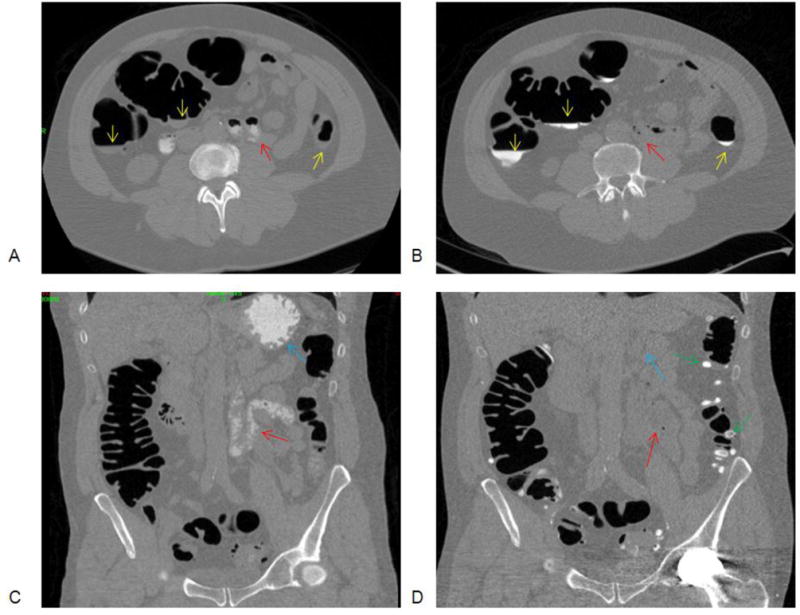
Transverse and coronal images from CTC examination performed same day CTC following incomplete OC (A,C) and separate exam at a later date using standard CTC prep (B,D). Layering untagged fluid is demonstrated on the same-day examination (A, yellow arrows) which was well opacified with standard CTC prep (B). Oral diatrizoate is demonstrated in the stomach and small bowel (A,C) (red, blue arrows) on the same-day examination with no contrast tagging in the colon. Also note tagged stool in the left colon due to the barium suspension administered with standard CTC prep (D, green arrows)
Figure 4. Selected images from CTC exams performed on two different dates on the same patient.

Transverse and coronal images from CTC examination performed same day CTC following incomplete OC (A,C) and separate exam at a later date using standard CTC prep (B,D). Frothy layering untagged fluid is demonstrated on the same day examination (A, yellow arrow) which was well opacified with standard CTC prep (B). All of the oral diatrizoate is demonstrated in the stomach and small bowel (A,C) (red) on the same-day examination with no contrast tagging in the colon. Contrast tagging has advanced to the distal colon with the standard prep (D, green arrow).
Discussion
Through the use of retrospective case review and an automated volumetric QA software tool that provides objective comparison of tagged residual fluid, we found that our dedicated CTC bowel preparation deferred for a separate day from OC resulted in a higher quality examination compared with CTC performed the same day following failed optical colonoscopy. In theory, oral contrast administration in CTC may improve both sensitivity and specificity for detecting colonic polyps (23, 25), although most evidence to date is indirect. However, the presence of positive oral luminal contrast tagging likely improves reader confidence. We consider contrast tagging of residual colonic fluid in the range of 500-1000 HU to be preferred, similar to what others have suggested (26). At lower densities, especially below 300 HU, polyps may be obscured by the dilute low-attenuation fluid, whereas fluid attenuation more than 1000 HU can begin to obscure polyps due to associated streak artifact which is magnified with low dose technique. Excessive residual luminal fluid impacts 3D CTC assessment for lesions because such fluid-filled portions cannot be evaluated without electronic cleansing (which we do not employ). In such cases, greater reliance will be placed solely on the 2D analysis, which is typically faster,(27–29) but may also result in reader fatigue in a high volume practice (30). In general, the best results at CTC are likely achieved when both 2D and 3D polyp detection strategies are employed.
In the same-day CTC cohort, significantly more fluid was identified in the colon, which was also considerably less dense. These results are an underestimation since only 80 of the 103 same-day examinations could be analyzed by the volumetric software. The primary reason for failure to analyze cases with the automated tool was due to low density of the luminal fluid in the colon, which was typically of larger volumes. The automated tool requires adequate opacification of the luminal fluid. In addition, estimating the percent of mucosal surface covered by fluid with this tool also requires contrast opacification (31). Visual analysis confirmed that in 11% of same-day patients, no contrast had progressed into the colon, and only 56% of patients had contrast extending to the rectum. These findings are concordant with findings by other authors using a similar regimen (17), who nonetheless concluded that adequate colonic opacification was achieved. However, that study lacked comparison to a cohort who underwent standard CTC bowel preparation on a separate day, as in our study.
The volumetric analysis tool could not be used to assess luminal fluid beyond the point where contrast extended because it was unable to identify or quantify unopacified fluid. Decreased fluid in these areas beyond tagging, with more fluid in the contrast-tagged colon may be due in part to the effects of diatrizoate on the colon, although unopacified luminal fluid in the distal colon may have also been removed during the preceding OC examination. As a hypertonic agent, diatrizoate draws fluid into the colon, which appears to affect same-day administration (2–3 hours before) more than the standard CTC prep, where the contrast is taken the evening before (at least 8 hours prior). Presumably, the low volumes of opacified fluid with the standard prep is due in part to the cathartic effect of the diatrizoate, and the increased time for the colon to pass the fluid which is drawn in by the hypertonic effect.
All patients in the standard CTC prep group had fluid tagging all the way to the rectum, compared with just over half of the cases in the same-day cohort. However, in some of the same-day cases with incomplete extension of the contrast, the contrast did reach the level reached by the colonoscope, arguably suggesting an adequate examination. Our study did not specifically address the issue of solid stool tagging with barium, which was only administered in the standard (separate day) CTC prep group. Tagging of solid adherent stool would presumably improve specificity in polyp characterization from stool, but this requires further dedicated investigation. Importantly, barium was not administered to the same-day CTC cohort. However, there is growing evidence that contrast coating of polyps (presumably with barium) is an important phenomenon associated with our standard CTC bowel preparation. It is particularly helpful for the detection of flat polyps that are more often located in the right colon (28,(32)). Given the increasingly recognized importance of serrated adenomas/polyps (SSA/SSP) and their often flat presentation, bowel preparations that promote contrast coating of the polyp mucosal surface are likely helpful to optimize detection of subtle polyps. Whether adequate contrast coating occurs in the same-day CTC situation is unknown.
This study highlights some of the tradeoffs that are inherent in performing same-day versus interval/future CTC following incomplete colonoscopy. Same-day CTC is generally more convenient for the patient as they do not have to make another appointment or undergo repeat bowel preparation. However, we have found that some of these patients are exhausted from the OC procedure and associated sedation, and would prefer to deal with the CTC exam on another day. Potential aspiration of diatrizoate related to sedation and slow transit related to ileus are additional potential concerns for same-day completion CTC. Although we wait at least 2–3 hours after ingestion of the iodinated oral contrast for same-day CTC, this delay may in fact be inadequate. However, waiting even longer would further reduce the patient convenience factor for same-day completion CTC.
In our clinical practice, we had a recent referral request for same-day CTC following a difficult incomplete OC examination. After further investigation, we found that the patient was actually symptomatic and later proved to have a full-thickness perforation that required urgent surgical repair. Had same-day CTC been performed in this case, the OC-related perforation might have been inappropriately ascribed to CTC. This case aside, the primary advantage of performing CTC on a separate day may be the superior bowel preparation, which might improve lesion detection accuracy. In addition, our low-volume CTC bowel prep is better tolerated than the standard PEG prep still in use by our gastroenterologists.
Based on our findings, we have developed a situational approach in considering when CTC should be performed following incomplete OC. If the endoscopist has only a limited specific clinical question, such as clearing the cecum of cancer or concern for a possible submucosal or extrinsic mass, a same-day examination may be appropriate. A discussion with the referring endoscopist is vital to assess the adequacy of bowel preparation during the colonoscopy. If the main reason for incomplete visualization of the colon is poor prep, then the same will likely be found on CTC, limiting its diagnostic yield. In this case, interval CTC should be performed with a dedicated CTC prep on a separate day. Furthermore, if the scope only reached the sigmoid colon, our current preference is to schedule a future CTC with dedicated prep as the majority of the colon has yet to be evaluated. Of course, patient preference and circumstance (eg, remote home location) will also play a role in the decision process, and we continue to perform same-day CTC in many cases, despite the disadvantages related to poor bowel prep quality. A third option that we did not explore in this study would consist of performing “next-day” CTC by keeping the patient on clear liquids and allowing for overnight oral contrast administration that would more closely approximate our standard CTC prep.
We acknowledge limitations to the current study. The first is the lack of comparison in actual lesion detection between the two groups. Given the low number of polyps detected and lack of confirmation in many cases (largely due to the difficulty with OC), statistically significant numbers would be difficult to obtain. Furthermore, the time interval (mean, 4.1 years) between same-day and separate-day CTC in the small sub-cohort with both examinations (n=15) complicates the interpretation of any new polyps detected on the latter study. Therefore, we were limited to the use bowel preparation quality as a proxy for diagnostic performance. An additional limitation is that this comparison applies to our specific CTC bowel preparation, which may differ from other practices. This uniform regimen was chosen to provide consistency for comparison. We did not attempt to quantify the effect of the different cohorts in terms of time required to interpret the examinations, or diagnostic confidence in the results. This would be interesting because evaluation of fluid-filled segments requires more 2D evaluation. Inherent limitations of the QA software employed include that it does not allow for scoring of residual adherent tagged solid stool or estimation of mucosal surface covered by unopacified fluid (22, 33, 34). The amount of residual adherent solid stool is another important factor in assessing the quality of bowel preparation at CTC, since its presence complicates 3D polyp detection (25, 35, 36). The QA software tool would need to be adapted further for assessment of residual adherent stool.
In conclusion, we found that performing CTC following failed optical colonoscopy on a separate day utilizing a dedicated CTC bowel prep results in a preferred distribution of administered oral contrast, greater contrast density in the colon, and less residual colonic fluid than same-day CTC with tagging. These findings have altered our practice pattern in terms of the preferred approach for completion CTC following incomplete OC.
Acknowledgments
Dr. Pickhardt is co-founder of VirtuoCTC and shareholder of Cellectar Biosciences. Dr. Kim is co-founder of VirtuoCTC, a consultant for Viatronix, and on the medical advisory board for Digital Artforms.
Footnotes
All other authors have no relevant financial disclosures
References
- 1.Hassan C, Pickhardt PJ, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168(7):696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. New England Journal of Medicine. 2008;359(12):1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357(14):1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349(23):2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109(11):2213–21. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 6.Copel L, Sosna J, Kruskal JB, Raptopoulos V, Farrell RJ, Morrin MM. CT colonography in 546 patients with incomplete colonoscopy. Radiology. 2007;244(2):471–8. doi: 10.1148/radiol.2442060837. [DOI] [PubMed] [Google Scholar]
- 7.Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189(4):774–9. doi: 10.2214/AJR.07.2048. [DOI] [PubMed] [Google Scholar]
- 8.Iafrate F, Hassan C, Zullo A, et al. CT colonography with reduced bowel preparation after incomplete colonoscopy in the elderly. Eur Radiol. 2008;18(7):1385–95. doi: 10.1007/s00330-008-0892-2. [DOI] [PubMed] [Google Scholar]
- 9.Macari M, Berman P, Dicker M, Milano A, Megibow AJ. Usefulness of CT colonography in patients with incomplete colonoscopy. American Journal of Roentgenology. 1999;173(3):561–4. doi: 10.2214/ajr.173.3.10470879. [DOI] [PubMed] [Google Scholar]
- 10.Morrin MM, Kruskal JB, Farrell RJ, Goldberg SN, McGee JB, Raptopoulos V. Endoluminal CT colonography after an incomplete endoscopic colonoscopy. American Journal of Roentgenology. 1999;172(4):913–8. doi: 10.2214/ajr.172.4.10587120. [DOI] [PubMed] [Google Scholar]
- 11.Halligan S, Wooldrage K, Dadswell E, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet. 2013;381(9873):1185–93. doi: 10.1016/S0140-6736(12)62124-2. [DOI] [PubMed] [Google Scholar]
- 12.Tolan DJM, Armstrong EM, Chapman AH. Replacing barium enema with CT Colonography in patients older than 70 years: The importance of detecting extracolonic abnormalities. American Journal of Roentgenology. 2007;189(5):1104–11. doi: 10.2214/AJR.07.2026. [DOI] [PubMed] [Google Scholar]
- 13.Duszak R, Jr, Kim DH, Pickhardt PJ. Expanding Utilization and Regional Coverage of Diagnostic CT Colonography: Early Medicare Claims Experience. Journal of the American College of Radiology. 2011;8(4):235–41. doi: 10.1016/j.jacr.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. Ca-a Cancer Journal for Clinicians. 2014;64(2):104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 15.Beebe TJ, Johnson CD, Stoner SM, Anderson KJ, Limburg PJ. Assessing attitudes toward laxative preparation in colorectal cancer screening and effects on future testing: potential receptivity to computed tomographic colonography. Mayo Clinic Proceedings. 2007;82(6):666–71. doi: 10.4065/82.6.666. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ. Colonic preparation for computed tomographic colonography: Understanding the relative advantages and disadvantages of a noncathartic approach. Mayo Clinic Proceedings. 2007;82(6):659–61. doi: 10.4065/82.6.659. [DOI] [PubMed] [Google Scholar]
- 17.Chang KJ, Rekhi SS, Jr, Anderson SW, Soto JA. Fluid tagging for CT colonography: effectiveness of a 2-hour iodinated oral preparation after incomplete optical colonoscopy. Journal of Computer Assisted Tomography. 2011;35(1):91–5. doi: 10.1097/RCT.0b013e3181f5a610. [DOI] [PubMed] [Google Scholar]
- 18.Bannas P, Bakke J, del Rio AM, Pickhardt PJ. Intra-individual comparison of magnesium citrate and sodium phosphate for bowel preparation at CT colonography: Automated volumetric analysis of residual fluid for quality assessment. Clin Radiol. 2014;69(11):1171–7. doi: 10.1016/j.crad.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannas P, Bakke J, Patrick JL, Pickhardt PJ. Automated volumetric analysis for comparison of oral sulfate solution (SUPREP) with established cathartic agents at CT colonography. Abdominal Imaging. 2015;40(1):11–8. doi: 10.1007/s00261-014-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick JL, Bakke JR, Bannas P, Kim DH, Lubner MG, Pickhardt PJ. Objective volumetric comparison of room air versus carbon dioxide for colonic distention at screening CT colonography. Abdom Imaging. 2015;40(2):231–6. doi: 10.1007/s00261-014-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Bakke J, Kuo J, et al. Volumetric Analysis of Colonic Distention According to Patient Position at CT Colonography: Diagnostic Value of the Right Lateral Decubitus Series. American Journal of Roentgenology. 2014;203(6):W623–W8. doi: 10.2214/AJR.13.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel Preparation for CT Colonography: Blinded Comparison of Magnesium Citrate and Sodium Phosphate for Catharsis. Radiology. 2010;254(1):138–44. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]
- 23.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189(2):290–8. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 24.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. American Journal of Roentgenology. 2006;186(6):1491–6. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: Advantages and pitfalls with primary three-dimensional evaluation. American Journal of Roentgenology. 2003;181(3):799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 26.Slater A, Taylor SA, Burling D, Gartner L, Scarth J, Halligan S. Colonic polyps: Effect of attenuation of tagged fluid and viewing window on conspicuity and measurement in vitro experiment with porcine colonic specimen. Radiology. 2006;240(1):101–9. doi: 10.1148/radiol.2401050984. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CD, Fletcher JG, MacCarty RL, et al. Effect of slice thickness and primary 2D versus 3D virtual dissection on colorectal lesion detection at CT colonography in 452 asymptomatic adults. AJR Am J Roentgenol. 2007;189(3):672–80. doi: 10.2214/AJR.07.2354. [DOI] [PubMed] [Google Scholar]
- 28.Macari M, Milano A, Lavelle M, Berman P, Megibow AJ. Comparison of time-efficient CT colonography with two- and three-dimensional colonic evaluation for detecting colorectal polyps. American Journal of Roentgenology. 2000;174(6):1543–9. doi: 10.2214/ajr.174.6.1741543. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SA, Halligan S, Slater A, et al. Polyp detection with CT colonography: Primary 3D endoluminal analysis versus primary 2D transverse analysis with computer-assisted reader software. Radiology. 2006;239(3):759–67. doi: 10.1148/radiol.2392050483. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT Colonography. American Journal of Roentgenology. 2007;189(6):1451–6. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]
- 31.Pickhardt PJ, Taylor AJ, Gopal DV. Surface visualization at 3D endoluminal CT colonography: Degree of coverage and implications for polyp detection. Gastroenterology. 2006;130(6):1582–7. doi: 10.1053/j.gastro.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Matkowskyj KA, Lubner MG, et al. Serrated polyps at CT colonography-based colorectal cancer screening: prevalence and characteristics of the serrated polyp spectrum. Radiology. doi: 10.1148/radiol.2016151608. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective blinded trial comparing 45-mL and 90-mL doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. Journal of Computer Assisted Tomography. 2007;31(1):53–8. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 34.Macari M, Lavelle M, Pedrosa I, et al. Effect of different bowel preparations on residual fluid at CT colonography. Radiology. 2001;218(1):274–7. doi: 10.1148/radiology.218.1.r01ja31274. [DOI] [PubMed] [Google Scholar]
- 35.Park SH, Ha HK, Kim MJ, et al. False-negative results at multi-detector row CT colonography: Multivariate analysis of causes for missed lesions. Radiology. 2005;235(2):495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Kim DH. CT Colonography: Pitfalls in Interpretation. Radiologic Clinics of North America. 2013;51(1):69–88. doi: 10.1016/j.rcl.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


