Abstract
Objective/Background
To compare sleep and circadian variability in adults with delayed sleep-wake phase disorder (DSWPD) to healthy controls.
Patients/Methods
Forty participants (22 DSWPD, 18 healthy controls) completed a 10-day protocol, consisting of DLMO assessments on two consecutive nights, a five-day study break, followed by two more DLMO assessments. All participants were instructed to sleep within one hour of their self-reported average sleep schedule for the last four days of the study break. We analyzed the participants’ wrist actigraphy data during these four days to examine intraindividual variability in sleep timing, duration and efficiency. We also examined shifts in the DLMO from before and after the study break.
Results and Conclusions
Under the same conditions, people with DSWPD had significantly more variable wake times and total sleep time than healthy controls (p≤0.015). Intraindividual variability in sleep onset time and sleep efficiency was similar between the two groups (p≥0.30). The DLMO was relatively stable across the study break, with only 11% of controls but 27% of DSWPDs showed more than a one hour shift in the DLMO. Only in the DSWPD sample was greater sleep variability associated with a larger shift in the DLMO (r=0.46, p=0.03). These results suggest that intraindividual variability in sleep can be higher in DSWPD versus healthy controls, and this may impact variability in the DLMO. DSWPD patients with higher intraindividual variability in sleep are more likely to have a shifting DLMO, which could impact sleep symptoms and the optimal timing of light and/or melatonin treatment for DSWPD.
Keywords: circadian, sleep, light, variability
1.0 Introduction
Delayed sleep-wake phase disorder (DSWPD) is a circadian rhythm sleep disorder. It is characterized by a significant delay in the timing of the major sleep episode, with habitual sleep onset and wake times occurring several hours later than desired socially acceptable times [1]. While people with DSWPD typically complain about not being able to fall asleep and difficulty waking at desired (earlier) times, their sleep duration and architecture is usually normal for age once sleep is initiated [1, 2]. DSWPD is likely due to a multitude of co-occurring factors, including: (1) a longer endogenous circadian period [3], (2) increased sensitivity or exposure to evening light [4], (3) decreased sensitivity or exposure to morning light [1], (4) reduced homeostatic sleep pressure in the evening [5], (5) comorbid features of an insomnia disorder [6], (6) light exposure associated with forced early awakenings causing phase delays [1], (7) a slower rise in evening melatonin levels [7], and (8) partial sleep deprivation which can reduce phase advances to morning light [8]. All these factors can drive the circadian promotion of sleep to later clock times, thus perpetuating and promoting DSWPD [9].
The most reliable measure of central circadian timing in humans is the dim light melatonin onset (DLMO) [10, 11]. Melatonin typically begins to rise in the 2–3 hours before the usual onset of nocturnal sleep [12], but must be measured in dim light as light can suppress melatonin secretion [13]. The assessment of the DLMO in DSWPD is encouraged to improve diagnostic accuracy [1, 14], and to optimize the timing of post-awakening light and afternoon/evening exogenous melatonin treatment of DSWPD [15–18]. Treatment recommendations [19], derived from the light and/or melatonin phase response curves [20, 21], often implicitly assume a stable DLMO in DSWPD[22]. To our knowledge, only one study has assessed the variability of the DLMO in people with DSWPD [23]. The week to week variability in the DLMO was examined in eight DSWPD and eight healthy controls that slept at times of their own choosing. The study found that 6/8 DSWPDs, but only 3/8 healthy controls showed a week to week DLMO shift of one hour or more, suggesting the possibility of increased variability in the DLMO in DSWPD (Figure 2 in [23]).
We recently completed a 10 day protocol in a larger sample of people with DSWPD and healthy controls [24, 25]. All participants completed two DLMO assessments, followed by a five-day study break, and then two more DLMO assessments. All participants were instructed to sleep within one hour of their self-reported average sleep schedule for the last four days of the study break. In this report, we examined the shift in the DLMO from immediately before to immediately after the study break. We also examined sleep timing during the study break, because shifts in sleep timing can shift the DLMO, presumably via associated changes in light exposure [26, 27]. We hypothesized that when DSWPD and healthy controls were assessed under the same conditions, there would be greater sleep and circadian variability in DSWPD as compared to healthy controls.
2.0 Materials and Methods
2.1 Participants
Data from 41 participants (21–52 years) were derived from two previous studies [24, 25] and included 23 participants with DSWPD and 18 healthy controls. However, a wrist monitor failed in one DSWPD participant, resulting in a final sample of 22 DSWPD and 18 healthy controls (Table 1). All participants selected for analysis were required to have valid DLMOs, and selected healthy controls were required to be ≤52 years, to match the oldest DSWPD participant. All participants were medication free (including antidepressants and antipsychotics) and based on their responses to screening questionnaires, had no medical disorders (Tasto Health Questionnaire [28]) and were considered low risk for obstructive sleep apnea (Berlin Sleep Apnea Questionnaire [29]), restless legs syndrome (IRLSSG consensus criteria for Restless Legs Syndrome [30]), and psychiatric disorders (Minnesota Multiphasic Personality Inventory-2 [31]). Each DSWPD participant underwent a telephone clinical interview with a board-certified sleep clinician (MP, JW) who confirmed they met the ICSD-2 criteria [32] for delayed sleep phase disorder. These criteria included: (1) a chronic or recurrent complaint of an inability to fall asleep at a desired conventional clock time, (2) an inability to awaken at a desired and socially acceptable time, (3) a delayed but otherwise stable sleep schedule, as verified with at least one week of sleep times recorded on a sleep diary (provided to sleep clinicians prior to telephone interview), (4) normal sleep quality and duration for age when allowed to choose a preferred sleep schedule and (5) sleep disturbance is not better explained by another current sleep disorder, medical, neurological, mental, substance use disorder or medication use. All participants passed a urine drug screen for common drugs of abuse and nicotine, and were not color blind as determined by the Ishihara test. All participants reported moderate caffeine and alcohol use (<300 mg/day of caffeine and <14 alcoholic drinks/week). All participants had not worked any night shifts in at least two months prior to the study and had not travelled across more than one time zone in the month preceding the study. Non-steroidal anti-inflammatory drugs (NSAIDs) were not permitted throughout the study as they can suppress melatonin [33]. All participants gave written informed consent prior to their participation. All participants expressing interest in treatment were referred for treatment at the end of the study. The study was approved by the Rush University Medical Center Institutional Review Board.
Table 1.
Baseline characteristics of the healthy control and delayed sleep-wake phase disorder participants.
| Controls Mean (S.D.) |
Delayed Sleep-Wake Phase Disorder Mean (S.D.) |
Group difference | |
|---|---|---|---|
| Sex | 9 M, 9 F | 13 M, 9 F | p=0.57 |
| Age (y) | 30.8 (7.3) | 28.0 (7.2) | p=0.16 |
| Body mass index (kg/m2) | 24.2 (3.1) | 23.4 (3.7) | p=0.48 |
| Race (White, Black, Asian, other) | 12 W, 3 B, 1 A, 2 O | 15 W, 0 B, 4 A, 3 O | p=0.17 |
| Employment (FT, PT, not working) | 6 FT, 8 PT, 4 NW | 4 FT, 8 PT, 10 NW | p=0.27 |
| Morning commitments (per week) | 2.8 (2.0) | 1.9 (2.3) | p=0.17 |
| Morningness-eveningness score | 50.4 (9.4) | 35.7 (6.8) | p<0.001 |
| Average prestudy sleep onset (h:mm) | 23:40 (0.94) | 02:44 (2.4) | p<0.001 |
| Average prestudy wake time (h:mm) | 07:47 (0.6) | 09:49 (1.4) | p<0.001 |
| Average prestudy sleep duration (h) | 8.1 (0.6) | 7.1 (2.9) | p=0.12 |
| DLMO before study break (h:mm) | 21:09 (1.3) | 23:09 (1.5) | p<0.001 |
FT = fulltime employment, PT = part-time employment.
Morning commitments were defined as regular commitments occurring before noon.
Bold highlights the group differences that reached statistical significance.
2.2 Protocol
Prior to the start of the study protocol, each participant noted their bedtime, estimated sleep onset latency, and wake time for a week with a daily sleep diary. Participants were not required to follow a fixed sleep-wake schedule during this week. From this, each participant’s average prestudy sleep onset time (bedtime + sleep onset latency) and wake time was calculated. All participants then participated in a 10-day protocol that consisted of two DLMO assessments, a five-day study break, and two more DLMO assessments. The five-day study break always occurred on a Thursday to Sunday. During the five-day study break, participants were instructed to sleep as desired on the first night, to recover from a two-hour delay in their bedtime the night before, as they sampled saliva in dim light for later determination of the DLMO. Thereafter, on the second, third, fourth and fifth nights of the study break, participants were to instructed to maintain their bedtime and wake times within a one-hour window (± 30 minutes), centered at their average prestudy sleep onset time and average prestudy wake time. This instruction was designed to stabilize their sleep and circadian timing before the DLMO was reassessed after the study break.
All participants wore a wrist actigraphy monitor (30 second epochs, Actiwatch Spectrum, Respironics, Bend, OR) on their non-dominant wrist throughout the 10-day study, and were instructed to press the event marker before and after sleep each day. To reduce study burden, participants were not required to complete sleep diaries during the study break.
The DLMOs collected immediately the evening before and evening after the study break were assessed from half-hourly saliva samples collected with Salivettes (Sarstedt, Newton, NC). The DLMOs were either collected in the laboratory in dim light (<5 lux) or at home (<50 lux). Others have shown that even in dark adapted individuals, minimal melatonin suppression occurs at light intensities <50 lux [34], and we have previously shown that saliva samples collected in <50 lux yield accurate DLMOs [24, 25]. All saliva samples were frozen at −20 °C after centrifuging and radioimmunoassayed (ALPCO, Inc., Macedon, NY, USA).
2.3 Data Analysis
The wrist actigraphy data was analyzed with the Actiware 6.0.7 program (Respironics, Bend, OR). The setting of nightly rest intervals for analysis was guided by the event markers, light data and activity levels [35]. The night-by-night actigraphic estimates of sleep onset time (clock time of the first epoch scored as sleep in each rest interval), wake time (clock time of the last epoch scored as sleep in each rest interval), total sleep time (number of minutes scored as sleep in each rest interval) and sleep efficiency (proportion of time from sleep onset to wake in each rest interval, scored as sleep, expressed as a percentage) were extracted. Due to the absence of sleep diary data, and an accurate “tried to fall asleep” time, sleep onset latency was not estimated. A DLMO was calculated for each phase assessment and defined as the clock time (with linear interpolation) when the melatonin concentration exceeded the mean of three low consecutive daytime values plus twice the standard deviation of these points [36]. This low threshold more closely tracks the initial rise of melatonin [37].
The baseline characteristics of the healthy controls and participants with DSWPD were compared with independent t-tests for continuous variables and Chi-squares for categorical variables. Global averages in sleep measures were compared with a MANOVA. Night-to-night variability in the sleep variables across nights 2–5 were assessed with the root mean squared successive difference (RMSSD) [38]. Statistical significance for all tests was determined at p<0.05 using two-tailed statistical tests unless otherwise stated.
3.0 Results
3.1 Baseline characteristics
The baseline characteristics of the healthy control and DSWPD participants are shown in Table 1. There were no group differences in sex, age, body mass index, race, employment status, or number of morning commitments. The average morningness-eveningness score derived from the Morningness-Eveningness Questionnaire [39] was significantly different between groups, revealing as expected, more eveningness in the DSWPD sample. Similarly, before the study started, the DSWPD participants reported falling asleep on average about three hours later and waking on average about two hours later than the controls. While not statistically significant, the DSWPD participants reported on average one hour less sleep per night than the healthy controls. Lastly, before the study break, the average DLMO of the DSWPD participants was two hours later than the average DLMO in the healthy controls.
3.2 Sleep
Sleep variables were first averaged across the last four nights of the study break to examine global group differences. As expected, the DSWPD participants had on average a significantly later sleep onset time (02:20 ± 1.2 vs. 00:10 ± 1.0, p<0.001) and wake time (9:42 ± 1.5 vs. 7:53 ± 1.3, p<0.001), than the healthy controls. There was no difference in total sleep time between the DSWPD participants and healthy controls (404.1 ± 50.6 vs. 425.6 ± 51.7, p=0.19). Sleep efficiency, as calculated after sleep onset, was on average equally good between groups (91.9 ±3.4 vs. 91.9 ±3.0, p=0.97).
The effects of weekend (Friday and Saturday nights) and weekdays (Sunday and Monday nights) on sleep variables in both groups were also examined. As expected, there was a group main effect by which DSWPD participants slept later than healthy controls (p<0.001), and a weekend effect by which both groups had a significantly later sleep onset and wake time on weekend versus weekdays (p=0.03). However, there was no group by weekend effect (p=0.44). On average, the DSWPDs went to sleep 30.2 minutes later and woke 36.8 minutes later on the weekend, whereas the healthy controls only went to sleep 7.7 minutes later and woke 27.2 minutes later on the weekend. There were no weekday versus weekend effects on total sleep time or sleep efficiency in either group (all ps>0.37).
The variability in the sleep variables during the study break are shown in Figures 1–4. The RMSSD data were first log transformed, before they were compared between groups, but the untransformed RMSSD data are reported. This analysis revealed that the DSWPD participants had significantly more variable wake times (1.31 ± 1.00 vs. 0.72 ±0.56, p=0.015) and significantly more variable total sleep time (80.62 ± 50.83 vs. 46.20 ± 30.28, p=0.012) than healthy controls. However, variability in sleep onset time (1.09 ± 0.76 vs. 0.84 ±0.53, p=0.30) and sleep efficiency (3.13 ± 1.73 vs. 3.56 ± 2.71, p=0.93) was similar between groups. There were no weekday versus weekend difference in RMSSD values for each sleep variable in either group (all ps>0.45). There was also no significant association between RMSSD values for each sleep variable and the number of morning commitments per week (all ps>0.34).
Figure 1.
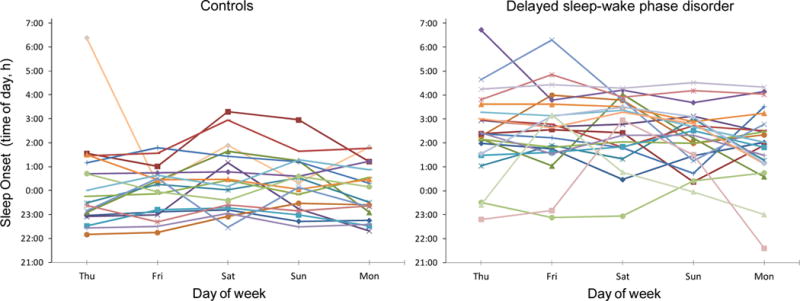
Sleep onset times during the study break in healthy controls (left) and participants with delayed sleep-wake phase disorder (DSWPD, right). Participants were permitted to sleep at any time on Thursday night to recover from study-induced sleep deprivation. After that, on Friday through Monday nights, participants were instructed to sleep within 30 minutes of their average prestudy sleep onset and wake times. The variability in sleep onset on Friday through Monday was not significantly different between the DSWPD participants and healthy controls (p=0.30). The same symbols and colors are used for the same participants in the other figures.
Figure 4.

Sleep efficiency (after sleep onset) during the study break in healthy controls (left) and participants with delayed sleep-wake phase disorder (DSWPD, right). Participants were permitted to sleep at any time on Thursday night to recover from study-induced sleep deprivation. After that, on Friday through Monday nights, participants were instructed to sleep within 30 minutes of their average prestudy sleep onset and wake times. The variability in sleep efficiency on Friday through Monday was not significantly different between the DSWPD participants versus healthy controls (p=0.93). The same symbols and colors are used for the same participants in the other figures.
3.3 Circadian Phase
There was no sex difference in the DLMO measured before and after the study break (ps>0.64). The phase angle difference between the average sleep onset time during the last 4 days of the study break and the DLMO assessed immediately after the study break, did not differ between the DSWPDs and healthy controls (3.0 ± 1.0 h vs. 3.4 ±1.3 h, p=0.35, respectively). The DLMOs before and after the study break are shown in Figure 5. As expected, the DSWPDs on average had later DLMOs than the healthy controls (p<0.001), but overall there was not a significant shift in the DLMO (p=0.42). There was a time by group interaction trend by which the DLMO delayed over the study break by average of 8.6 minutes in the DSWPD participants, whereas the DLMO advanced in the healthy controls by an average of 22.0 minutes (p=0.071), although the magnitude of these changes in the DLMO were small. As hypothesized, only two healthy controls but six DSWPD participants had more than a one-hour shift in their DLMOs from before to after the study break, but this did not reach statistical significance (one-tailed Fisher’s exact test p=0.19). Furthermore, no healthy controls had more than a 1.5-hour shift in their DLMO while three DSWPD participants did, but this also did not reach statistical significance (one-tailed Fisher’s exact test p=0.16). The only sleep variability parameter that significantly correlated with the shift in the DLMO was sleep onset variability, which was positively associated with a larger shift in the DLMO (r=0.46, p=0.03), but only in the DSWPD participants.
Figure 5.
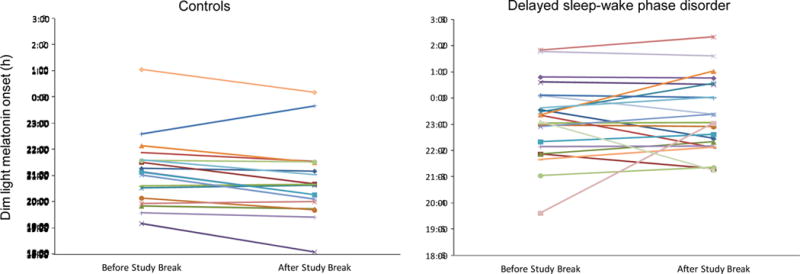
The clock time of the dim light melatonin onsets (DLMOs) measured immediately before and after the 5 day study break in healthy controls (left) and participants with delayed sleep-wake phase disorder (DSWPD, right). Participants were permitted to sleep at any time on the first night of the study break, but for the remaining 4 nights participants were instructed to sleep within 30 minutes of their average prestudy sleep onset and wake times. Only 2 healthy controls but 6 DSWPD participants had more than a 1 hour shift in the DLMO. The same symbols and colors are used for the same participants in the other figures.
4.0 Discussion
In this study, we examined sleep and circadian variability in people with DSWPD as compared to controls. All participants received the same instruction: to maintain their sleep schedule for four days within a one-hour window centered around their prestudy average sleep onset and wake times. We found increased sleep and circadian variability in DSWPD participants versus healthy controls even with this instructed stabilization of sleep schedule. Specifically, DSWPD participants were more variable in their wake times and total sleep time than healthy controls. These differences are consistent with the observation that people with DSWPD often wake later when their social schedule permits, but force themselves to wake up earlier, to meet morning social obligations [22]. This behavior would be expected to lead to increased variability in wake times, and consequently increased variability in total sleep time in DSWPD. Of note, DSWPD participants did not show increased variability in sleep onset times or sleep efficiency (after sleep onset) as compared to healthy controls, consistent with the notion that their delayed sleep onset is relatively stable [1] and their sleep, once initiated, is usually normal for age [1, 2]. Indeed, both the DSWPDs and healthy controls mostly had high sleep efficiencies after initially falling asleep. We also found a higher prevalence of a shift in the DLMO from before to after the study break in participants with DSWPD than in healthy controls, consistent with higher week to week DLMO variability reported in an earlier study [23]. Overall almost a third of DSWPD participants showed a shift in the DLMO of more than one hour during the study break despite an instruction to maintain a stable sleep schedule. However, the shift in the DLMO was only positively correlated with variability in sleep onset (and not wake time) and only in the DSWPD participants. It is not clear why this is the case, but we note that due to our instruction to all participants to stabilize their sleep schedule, the variability in sleep and circadian timing observed in this study is likely significantly less than what would naturally occur in the field.
While we did not measure any clinical outcomes in this study, the increased sleep variability in DSWPD could be clinically significant, as others have reported that increased sleep variability is associated with worse health outcomes, including depression [40], insomnia [41], obesity [42] and insulin resistance [43]. Future studies could examine the relationship between sleep variability in DSWPD and clinical outcomes. Additionally, it is possible that the health benefits of post-awakening light and/or afternoon/evening melatonin for DSWPD extend beyond eliciting a circadian phase advance (and the associated increase in sleep duration), to also enabling a more stable sleep schedule and reduced sleep variability. Currently, the collection of at least a week of sleep diary and/or wrist actigraphy monitoring to “demonstrate a delay in the timing of the habitual sleep period” is suggested as part of the diagnosis of DSWPD [1]. When considering treatment, we encourage clinicians to also examine the variability in sleep timing, as this is likely to influence the variability in the DLMO. A DSWPD patient with higher sleep variability is more likely to have a shifting, rather than stable DLMO. Thus, if the DLMO is used to guide the timing of post-awakening light and/or afternoon/evening melatonin treatment [19–21], treatment may be optimized if sleep timing variability is also considered. However, we note that the additional consideration of sleep variability in treating DSWPD requires more examination.
We observed a range in the timing of the DLMO in our sample of DSWPD participants (Figure 5), who underwent a telephone clinical interview with a board-certified sleep clinician who confirmed that they met the ICSD-2 criteria for DSWPD [32]. The range in DLMO highlights the importance of measuring the DLMO to assist in the diagnosis of DSWPD, which is increasingly encouraged [1, 14]. One DSWPD participant had a DLMO as early as 19:37. This participant was employed fulltime and reported an average bedtime 01:00 and wake time of 7:00. If the clinician had known the timing of his DLMO, they would have suspected insomnia rather than DSPD. This individual highlights the usefulness of the DLMO in diagnosing DSWPD.
In this study the DSWPD participants did not significantly differ from the sample of healthy controls in a number of important factors, including age, sex, body mass index, race, employment status, and number of morning commitments. These similarities between the two groups increase our confidence that the differences in sleep and circadian variability were due to DSWPD, and not some external related factor. Nonetheless, only 25% of our samples were employed full time and on average the healthy controls and DSWPD participants reported only 2–3 morning commitments per week (Table 1). The variability in sleep and circadian timing could be expected to be greater in DSWPD patients who work full time or have more morning commitments. Notably, we assessed objective sleep variability with wrist actigraphy and did not rely on subjective sleep reports, which can be less accurate. Nonetheless, we acknowledge several weaknesses in our study, including that we were not able to assess sleep across an entire seven-day week (although we did assess some weekdays and weekends). Also, we assessed sleep after a night where participants could sleep whenever they wanted to, in order to recover from some study-induced sleep deprivation, and this could have potentially influenced the sleep and circadian variability during the study break. However, both the DSWPD participants and healthy controls were assessed in the same conditions, increasing the likelihood that DSWPD is indeed associated with greater sleep and circadian variability. Additionally, as prestudy sleep was assessed with a sleep diary and not wrist actigraphy, it remains possible that participants may have misrepresented their sleep before the study began, thereby making it more difficult for them to follow the fixed sleep schedule during the study break. However, there was no incentive for participants to misrepresent their sleep. We also note that while our sample of 22 DSWPD participants is the largest sample to date to examine sleep and circadian variability in DSWPD, this relatively small sample size may limit the statistical power and generalizability of our findings. We therefore encourage other researchers with wrist actigraphy or multiple DLMO assessments in larger samples of DSWPD participants to examine their data with a focus on sleep and circadian variability. In conclusion, the results of this study suggest increased sleep and circadian variability in DSWPD as compared to healthy controls. Increased sleep variability is likely to lead to increased variability in the DLMO, and this should be taken into consideration when timing light and/or melatonin treatments relative to the DLMO to treat DSWPD.
Supplementary Material
Figure 2.
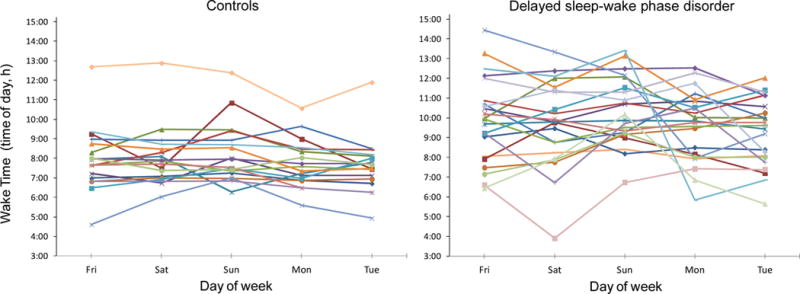
Wake times during the study break in healthy controls (left) and participants with delayed sleep-wake phase disorder (DSWPD, right). Participants were permitted to wake at any time on Friday morning to recover from study-induced sleep deprivation. After that, on Saturday through Tuesday mornings, participants were instructed to sleep within 30 minutes of their average prestudy sleep onset and wake times. The variability in wake time on Saturday through Tuesday was significantly higher in the DSWPD participants versus healthy controls (p=0.015). The same symbols and colors are used for the same participants in the other figures.
Figure 3.
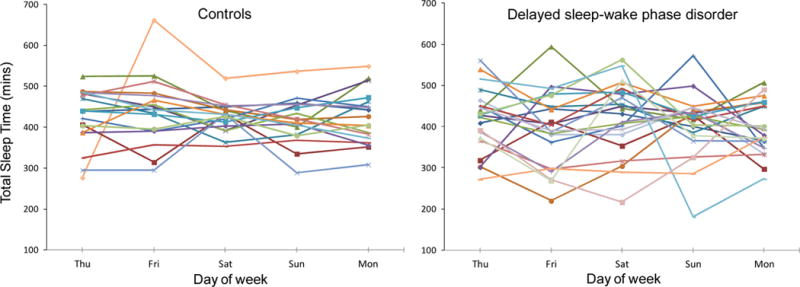
Total sleep time during the study break in healthy controls (left) and participants with delayed sleep-wake phase disorder (DSWPD, right). Participants were permitted to sleep at any time on Thursday night to recover from study-induced sleep deprivation. After that, on Friday through Monday nights, participants were instructed to sleep within 30 minutes of their average prestudy sleep onset and wake times. The variability in total sleep time on Friday through Monday was significantly higher in the DSWPD participants versus healthy controls (p=0.012). The same symbols and colors are used for the same participants in the other figures.
Delayed Sleep Wake Phase Disorder is associated with greater sleep variability
Greater sleep variability may contribute to greater variability in circadian timing
When treating Delayed Sleep Wake Phase Disorder consider sleep variability
Acknowledgments
We thank Marissa Dziepak, Amy Feehan, Jazmin Garcia, Toni Iurcotta, Julia Kleinhenz, Athanasios Kondilis, Devon Langston, Thomas Molina, Brock Peiffer, Neha Singla, Michael Steinert, Christina Suh, Haein Sung, Asantewaa Ture, and Gabriela Velazquez for their assistance with data collection and analysis. This research was supported by an R01 grant (AT007104) from the National Center for Complementary and Integrative Health of the National Institutes of Health to HJB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary and Integrative Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: All authors report none.
Clinical trial: Circadian Phase Assessments at Home, http://clinicaltrials.gov/show/NCT01487252, NCT01487252.
References
- 1.American Academy of Sleep Medicine. The International Classification of Sleep Disorders-3 Diagnostic and Coding Manual. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Alvarez B, Dahlitz MJ, Vignau J, Parkes JD. The delayed sleep phase syndrome: clinical and investigative findings in 14 subjects. J Neurol Neurosurg Psychiatry. 1992;55:665–70. doi: 10.1136/jnnp.55.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson S, Lack L. Circadian melatonin and temperature taus in delayed sleep-wake phase disorder and non-24-hour sleep-wake rhythm disorder patients: An ultradian constant routine study. J Biol Rhythms. 2016;31:387–405. doi: 10.1177/0748730416650069. [DOI] [PubMed] [Google Scholar]
- 4.Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiol Int. 2001;18:263–71. doi: 10.1081/cbi-100103190. [DOI] [PubMed] [Google Scholar]
- 5.Uchiyama M, Okawa M, Shibui K, Liu X, Hayakawa T, Kamei Y, Takahashi K. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23:553–8. [PubMed] [Google Scholar]
- 6.Richardson CE, Gradisar M, Barbero SC. Are cognitive “insomnia” processes involved in the development and maintenance of delayed sleep wake phase disorder? Sleep Med Rev. 2016;26:1–8. doi: 10.1016/j.smrv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson SA, Kennaway DJ, Lack L. Nocturnal melatonin profiles in patients with delayed sleep-wake phase disorder and control sleepers. J Biol Rhythms. 2015;30:437–48. doi: 10.1177/0748730415591753. [DOI] [PubMed] [Google Scholar]
- 8.Burgess HJ. Partial sleep deprivation reduces phase advances to light in humans. J Biol Rhythms. 2010;25:460–8. doi: 10.1177/0748730410385544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micic G, Lovato N, Gradisar M, Ferguson SA, Burgess HJ, Lack LC. The etiology of delayed sleep phase disorder. Sleep Med Rev. 2016;27:29–38. doi: 10.1016/j.smrv.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 11.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–93. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 12.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 14.Murray JM, Sletten TL, Magee M, Gordon C, Lovato N, Bartlett DJ, Kennaway DJ, Lack LC, Grunstein RR, Lockley SW, Rajaratnam SM. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. doi: 10.1093/sleep/zsw002. in press. [DOI] [PubMed] [Google Scholar]
- 15.Lockley SW. Timed melatonin treatment for delayed sleep phase syndrome: the importance of knowing circadian phase. Sleep. 2005;28:1214–6. doi: 10.1093/sleep/28.10.1214. [DOI] [PubMed] [Google Scholar]
- 16.Keijzer H, Smits MG, Duffy JF, Curfs LM. Why the dim light melatonin onset (DLMO) should be measured before treatment of patients with circadian rhythm sleep disorders. Sleep Med Rev. 2014;18:333–9. doi: 10.1016/j.smrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Strollo PJ, Jr, Badr MS, Coppola MP, Fleishman SA, Jacobowitz O, Kushida CA. The future of sleep medicine. Sleep. 2011;34:1613–9. doi: 10.5665/sleep.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13:47–60. doi: 10.1016/j.smrv.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: Advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015;11:1199–236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol. 2012;590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magee M, Marbas EM, Wright KP, Jr, Rajaratnam SM, Broussard JL. Diagnosis, cause, and treatment approaches for delayed sleep-wake phase disorder. Sleep Med Clin. 2016;11:389–401. doi: 10.1016/j.jsmc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29:1075–80. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]
- 24.Burgess HJ, Wyatt JK, Park M, Fogg LF. Home circadian phase assessments with measures of compliance yield accurate dim light melatonin onsets. Sleep. 2015;38:889–97. doi: 10.5665/sleep.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess HJ, Park M, Wyatt J, Fogg LF. Home dim light melatonin onsets with measures of compliance in delayed sleep phase disorder. J Sleep Res. 2016;25:314–7. doi: 10.1111/jsr.12384. [DOI] [PubMed] [Google Scholar]
- 26.Burgess HJ, Eastman CI. Early versus late bedtimes phase shift the human dim light melatonin rhythm despite a fixed morning lights on time. Neurosci Lett. 2004;356:115–8. doi: 10.1016/j.neulet.2003.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgess HJ, Eastman CI. A late wake time phase delays the human dim light melatonin rhythm. Neurosci Lett. 2006;395:191–5. doi: 10.1016/j.neulet.2005.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasto DL, Colligan MJ, Skjei EW, Polly SJ. Health Consequences of Shift Work. Cincinnati: NIOSH Publication #78-154; 1978. [Google Scholar]
- 29.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 30.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med. 2014;15:860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Butcher JN, Dahlstrom WG, Graham JR, Tellegen A, Kaemmer B. MMPI-2 (Minnesota Multiphasic Personality Inventory-2): Manual for Administration and Scoring. Minneapolis: University of Minnesota Press; 1989. [Google Scholar]
- 32.American Academy of Sleep Medicine. The International Classification of Sleep Disorders-Revised: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 33.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans. Physiol Behav. 1996;59:133–9. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 34.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SR, Weng J, Rueschman M, Dudley KA, Loredo JS, Mossavar-Rahmani Y, Ramirez M, Ramos AR, Reid K, Seiger AN, Sotres-Alvarez D, Zee PC, Wang R. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38:1497–503. doi: 10.5665/sleep.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 37.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28:714–8. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straus LD, Drummond SP, Nappi CM, Jenkins MM, Norman SB. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress. 2015;28:8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 40.Suh S, Nowakowski S, Bernert RA, Ong JC, Siebern AT, Dowdle CL, Manber R. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13:469–75. doi: 10.1016/j.sleep.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, Vgontzas AN, Liao D. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16:1489–94. doi: 10.1016/j.sleep.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor BJ, Matthews KA, Hasler BP, Roecklein KA, Kline CE, Buysse DJ, Kravitz HM, Tiani AG, Harlow SD, Hall MH. Bedtime variability and metabolic health in midlife women: The SWAN Sleep Study. Sleep. 2016;39:457–65. doi: 10.5665/sleep.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


